Abstract
We explored in-gap states (IGSs) in perovskite oxide heterojunction films. We report that IGSs in these films play a crucial role in determining the formation and properties of interfacial two-dimensional electron gas (2DEG). We report that electron trapping by IGSs opposes charge transfer from the film to the interface. The IGS in films yielded insulating interfaces with polar discontinuity and explained low interface carrier density of conducting interfaces. An ion trapping model was proposed to explain the physics of the IGSs and some experimental findings, such as the unexpected formation of 2DEG at the initially insulating LaCrO3/SrTiO3 interface and the influence of substitution layers on 2DEG.
Similar content being viewed by others
Introduction
The discovery of two-dimensional electron gas (2DEG) at perovskite oxide interfaces paved the way to observations of new phenomena. For example, the LaAlO3/SrTiO3 (LAO/STO) interface1 has been shown to exhibit two-dimensional (2D) superconductivity2,3,4,5, ferromagnetism6,7,8,9,10, as well as coexistence of these two phases11,12,13. Despite more than a decade of extensive research, the mechanism of 2DEG formation is still debated14. The polar catastrophe model1,15 is the most widely accepted model (Fig. S1). This model postulates that LAO is polar, with alternating sheets of positive and negative charge in the (001) direction, while STO is not polar. To accommodate the diverging potential caused by the polar discontinuity at the LAO/STO interface, about one half of the electrons and holes accumulate at the interface, forming the 2DEG. This model successfully explains the origin of 2DEG and why the 2DEG at the n-type LAO/STO interface requires a critical thickness of 4 unit cells (u.c.) of LAO while STO has to be TiO2-terminated. However, p-type interfaces1 and some n-type interfaces are not conductive, (e.g., LaCrO3/STO16 and LaMnO3/STO17). Furthermore, the predicted electrical field within the critical thickness is absent18,19, and the observed interface carrier density (n s ) at the LAO/STO interface is much lower (<10%) than the theoretical value20.
Although significant progress has been made in explaining the formation of 2DEG at some interfaces, little attention has been devoted to insulating interfaces with polar discontinuity. The formation mechanism of insulating interfaces must play a vital role in the 2DEG formation and is as important as the polar catastrophe model. Characterization of this mechanism is important for successful formulation of the 2DEG formation theory.
The in-gap state (IGS) is a promising candidate mechanism to fill this gap in the physical understanding. IGSs can absorb the electrons that transfer to the STO conduction band and form 2DEG in the polar catastrophe model. Thus, IGSs can yield a weak electric field in films, a low n s , and even an insulating interface. Current research on IGSs focuses on IGSs in substrates, which are generated by Ti ions in the near-interfacial STO21,22,23. These states can be found at both insulating and conducting LAO/STO21,23 interfaces. However, these substrate IGSs cannot explain the insulation properties of interfaces with polar discontinuity, because the difference between these STO-based interfaces is in their films, rather than substrates. Therefore, it is conceivable that IGSs in films play a role in the formation of interfacial 2DEG. In this study, we thoroughly explored film IGSs both experimentally and theoretically, and argued that, combining our results with the polar catastrophe model, a relatively complete theory can be achieved.
Results and Discussions
The 2DEG at LaCrO3/SrTiO3 interfaces
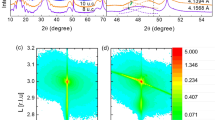
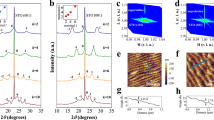
To explore the physical processes yielding insulating interfaces, we grew LaCrO3 (LCO) films on TiO2-terminated (001) STO substrates, at different oxygen partial pressures (P ox ), ranging from 6 × 10−8 to 1 × 10−6 Torr, using pulsed laser deposition (seen in sample growth). Previously, no 2DEG was observed at the LCO/STO interface16,24. To our surprise, 2DEG formed at the LCO/STO interface when the samples were grown at a very low P ox , under 9.0 × 10−7 Torr (Fig. 1(a)), and the STO substrates were insulating in all cases. The critical thickness for the 2DEG formation was also 4 u.c. (the illustration in Fig. 1(d)) and the n s of LCO/STO is similar with the previous reports on LAO/STO20 (Fig. 1(b)). At the same time, the films themselves exhibited different behaviors, i.e., in some samples they were semiconducting, owing to the high densities of oxygen vacancies (n ox ) in these films (Fig. S2(b)). X-ray photoelectron spectroscopy (XPS) measurements show a high percentage (~12%) of Cr2+ (Fig. 2(f)) and indicate a high n ox in LCO film. The Cr5+ cations resulted from the oxidation of Cr3+ in the near-surface region of LCO when the sample was exposed to air before the XPS measurement25,26. X-ray diffractometry (XRD) measurements indicated that the lattice constant of LCO obviously increased when the films were grown at a lower P ox (Fig. 1(d)). That is owing to the high n ox in LCO films27,28 and the formation of Cr2+ cations (Fig. 2(f)) which has a bigger radius29 than Cr3+.
Properties of LCO/STO heterojunctions. (a) Interfacial resistance-temperature curves for LCO/STO heterojunctions grown at different P ox (Torr). (b) Interfacial carrier density-temperature curves for LCO/STO heterojunctions grown at different P ox (Torr). (c) Absorption coefficients (α) of different LCO films, after subtracting the influence of STO. The red and yellow curves are the data for 2 u.c. (sample 1) and 17 u.c. (sample 2) LCO films grown at low P ox (7 × 10−7 Torr), and the green curve is the data for 30 u.c. LCO films (sample 3) grown at high P ox (4 × 10−6 Torr). (d) The lattice constant of the LCO film grown at different oxygen partial pressures, calculated from XRD data (see methods). The illustration in (d) is the interface resistance of LCO/STO with different LCO thicknesses when grown in 2 × 10−7 Torr. The critical thickness of 2DEG formation is 4 u.c.
The XPS data. The core level spectra of Sr 3d5/2 (a,b) and La 4d5/2 (d,e) for LCO/STO with different films thicknesses when the films were grown at a low P ox (a,d) and high P ox (b,e). The LCO(0 u.c.)/STO sample in (a) and (b) is bare STO. (c) The core level spectra of Ti 2p3/2 of LCO(1 u.c.)/STO (grown in 7 × 10−7 Torr). The black dashed lines are the binding energy of Ti3+ if exist. (f) The core level spectra of Cr 2p3/2 of LCO(20 u.c.)/STO (grown in 7 × 10−7 Torr). (g–i) The VBS of STO (g), LCO(20 u.c.)/STO grown in 7 × 10−7 Torr (h) and LCO(20 u.c.)/STO grown in 4 × 10−6 Torr (i).
The oxygen vacancies in STO substrate may dominate the conduction of the interface due to the low P ox 30,31. To explore the influence of the oxygen vacancies, we annealed some samples with 2DEG in pure oxygen of one atmospheric pressure for 4 hours (400 °C); after annealing, 2DEG still existed. We also measured the valence of Ti cations of LCO(1 u.c.)/STO grown in 7 × 10−7 Torr (Fig. 2(c)) using XPS. To avoid the influence of the polar catastrophe model (the critical thickness of charge transfer is 4 u.c.1,15), the LCO thickness is only 1 u.c. We found there are almost no Ti3+ signals. That means n ox in STO substrate should be very low. In addition, we found that the temperature dependence of n s of the LCO/STO interface is different from oxygen-defects induced conduction of STO32 (Fig. 1(b)) and the critical thickness of 2DEG formation (4 u.c.) cannot be explained by oxygen defects. These findings suggest that oxygen defects may be not the main mechanism of 2DEG formation at the LCO/STO interface. For a more in-depth investigation, we grew LaMnO3 LaCoO3 and STO films on STO, under the same growth conditions as those of LCO (the P ox was 6 × 10−8 Torr), and observed no conducting interface. As we know, oxygen outwards diffusion is film dependent, and the film will influence the n ox formed in STO side. Previous reports have revealed that the oxygen migration barriers energy (E m , the energy barriers for hopping) of LCO is larger than E m of LaMnO3, LaCoO3 and STO33,34. So that, the n ox in STO side of LCO/STO may be slightly lower than that of LaMnO3/STO LaCoO3/STO. We also annealed the STO substrate under the growth condition of LCO films, at a P ox of 6 × 10−8 Torr, for 2 hours, following which the STO substrate was still found to be insulating. In fact, we found that the STO substrate becomes conductive only when the P ox is less than 2 × 10−8 Torr (annealed at 830 °C for 2 hours). If the LCO/STO interface becomes conducting owing to oxygen defects, the LaMnO3/STO LaCoO3/STO and STO/STO interfaces, as well as the annealed STO substrate, should all have been conducting; however, this was not the case. Based on these results, we concluded that oxygen defects are necessary but not sufficient to explain the formation of 2DEG at the LCO/STO interface.
The IGS and band alignment of LCO/STO
We measured the optical transmission spectra of bare STO and of the LCO/STO heterojunctions by a UV-VIS-IR recording spectrophotometer and calculated the absorption coefficients (α) of them (Fig. 1(c)). After subtracting the contribution of STO, we observed several absorption peaks within the band gaps of LCO (1.6 eV16) and STO (3.2 eV14). These peaks were observed in thick films (regardless the interfacial 2DEG formation), while they were negligible in thin films, suggesting that the peaks are caused by the IGSs in the LCO films, rather than by the substrates or interfaces. We can find sample 3 (30 u.c.) has a lower α than sample 2 (17 u.c.) which is thinner and grown at a lower P ox than sample 3. If the density of IGSs is same for all samples, sample 3 should have a higher α. This abnormal phenomenon means P ox or oxygen vacancies may have a great influence on IGSs. Many reports have found that the ions neighboring oxygen vacancies can introduce IGSs, such as the Ti ions in STO34,35,36,37, the Al and La ion in LAO36 and so on. Due to the low P ox , we think the IGSs in the LCO films should result from the La and Cr ions neighboring the oxygen vacancies, and the oxygen vacancies are necessary for the formation of IGSs in films. Because sample 2 was grown at a lower P ox , the density of oxygen vacancies and IGSs should be high than sample 3, so that sample 2 has a higher α.
We also measured the band bending in STO substrates and LCO films, using XPS. The core level shift with film thickness changing can be used to quantitatively determine band bending of heterojunctions14,38. Here we use the core level shift of A-site cations (Sr 3d5/2 and La 4d5/2, Fig. 2(a,b,d,e)) to determine the band bending in STO and LCO16,18,39. In fact, we found the core level shifts of A-site and B-site cations are very similar (Fig. S2(d–h)). With these data, downward band bending toward the interface was found for both STO and LCO film. The valence band maximum (VBM) of STO and LCO were determined by extrapolating the leading edge of the valence band spectra (VBS) of STO substrate38,39 and LCO(20 u.c.)/STO (Fig. 2(g–i)). Because oxygen pressure can influence VBM of LCO (Fig. 2(h,i)), we use 0.26 eV as the VBM of LCO for the LCO films grown in 7 × 10−7 Torr (Fig. 2(h)) and use 0.35 eV as the VBM of LCO for the LCO films grown in 4 × 10−6 Torr (Fig. 2(i)). Together with the absorption coefficients data, this analysis allowed us to map the band structure of LCO/STO, including the IGSs in the LCO films (Fig. 3).
Band alignments of LCO/STO. Band alignment of the LCO/STO interface for a film grown at a high P ox (a) and low P ox (b). Here we only show the IGSs located in the range of 0.55–0.86 eV (Fig. 1(c)). The illustration in (b) is the schematic diagram of the electrons distribution (upper row) and the corresponding potential (second row) near the LCO/STO interface. The green balls in the illustration are electrons.
The band bending on STO side result from the distribution of transferred electrons on STO side40 and the mismatch between film and substrate which can lower the energy of d xy of Ti7. The band bending on LCO side can be understood by combining IGSs and the polar catastrophe model. As described in polar catastrophe model, the electric potential caused by the polar-discontinuity at interface should be eliminated by the charge transfer from film to the interface15 (Fig. S1(a,b)). However, the IGSs in films can trap a large part of transferred electrons, weaken the charge transfer and make the electric potential cannot be completely eliminated. The residual potential result in a band bending on LCO sides (illustration in Fig. 3(b)).
We can find that when the LCO films were grown at a high P ox (Fig. 3(b)), the near-interfacial IGSs in these films are below the Fermi energy, while the STO conduction band is higher than the Fermi energy. Thus, electrons are localized in the IGS and could not move to the STO conduction band, preventing the formation of 2DEG. On the other hand, the IGS in the films and the STO conduction band near the interface are both below the Fermi energy when the films were grown at a low P ox (Fig. 3(a)). As a result, 2DEG formed at the LCO/STO interface, as we have observed (Fig. 1(a,b)). Therefore, because some electrons are localized in the IGSs in these films, n s should be lower than the theoretical value of the polar catastrophe model, as found previously20.
Strangely, we found that there exists a band bending on the STO side when no 2DEG formed at the interface, and the wells on STO side are very deep (Fig. 3). Considering that the band bending on STO side mainly results from the transferred electrons40, the former means some electrons still can transfer from film to STO side even when the LCO films were grown in a relatively high P ox . However, because the IGSs in STO can trap a lot of electrons34,35,36,37, these transferred electrons are localized and the interface is still insulating. We think the latter should result from the oxygen vacancies induced by the low P ox . Firstly, we found the oxygen vacancies can introduce a band bending on STO side (Fig. S2(c)). Secondly, oxygen vacancies can increase the dielectric constant of STO41, result in a larger distribution length of electrons on STO side42,43 and then enhance the band bending on STO side40. As a result of this two reasons, the wells on STO side become very deep.
The ion trapping model
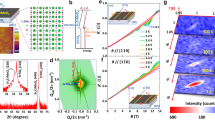
From the absorption coefficient results, it was very confusing to find that the IGSs in different films are very similar to thick films. Thus, we expect the charge transfer to be affected by the detailed characteristics of IGSs. These characteristics should be material-related and should compete with the charge transfer owing to the polar discontinuity in the polar catastrophe model. To determine these key characteristics of IGSs, we analyzed the A3+B3+O3/STO interface that has been studied previously16,17,44,45,46,47,48,49,50,51. We summarized these reports in Fig. 4(a) and found a strong relationship between the 2DEG formation and the third ionization energy (I 3) of the film B site cations. 2DEG can form only if I 3 is less than or equal to 2963 KJ/mol (I 3 of Ga3+)29. As is known, the ionization energy is the energy required to remove one electron, and it can also be regarded as a propensity to trap an electron by atoms/cations. We suppose that such an electron trapping propensity of cations is the key determinant of IGSs, and cations yield IGSs in films just like substrate Ti cations are at the origin of substrate IGSs21,22,23,34,35,36,37. Here, we propose an ion trapping model and try to explain how the IGSs in films work.
Relationship between 2DEG and ionization energy, and the schematic of IPW. (a) The interface electrical conductivity16,17,44,45,46,47,48,49,50,51 and the third ionization energy (I 3) of B site cations (Table S1), for different A3+B3+O3/STO heterojunctions have been reported. (b) Schematic of the cation IPW; (c–e) The r dependence of the potential energy of one electron on the position marked by a light blue dashed curve in (b) for E = 0 (c), 2EeR > eU (d), and 2EeR = eU (e). The situation considering the actual electric field in the polar catastrophe model is shown in Fig. S1.
Our model utilizes the concept of the ion potential well (IPW), which is the IGS in a film. IPWs are generated by film cations/anions and can trap electrons/holes, preventing them from reaching the interface and thus weakening/destroying 2DEG. The competition between the transfer of charges (polar catastrophe model) and their trapping by ions (ion trapping model) determines the formation of interfacial 2DEG. Different ions have different IPWs, which explains why some interfaces are conductive while others are insulating. We emphasize that a polar system is actually not needed for IPWs, but it is necessary for the formation of 2DEG as described in the polar catastrophe model1,15. Our model is simple and does not take into account complex quantum mechanisms. This is somewhat similar to the polar catastrophe model. Our model is a simple try, with it we provide a new and effective way to understand the formation of 2DEG.
Because the trapped charges will move around the ion core and cannot enter into the ion core due to the Coulomb repulsion, we suppose that an IPW is a spherical-shell well with a depth of eU (Fig. 4(b)) and a thickness of d. We also assume that if the distance between one charge and one IPW is less than the trapping radius R, this charge will be trapped by the IPW: this charge will be located within the IPW’s thickness d. The trapped charge cannot escape from the IPW unless it is sufficiently energetic. We emphasize that charge transfer is driven by the electric field caused by the polar discontinuity at the interface (E p ), as described by the polar catastrophe model. Here, we shall use the cation IPW to explain how our model works (Fig. 4(c–e)). If an electron that is trapped by the cation IPW moves a distance 2 R in the direction opposite to that of the electric field (E), its energy will increase by 2EeR. Here, e is the electron charge, and average E p is the primary part of E. For 2EeR ≤ eU, the electron cannot escape from the IPW, and 2DEG does not form at the interface (Fig. 4(c)). For 2EeR > eU, the electron can escape from the IPW, moving to the interface (Fig. 4(d)); thus, 2EeR > eU is the condition for 2DEG formation. The escaped charge generates an additional electric field (E i ) and weakens E, so that when 2EeR = eU no more electrons can escape (Fig. 4(e)). 2EeR = eU is the equilibrium condition between charge transfer and charge trapping.
Model details
As discussed above, the electric field in polar-non-polar heterojunctions has two main components (Eq. (1)), one owing to polar discontinuity and the other owing to the charges that move to the interface.
The quantity ε r is the relative dielectric constant of the film. n i is the real interface charge density. n p is the theoretical interfacial charge density in the polar catastrophe model, which is ~3.28 × 1014/cm2 for A3 +B3+O3/STO(001) heterojunctions. It is necessary to distinguish between n i and the real interface carrier density (n s ), because not all charges at the interface contribute to conductivity, owing to the IGS in STO19,22.
The IPW depth eU and the trapping radius R are the key factors of the ion trapping model. In our calculations, we assumed that eU results from the Coulomb interaction. By a logical extension of the relationship between 2DEG and ionization energy (Fig. 4(a)), the ionization energy of cations or the electronic affinity of anions (T/ε r ) can be used as a measure of the trapping ability of cations or anions, the main part of eU. Considering the valence of A or B site cations and O2− anions, T is the third ionization energy (I 3) for cations, the second electronic affinity (A 2) for O2− anions at the A3+B3+O3/SrTiO3 interface (Table S1). On the other hand, the Coulomb potential energy created by all ions in the film also affects eU. This potential energy exists everywhere in the film and is the background potential energy (eU 0(r)/ε r ), where r is the charge-IPW distance. It is necessary to subtract eU 0(r)/ε r from T/ε r to obtain the effective eU (Eq. (2)).
To calculate eU 0, we coded a simple program (see Program details). We found that eU 0(r)/ε r is anisotropic and attains a minimum at r = R (Fig. S4(f)). Here, we only focus on the [001] orientation and take the maximum value of \(eU\,(T/{\varepsilon }_{r}-e{U}_{0}(R)/{\varepsilon }_{r})\) as the effective depth. Thus, eU can be regarded as a function of R. With the value of eU, 2EeR = eU and Eq. (1), we can calculate n i (Eq. (3)) and the percentage trapping of electrons (k) (Eq. (4)) of one type of IPW (see details in Supplementary Information).
The parameters k and n i are independent of the film thickness or the number of IPWs, resulting in a constant n s , which is in agreement with the experimental values20.
2DEG can form only when k < 100%, so the condition for 2DEG formation can be written as Eq. (5).
Equation (5) is independent of ε r and T is the only variable factor for different ions, so T of A or B site cations (T = I 3 for A3+ or B3+ cations) is the most important factor that determines the formation of 2DEG as shown in Fig. 4(a). R is the only unknown factor, which can be indirectly determined experimentally. To simplify our model, we assume that ions at the same sites (A, B, or O2−) have the same R. For ε r = 1, the left side of Eq. (5) is the energy increment (equivalent to 2EeR), which is ion-independent for the same-site-ions, while the right side of Eq. (5) is the IPW depth of different ions. To obtain R, we plot the two sides of Eq. (5) as a function of R, for A site ions, B site ions, and O2− ions in the [001] direction (Fig. 5). 2DEG was discovered in LaGaO3/STO17,44, NdGaO3/STO44, NdAlO3/STO44, PrAlO3/STO44, LaVO3/STO45, LaTiO3/STO46,47, DyScO3/STO48, SmTiO3/STO52 and GdTiO3/STO49, but it was not observed in LaCrO3/STO16,50, LaMnO3/STO17, BiMnO3/STO51, and LaCoO3/STO. For all types of ions in these films, if the IPW depth (the colored curves in Fig. 5) is larger than the energy increment (the black curve in Fig. 5) for a certain value of R, no charge can escape from the IPW, and 2DEG will not form at the interface. Otherwise, 2DEG will form. It should be noted that, because here our model only focuses on the perovskite A3+B3+O3/STO interfaces, the other kinds of interfaces cannot be included, such as gamma-Al2O3/STO53, CaZrO3/SrTiO3 54 and LSAT/STO55.
Simulation results for the two sides of Eq. (5) ([001] direction) as a function of R. (a–c) The situation for A3+, O2−, and B3+ IPWs. (d) The two sides of Eq. (5) for Cr3+, Co3+, Mn3+ (solid lines) and Cr3+ ex, Co3+ ex, Mn3+ ex (dashed lines). Cr3+ ex, Co3+ ex and Mn3+ ex are the IPW depth when considering the influence of lattice expansion. The IPW depths for different ions are color-coded curves, and the energy increase (2EeR) is the black curve with circles. The dashed curves in (a,c), and (d) are the value of R. In (b), the dashed curves indicate the ion radius of O2− (1.4 Å) and R (1.032 Å) at the crossing point between the IPW depth and energy change curves.
For B site cation IPWs, we observed that Mn3+, Co3+, Cr3+ have deeper IPWs than others, while Ga3+ and Cr3+ have very similar IPW depths (Fig. 5(c)). The trapping radius R should be located between the intersections of the IPW depth and energy increment, for Ga3+ and Cr3+ (Fig. 5(c)). In this article, we set R = 0.636 Å for B cite IPWs. For the A site cation IPWs, we found that the IPW depth are always less than the energy increment (Fig. 5(a)). This indicates that B site cation IPWs are stronger and play a more important role than A site cations in 2DEG formation. This is consistent with our findings (Fig. 4(a)). Here, we temporarily set R = 1.58 Å; at this value of R, the IPW depth and the energy increment are the most similar for all of the listed cations. The curves of O2− IPWs considering Eq. (5) in the p-type heterojunction are plotted in Fig. 5(b). Note that holes in the p-type heterojunctions can reach the interface only if R is below 1.032 Å. But, the R-value of the O2− IPW should be larger because the radius of O2− (1.4 Å29) is significantly larger. This result suggests that the O2− IPW is too deep and p-type 2DEG cannot form as have been found1. We also calculated the O2− IPW in the [110] and [111] directions, and found that, theoretically, p-type 2DEG may form at the (111) interface (Fig. S5).
The origin of 2DEG at LCO/STO interfaces
Inspired by the lattice enhancement of the LCO film (Fig. 1(d)), we studied the influence of the lattice constant on eU 0(R)/ε r . We found that eU 0(R)/ε r increases with increasing the lattice constant (Fig. S7(c)), decreasing the IPW depth eU. Nevertheless, using our method to calculate eU 0(R)/ε r , we found that oxygen vacancies cannot directly influence eU 0(R)/ε r because the electrons generated by oxygen vacancies have the same influence on eU 0(R)/ε r as O2− ions. For example, the decrease in eU of LCO was 0.6 eV/ε r when the lattice parameter was 3.957 Å (at 8 × 10−7 Torr) (Fig. 1(d)). This is sufficiently large for 2DEG formation at the LaCrO3/STO interface (Cr3+ ex in Fig. 5(d)), as the IPW depth of Cr3+ is near the critical value (~7.5/ε r eV). Apparently, oxygen vacancies and the induced lattice expansion are the keys to the formation of 2DEG in LCO/STO. But, for the other initially insulating interfaces, such as LaMnO3/STO and LaCoO3/STO, the change in the IPW depth caused by the lattice expansionwas relatively small compared with the required change of ~2.7 eV/ε r when considering a lattice parameter of 4 Å (Co3+ ex and Mn3+ ex in Fig. 5(d)). Thus, we determined that an insulator-metal transition caused by the introduction of oxygen vacancies cannot occur for these heterojunctions.
It is worthy to notice that eU is gradually varying with P ox because of the gradual change of LCO lattice constant with P ox (Fig. 1(d)). So that, there does not exist a critical P ox that the charge escaping from IPWs and the insulator-metal transition can happen. The interfacial conductivity and the probability of charges escaping from IPWs are gradually varying with the P ox . That is the reason for the gradual change of interfacial resistance (Fig. 1(a)) and why a charge transfer can happen under a relatively high P ox and result in a band bending on STO side (Fig. 3(a)).
The trapping radios
Using Eq. (4) and the as-determined R, the trapping ratios k were calculated for different ions (Table 1). The value of n i is determined by the cation’s IPW (A or B) with the strongest trapping ability (highest k) in the ABO3 film. It is worthy to notice that the calculated n i somewhat deviates from experimental values, because our calculations use the same R. For LaCrO3/STO, LaMnO3/STO, and LaCoO3/STO, Cr3+ IPW, Mn3+ IPW, and Co3+ IPW exhibit 100% trapping efficiency (Table 1); thus, 2DEG does not form. For LaAlO3 films, Al3+ IPW (k = 67.9%) is more efficient than La3+ IPW (k = 61.1%). The theoretical value of the interface charge density n i is 1.05 × 1014/cm2 and it corresponds to 0.16 Ti3+/u.c.2. This is in agreement with experimental findings19,22. Since many electrons on the STO side are localized19,56, n s is much lower than n i .
The influence of substitution layers on LAO/STO interfaces
The above discussion suggests that the IGSs in these films are a key factor deciding the formation and properties of 2DEG. Thus, by substituting some unit layers of a film with a heterojunction with 2DEG by other perovskite oxides, it could be possible to modulate the properties of 2DEG. Here, we replaced one unit layer of LAO(4 u.c.)/STO films with NdGaO3, LaGaO3, and NdAlO3. As expected, such substitutions affected n s . A strong relationship between n s and substitution layers was found (Fig. 6).
Influence of substitution layers on n s . (a) The influence of substitution oxide layers on n s . (b–d) The relationships between n s and substitution oxides, for replacing the first (b), third (c), and fourth (d) unit layers. The parameters k A and k B are k (trapping percentage, Eq. (4)) of A site and B site cations of the substitution layers. (k A + k B)/2 is the average k value.
When the first unit layer (closest to the interface) was replaced, n s depended on the lattice mismatch between the substitution oxide and the substrate (Fig. 6(b)), in agreement with previous results57,58. The influence of the substitution layer on n s when the third and fourth layers were replaced can be explained in terms of the average k values of A site and B site cation IPWs (Fig. 6(c,d)). We found that n s is inversely proportional to the average k, as our model predicted (Eq. (3)). The effects of IPWs on the fourth and third layers were more pronounced than for other layers, because the former are the first and second layers, correspondingly, which the transferred electrons pass through owing to the 4 u.c. critical thickness of charge transfer. Interestingly, this result shows that A site and B site cation IPWs play the same role when only one unit layer is considered; yet, this is different from thick films. The influence of the substitution layer on n s when the second layer is replaced is the mixture of the effects of lattice mismatch and IPWs (Fig. 6(a)). Generally, some oxygen vacancies will form in films during film growth due to the low P ox and the high temperature. They can induce IPWs in films and influence the formation of 2DEG. Also, oxygen vacancies can form in STO substrates during films growth due to the oxygen diffusion, the bombardment by the plasma plume and so on30. By applying the ion trapping model to substrates (Fig. S6), we suggest that the IPWs induced by oxygen vacancies can give rise to IGSs and electron localization in substrates as have been found34,35,36,37. These results suggest that the IGS is a widespread and important factor in the formation of 2DEG, and we suggest that if the film has a perfect crystalline quality, a theoretical n s may be achieved.
Conclusions
We studied IGSs in films and found that these states are necessary for obtaining insulating interfaces with polar discontinuity. Oxygen vacancies should be necessary for the formation of IGSs in films. We propose the ion trapping model to explain how the IGS works. Our model is simple and phenomenological, defects, lattice distortion and some other factors are neglected. We think our research is a simple try, a deep theoretical research should be performed in future. Although our model is simple, it matched the experiments very well. Combining the polar catastrophe model and our model, the origin of interfacial 2DEG can be clearly understood. The 2DEG formed at the initially insulating LCO/STO interface and the influence of the substitution layer on n s can also be explained.
Methods
Sample growth
Films of LaCrO3, LaCoO3, LaMnO3, and SrTiO3 were grown at 830 °C, at different oxygen partial pressures, using pulsed laser deposition with laser energy of 0.9 J/cm2 and frequency of 1 Hz. The film growth was monitored using reflection high energy electron diffraction (RHEED). The substrate was TiO2-terminated (001) SrTiO3. AFM images of the substrate before deposition and of the LaCrO3 film are shown in Fig. 7(a,c). The XRD patterns of the films (Fig. 7(b)) indicating that the films were well epitaxial with (001) single phase character. We also found the lattice constant of the LaCrO3 film increases with decreasing oxygen partial pressure during growth (Fig. 7(b)).
(a) AFM image of SrTiO3 substrates. (b) XRD patterns of LCO/STO (001) heterojunctions prepared in different growth oxygen pressure (Torr). (c) AFM image of the LaCrO3 film grown on (001) SrTiO3 in a partial P ox of 6 × 10−8 Torr. (d) RHEED intensity oscillations of the specularly reflected beam for the growth of LaCrO3 (4 u.c.).
Program details
We coded a computer program to calculate the potential energy generated by different ions in a finite volume, given the distance (r) between the charge (one electron or hole) and IPW with ε r = 1. The absolute value of the summation for all ions was eU 0(r). In the calculation, we considered a volume of 1 × 106 u.c.3 with the lattice constant of SrTiO3 (3.905 Å). Our calculations show a convergence trend with the increase in volume (Fig. S3), indicating that our simulation is coarse but functional. The arrangement of ions was different for different crystallographic directions; hence, eU 0(r) was anisotropic (Fig. S4). The calculation method of our simulation was as follows:
Here, \({\mathop{r}\limits^{\rightharpoonup }}_{[h,k,l]}\) \((|{\mathop{r}\limits^{\rightharpoonup }}_{[h,k,l]}|=r)\) is the position of the charge to the IPW, in the [h, k, l] direction. The distances \(|{\mathop{r}\limits^{\rightharpoonup }}_{{A}^{3+}}-{\mathop{r}\limits^{\rightharpoonup }}_{[h,k,l]}|\), \(|{\mathop{r}\limits^{\rightharpoonup }}_{{B}^{3+}}-{\mathop{r}\limits^{\rightharpoonup }}_{[h,k,l]}|\) and \(|{\mathop{r}\limits^{\rightharpoonup }}_{{O}^{2-}}-{\mathop{r}\limits^{\rightharpoonup }}_{[h,k,l]}|\) are the distances between the positions of A3+, B3+, and O2− ions (\({\mathop{r}\limits^{\rightharpoonup }}_{{A}^{3+}}\), \({\mathop{r}\limits^{\rightharpoonup }}_{{B}^{3+}}\) or \({\mathop{r}\limits^{\rightharpoonup }}_{{O}^{2-}}\)) and the position of charge to the IPW \(({\mathop{r}\limits^{\rightharpoonup }}_{[h,k,l]})\). Our simulation considers ions from the film’s bulk. Surface states of the film and ions of the substrate (SrTiO3) are neglected because they are expected to have a weak and indirect influence on eU0 and IPWs. Film thickness was not taken into consideration.
References
Ohtomo, A. & Hwang, H. Y. A high-mobility electron gas at the LaAlO3/SrTiO3 heterointerface. Nature 427, 423–426 (2004).
Richter, C. et al. Interface superconductor with gap behaviour like a high-temperature superconductor. Nature 502, 528–531 (2013).
Reyren, N. et al. Superconducting Interfaces Between Insulating Oxides. Science 317, 1196–1199 (2007).
Han, Y.-L. et al. Two-dimensional superconductivity at (110) LaAlO3/SrTiO3 interfaces. Appl. Phys. Lett. 105, 192603 (2014).
Biscaras, J. et al. Multiple quantum criticality in a two-dimensional superconductor. Nat. Mater. 12, 542–548 (2013).
Kalisky, B. et al. Critical thickness for ferromagnetism in LaAlO3/SrTiO3 heterostructures. Nat. Commun. 3 (2012).
Banerjee, S. Ferromagnetic exchange, spin-orbit coupling and spiral magnetism at the LaAlO3/SrTiO3 interface. Nat. Phys. 9, 626–630 (2013).
Brinkman, A. et al. Magnetic effects at the interface between non-magnetic oxides. Nat. Mater. 6, 493–496 (2007).
Gabay, M. & Triscone, J.-M. Oxide heterostructures: Hund rules with a twist. Nat. Phys. 9, 610–611 (2013).
Millis, A. J. Oxide interfaces: Moment of magnetism. Nat. Phys. 7, 749–750 (2011).
Dikin, D. A. et al. Coexistence of Superconductivity and Ferromagnetism in Two Dimensions. Phys. Rev. Lett. 107, 056802 (2011).
Bert, J. A. et al. Direct imaging of the coexistence of ferromagnetism and superconductivity at the LaAlO3/SrTiO3 interface. Nat. Phys. 7, 767–771 (2011).
Li, L., Richter, C., Mannhart, J. & Ashoori, R. C. Coexistence of magnetic order and two-dimensional superconductivity at LaAlO3/SrTiO3 interfaces. Nat. Phys. 7, 762–766 (2011).
Han, Y.-L. et al. Reconstruction of electrostatic field at the interface leads to formation of two-dimensional electron gas at multivalent (110) LaAlO3/SrTiO3 interfaces. Phys. Rev. B 92, 115304 (2015).
Nakagawa, N., Hwang, H. Y. & Muller, D. A. Why some interfaces cannot be sharp. Nat. Mater. 5, 204–209 (2006).
Chambers, S. A. et al. Band Alignment, Built-In Potential, and the Absence of Conductivity at the LaCrO3/SrTiO3 (001) Heterojunction. Phys. Rev. Lett. 107, 206802 (2011).
Perna, P. et al. Conducting interfaces between band insulating oxides: The LaGaO3/SrTiO3 heterostructure. Appl. Phys. Lett. 97, 152111 (2010).
Segal, Y., Ngai, J. H., Reiner, J. W., Walker, F. J. & Ahn, C. H. X-ray photoemission studies of the metal-insulator transition in LaAlO3/SrTiO3 structures grown by molecular beam epitaxy. Phys. Rev. B 80, 241107 (2009).
Takizawa, M., Tsuda, S., Susaki, T., Hwang, H. Y. & Fujimori, A. Electronic charges and electric potential at LaAlO3/SrTiO3 interfaces studied by core-level photoemission spectroscopy. Phys. Rev. B 84, 245124 (2011).
Thiel, S., Hammerl, G., Schmehl, A., Schneider, C. W. & Mannhart, J. Tunable Quasi-Two-Dimensional Electron Gases in Oxide Heterostructures. Science 313, 1942–1945 (2006).
Drera, G. et al. Spectroscopic evidence of in-gap states at the SrTiO3/LaAlO3 ultrathin interfaces. Appl. Phys. Lett. 98, 052907 (2011).
Koitzsch, A. et al. In-gap electronic structure of LaAlO3/SrTiO3 heterointerfaces investigated by soft x-ray spectroscopy. Phys. Rev. B 84, 245121 (2011).
Ristic, Z. et al. Photodoping and in-gap interface states across the metal-insulator transition in LaAlO3/SrTiO3 heterostructures. Phys. Rev. B 86, 045127 (2012).
Pramod Kumar, P. P. Metal-to-insulator transition in LaAl1−xCrxO3/SrTiO3 oxide heterostructures guided by electronic reconstruction. Phys. Rev. B 91, 115127 (2015).
Qiao, L. et al. The Impacts of Cation Stoichiometry and Substrate Surface Quality on Nucleation, Structure, Defect Formation, and Intermixing in Complex Oxide Heteroepitaxy–LaCrO3 on SrTiO3(001). Adv. Funct. Mater. 23, 2953–2963 (2013).
Qiao, L. et al. LaCrO3 heteroepitaxy on SrTiO3(001) by molecular beam epitaxy. Appl. Phys. Lett. 99, 061904 (2011).
Kim, H. S. & Christen, H. M. Controlling the magnetic properties of LaMnO3 thin films on SrTiO3(100) by deposition in a O2/Ar gas mixture. J. Phys. Condens. Matter 22, 146007 (2010).
Armstrong, T. R., Stevenson, J. W., McCready, D. E., Paulik, S. W. & Raney, P. E. The effect of reducing environments on the stability of acceptor substituted yttrium chromite. Solid State Ion. 92, 213–223 (1996).
Catherine E. Housecroft, A G. Sharpe. Inorganic Chemistry. (2012).
Chen, Y. et al. Metallic and Insulating Interfaces of Amorphous SrTiO3-Based Oxide Heterostructures. Nano Lett. 11, 3774–3778 (2011).
Kalabukhov, A. et al. Effect of oxygen vacancies in the SrTiO3 substrate on the electrical properties of the LaAlO3∕SrTiO3 interface. Phys. Rev. B 75, 121404 (2007).
Liu, Z. Q. et al. Metal-Insulator Transition in SrTiO3-x Thin Films Induced by Frozen-Out Carriers. Phys. Rev. Lett. 107, 146802 (2011).
Mayeshiba, T. & Morgan, D. Strain effects on oxygen migration in perovskites. Phys. Chem. Chem. Phys. 17, 2715–2721 (2014).
Carrasco, J. et al. First-principles calculations of the atomic and electronic structure of F centers in the bulk and on the (001) surface of SrTiO3. Phys. Rev. B 73, 064106 (2006).
Altmeyer, M. et al. Magnetism, Spin Texture, and In-Gap States: Atomic Specialization at the Surface of Oxygen-Deficient SrTiO3. Phys. Rev. Lett. 116, 157203 (2016).
Mitra, C., Lin, C., Robertson, J. & Demkov, A. A. Electronic structure of oxygen vacancies in SrTiO3 and LaAlO3. Phys. Rev. B 86, 155105 (2012).
Ricci, D., Bano, G., Pacchioni, G. & Illas, F. Electronic structure of a neutral oxygen vacancy in SrTiO3. Phys. Rev. B 68, 224105 (2003).
Yoshimatsu, K., Yasuhara, R., Kumigashira, H. & Oshima, M. Origin of Metallic States at the Heterointerface between the Band Insulators LaAlO3 and SrTiO3. Phys. Rev. Lett. 101, 026802 (2008).
Berner, G. et al. Band alignment in LaAlO3/SrTiO3 oxide heterostructures inferred from hard x-ray photoelectron spectroscopy. Phys. Rev. B 88, 115111 (2013).
Luca, G. D. et al. Potential-well depth at amorphous-LaAlO3/crystalline-SrTiO3 interfaces measured by optical second harmonic generation. Appl. Phys. Lett. 104, 261603 (2014).
Liu, X. Z., Tao, B. W. & Li, Y. R. Effect of oxygen vacancies on nonlinear dielectric properties of SrTiO3 thin films. J. Mater. Sci. 42, 389–392 (2007).
Siemons, W. et al. Origin of Charge Density at LaAlO3 on SrTiO3 Heterointerfaces: Possibility of Intrinsic Doping. Phys. Rev. Lett. 98, 196802 (2007).
Sakudo, T. & Unoki, H. Dielectric Properties of SrTiO3 at Low Temperatures. Phys. Rev. Lett. 26, 851–853 (1971).
Li, C. et al. Tailoring the Two Dimensional Electron Gas at Polar ABO3/SrTiO3 Interfaces for Oxide Electronics. Sci. Rep. 5, 13314 (2015).
Hotta, Y., Susaki, T. & Hwang, H. Y. Polar Discontinuity Doping of the LaVO3/SrTiO3 Interface. Phys. Rev. Lett. 99, 236805 (2007).
Biscaras, J. et al. Two-Dimensional Superconducting Phase in LaTiO3/SrTiO3 Heterostructures Induced by High-Mobility Carrier Doping. Phys. Rev. Lett. 108, 247004 (2012).
Biscaras, J. et al. Two-dimensional superconductivity at a Mott insulator/band insulator interface LaTiO3/SrTiO3. Nat. Commun. 1, 89 (2010).
Li, D. F., Wang, Y. & Dai, J. Y. Tunable electronic transport properties of DyScO3/SrTiO3 polar heterointerface. Appl. Phys. Lett. 98, 122108 (2011).
Moetakef, P. et al. Transport in ferromagnetic GdTiO3/SrTiO3 heterostructures. Appl. Phys. Lett. 98, 112110 (2011).
Colby, R. et al. Cation intermixing and electronic deviations at the insulating LaCrO3/SrTiO3 (001) interface. Phys. Rev. B 88, 155325 (2013).
Salluzzo, M. et al. Origin of Interface Magnetism in BiMnO3/SrTiO3 and LaAlO3/SrTiO3 Heterostructures. Phys. Rev. Lett. 111, 087204 (2013).
Zhang, J. Y. et al. Correlation between metal-insulator transitions and structural distortions in high-electron-density SrTiO3 quantum wells. Phys. Rev. B 89, 075140 (2014).
Chen, Y. Z. et al. A high-mobility two-dimensional electron gas at the spinel/perovskite interface of γ-Al2O3/SrTiO3. Nat. Commun. 4, 1371 (2013).
Chen, Y. et al. Creation of High Mobility Two-Dimensional Electron Gases via Strain Induced Polarization at an Otherwise Nonpolar Complex Oxide Interface. Nano Lett. 15, 1849–1854 (2015).
Gunkel, F. et al. Thermodynamic Ground States of Complex Oxide Heterointerfaces. ACS Appl. Mater. Interfaces 9, 1086–1092 (2017).
Popović, Z. S., Satpathy, S. & Martin, R. M. Origin of the Two-Dimensional Electron Gas Carrier Density at the LaAlO3 on SrTiO3 Interface. Phys. Rev. Lett. 101, 256801 (2008).
Annadi, A. et al. Electronic correlation and strain effects at the interfaces between polar and nonpolar complex oxides. Phys. Rev. B 86, 085450 (2012).
Bark, C. W. et al. Tailoring a two-dimensional electron gas at the LaAlO3/SrTiO3 (001) interface by epitaxial strain. Proc. Natl. Acad. Sci. 108, 4720–4724 (2011).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos 11674031, 11474022, 11474024, 11422430, and 11374035) and the National Basic Research Program of China (Grant Nos 2014CB920903, 2013CB921701 and 2013CBA01603). PB acknowledges UEFISCDI PNIIPCCE contract No. 3/2012, Romania and MEC-UEFISCDI, project POC 37_697 no. 28/01.09.2016 REBMAT, Romania.
Author information
Authors and Affiliations
Contributions
Jia-Cai Nie and Cheng-Jian Li proposed and designed experiments. Cheng-Jian Li, Hong-Xia Xue, Guo-Liang Qu, Ming-rui Liu, Wei-min Jiang and Wei-ming Lü prepared the samples with the assistance from and Sheng-Chun Shen, Yan-Peng Hong and Xin-Xin Wang. Cheng-Jian Li, Hong-Xia Xue and Wei-ming Lü performed the measurements with the assistance from and Sheng-Chun Shen, Yan-Peng Hong and Xin-Xin Wang. Results were analyzed by Cheng-Jian Li and Jia-Cai Nie. The manuscript was written by Cheng-Jian Li and Petre Badica and discussed with Wei-ming Lü. All authors discussed and contributed with comments regarding results and the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
41598_2017_18583_MOESM1_ESM.pdf
Supplementary Information: Influence of In-Gap States on the Formation of Two-Dimensional Election Gas at ABO3/SrTiO3 Interfaces
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, CJ., Xue, HX., Qu, GL. et al. Influence of In-Gap States on the Formation of Two-Dimensional Election Gas at ABO3/SrTiO3 Interfaces. Sci Rep 8, 195 (2018). https://doi.org/10.1038/s41598-017-18583-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-18583-5
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.