Abstract
To investigate the contribution of adrenomedullin (AM) and its gene-related peptide, proadrenomedullin N-terminal 20 peptide (PAMP), to the progression and potential treatment of colon cancer we studied the effects of four small molecules (SM) related to AM and PAMP on a mouse model of colon cancer. For each SM, four experimental groups of male mice were used: (i) Control group; (ii) SM group; (iii) DSS group (injected with azoxymethane [AOM] and drank dextran sulfate sodium [DSS]); and (iv) DSS + SM group (treated with AOM, DSS, and the SM). None of the mice in groups i and ii developed tumors, whereas all mice in groups iii and iv developed colon neoplasias. No significant differences were found among mice treated with PAMP modulators (87877 and 106221). Mice that received the AM negative modulator, 16311, had worse colitis symptoms than their control counterparts, whereas mice injected with the AM positive modulator, 145425, had a lower number of tumors than their controls. SM 145425 regulated the expression of proliferation marker Lgr5 and had an impact on microbiota, preventing the DSS-elicited increase of the Bacteroides/Prevotella ratio. These results suggest that treatment with AM or with positive modulator SMs may represent a novel strategy for colon cancer.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer worldwide. It is responsible for the death of about 200,000 people each year in Europe1 and it is expected to cause about 50,260 deaths and 135,430 new cases during 2017 in the United States2. Standard treatment consists on a combination of neoadjuvant chemoradiotherapy followed by surgery, but the response to this treatment and patient survival are heterogeneous3.
Adrenomedullin (AM) is a 52 amino acid peptide, in humans, that belongs to the calcitonin/calcitonin gene-related peptide family, which comprises also amylin and another peptide with high homology to AM, known as AM2 or intermedin4,5. AM is synthesized as part of a larger precursor molecule, termed preproadrenomedullin. This precursor consists of 185 amino acids in humans and contains a 21-amino acid N-terminal signal peptide that immediately precedes a 20-amino acid amidated peptide, designated proadrenomedullin N-terminal 20 peptide or PAMP. AM exerts its actions through a combination of the calcitonin receptor like receptor or CLR; and either receptor activity-modifying protein 2 (RAMP2) or RAMP3 (known as AM1 and AM2 receptors, respectively)4.
AM and PAMP are expressed throughout the gastrointestinal tract, being specially abundant in the neuroendocrine cells of the gastrointestinal mucosa; in the enterochromaffin-like and chief cells of the gastric fundus; and in the submucosa of the duodenum, ileum, and colon. This wide distribution in the gastrointestinal tract suggests that AM and PAMP may act as gut hormones regulating many physiological and pathological conditions6.
AM is widely expressed in a variety of tumor types7 and several studies have provided evidence that AM is involved in tumor initiation and progression8.
AM ligand and receptor overexpression in colonic cancers has been previously reported9,10,11,12,13 and a correlation between higher AM levels and lower disease-free survival has been described10,14,15,16. Furthermore, antibodies against either the peptide or the receptor reduce the growth of xenografted tumors17,18. Nevertheless, there is some controversy on whether AM is good or bad for colon cancer patients since application of the peptide clearly reduces inflammation and clinical severity in human and mouse models of colitis19,20,21, which is an important risk factor for colon cancer. In addition, AM has a protective role in gastrointestinal diseases10,22, and ameliorates the severity of these gut pathologies15,23.
Furthermore, AM and PAMP are antimicrobial peptides found in most epithelial surfaces and body secretions24,25 and their presence or absence modifies the composition of gut microbiota20.
Several pharmacological modulators of AM and PAMP have been described and they can be used to intervene in all physiological and pathological conditions where these peptides play a role. These modulators include monoclonal antibodies26, polyclonal antibodies against either the peptide27,28 or the receptors17, the peptide fragments AM22-5229 or PAMP12-2030, and small interfering RNAs31. In addition, several small molecules (SM) have been identified which can either increase or decrease AM or PAMP functions30,32.
To further investigate the contribution of AM and its gene-related peptide, PAMP, to the progression and potential treatment of colon cancer we have studied the effects of several SM related to AM and PAMP on a mouse model of colon cancer. Four SM were tested: 16311 (a negative modulator of AM), 145425 (a positive modulator of AM), 87877 (a negative modulator of PAMP), and 106221 (a positive modulator of PAMP)30,32.
Results
PAMP-related SMs do not modify cancer phenotype
PAMP modulators (87877 & 106221) did not modify colon cancer status or any of the other parameters tested (data not shown), indicating that PAMP may not play a major role in colon cancer or that the PAMP-related SMs were not very effective.
SM 16311, but not 145425, increases weight loss and severity index in AOM-and DSS-treated mice
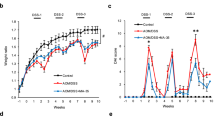
The aim of this work was to analyze the impact of AM on colitis-associated CRC initiation and progression. This protocol allowed us to investigate the effect of SM modulators of AM on the severity of DSS-induced colitis. Control groups (Control and SM-treated) maintained body weight (Fig. 1A,C) and did not suffer major clinical symptoms (not shown). As expected, DSS treated mice experienced weight loss (18.3% over initial weight) (ANOVA+ Bonferroni p < 0.001) (Fig. 1A,C), and worse colitis symptoms such as dehydration, diarrhea, and rectal bleeding compared to control/untreated counterparts (Fig. 1B,D) (ANOVA + Bonferroni p < 0.001). In addition, mice that received DSS + 16311 experienced a significantly deeper weight loss (more than 20% over initial weight) (Fig. 1A), and more severe colitis symptoms (Fig. 1B) than mice treated with DSS. In contrast, 145425-treatment did not show any difference on these parameters over the DSS treatment (Fig. 1C,D).
SM 16311 had a negative influence in the health status of DSS-treated mice. Body weight changes were recorded weekly in the four experimental groups treated with 16311 (A) or with 145425 (C) and are represented as weight gain. Mice treated with AOM and 3 cycles of DSS present weight loses following each cycle. SM 16311-treated mice experienced a significantly higher percentage of weight loss than their DSS counterparts. Colitis symptoms were scored on a 0–12 point scale in 16311 (B) and 145425 (D)-treated mice. Mice not receiving DSS had a 0 index at all times and are not shown. SM 16311-treated mice exhibited severe colitis symptoms reaching higher scores than their DSS-treated counterparts (B). SM 145425 had no effect on any of the parameters studied (C,D). Data are shown as mean ± SEM. ANOVA test. Asterisks represent time points at which statistically significant differences between 16311-treated and untreated mice exposed to DSS were found (Bonferroni); *p < 0.05.
AM positive modulator, 145425, reduces tumor burden and colon weight/length ratio
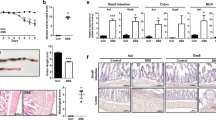
At the macroscopic level, untreated animals present the characteristic size and oval shape of mice feces (Fig. 2). Treatment with the SMs had no effects on feces or intestinal morphology (Fig. 2). However, DSS administration caused gut pathology characterized by more liquid feces, macroscopic inflammation, and colonic thickening. SM 16311 worsened (Fig. 2) but 145425 improved (Fig. 2) the macroscopic appearance of the colon in comparison with DSS-treated animals. All mice in DSS-treated groups (DSS and DSS+SM) developed colon neoplasias that were classified either as adenomas, carcinomas in situ, or adenocarcinomas (Table 1). Number of tumors (Fig. 3A,C) and the colon weight/length ratio (Fig. 3B,D) was recorded in the four groups. None of the mice belonging to control groups (Control and SM) developed any tumor or had any pathological finding, indicating that the SMs do not present overt toxicity. As expected, treatment with DSS resulted in a significant increase on the number of tumors (p < 0.001) and on the colon weight/length ratio (p < 0.001). SM 16311 had no effect on these parameters (Fig. 3A,B) whereas 145425 significantly reduced the number of tumors (p < 0.001) (Fig. 3C) and the colon weight/length ratio (p < 0.05) (Fig. 3D).
Effect of 16311 & 145425 on DSS-induced colon injury. Macroscopic aspect of the colon in the four experimental groups treated with either 16311 or 145425. The four experimental groups were: untreated (Control), SM-treated (SM), DSS-treated (DSS), SM and DSS-treated (DSS+SM). DSS treatment caused local inflammation on the colon. However, this inflammatory response was aggravated by 16311 and prevented in 145425-treated mice. Histological images were stained with hematoxylin-eosin. Histological analysis of untreated or SM-treated mice did not show any abnormality. The colon of the DSS group had numerous atypic cells and large areas occupied by polyps and tumors accompanied by lymphocyte infiltrates. After 16311 treatment some tumors invaded the submucosa (T1). When 145425 was present most tumors were classified as carcinoma in situ (Tis). Scale bar = 200 µm.
Number of tumors and relative weight of the colon. Number of tumors (A,C) and relative weight of the colon (B,D) was calculated in the four experimental groups. There is a significant increase in the number of tumors and weight/length ratio of the colon in all DSS-treated mice when compared with their respective controls. SM 145425 significantly reduced the number of tumors in treated mice (C). The reduction in the number of tumors was also evidenced by a reduction in colon weight (D). SM 16311 had no significant effect on any of these parameters (A,B). Data are shown as mean ± SEM. Kruskal‐Wallis test followed by Mann Whitney. Asterisks represent statistically significant differences with mice not receiving DSS; *p < 0.05; **p < 0.01; ***p < 0.001. Ampersands indicate statistically significant differences between DSS treated mice; &p < 0.05; &&&p < 0.001.
SMs do not modify the expression of inflammatory cytokines in the colon
In an attempt to identify the potential mechanism of AM modulators in the immune response, we examined gene expression patterns of different inflammatory cytokines. In DSS-treated mice, there was a significant increase in IFN-γ, TNF-α, IL-6, and IL-10 levels compared with their sham groups. The levels of these cytokines were not significantly affected by SM treatment (Supplementary Figure S1A–H).
SMs modulate the expression of AM and AM2 in the colon
We also measured the mRNA expression levels of AM and AM2 in colon samples from all the experimental groups. SM 16311 significantly decreased the levels of AM in DSS-treated samples but had no influence on AM2 expression (Supplementary Figure S2A-B). Conversely, SM 145425 significantly elevated the levels of AM in DSS-treated tissues (Supplementary Figure S2C). Concerning AM2 expression, SM 145425 reduced AM2 levels in untreated animals but had no significant effects on DSS-treated mice (Supplementary Figure S2D).
SM 145425 prevents DSS-related changes in Lgr5 and Erbb2 expression
In DSS-treated mice, there was an increase in Lgr5 and a decrease in Erbb2 gene expression levels compared with their sham groups (Fig. 4A). SM 16311 did not significantly modify the expression of either Lgr5 or Erbb2 (Fig. 4A,B). A significant decrease of Lgr5 and a significant increase of Erbb2 was induced by 145425 administration in DSS-treated mice (p < 0.05) (Fig. 4C,D). Since 145425 seems to offer a therapeutic potential, we further investigated the influence of this SM in histopathology and microbiota modulation.
Gene expression in the colon. Stem cell marker Lgr5 significantly increased with DSS treatment (A,C), indicating extensive proliferation. This marker was significantly reduced by treatment with 145425 (C). On the other hand, Erbb2 had the reverse behavior, decreasing by DSS and being modulated by 145425 (D). Moreover, treatment with 16311 did not affect any of these two genes studied when compared with their respective DSS-treated controls (A,B). Data are shown as mean ± SEM. Kruskal‐Wallis test followed by Mann Whitney; *p < 0.05; **p < 0.01; ***p < 0.001. Ampersands indicate statistically significant differences between DSS treated mice; &p < 0.05.
SM 145425 reduces tumor growth in DSS treated mice
Macroscopic inspection of colon and rectum provided evidence of different degrees of colonic inflammation and tumor burden in DSS-treated animals. Mice that were not exposed to DSS had a healthy mucosa (Fig. 5A), whereas DSS treatment resulted in a thickening of the mucosa and the presence of numerous polyps and tumors (Fig. 5A). Treatment with 145425 reduced the number of tumoral structures in the colon (Fig. 5A). In addition, a low number of proliferating (Fig. 5B) and apoptotic (Fig. 5C) cells were detected in control animals but this numbers greatly increased in the DSS groups. The number of proliferating and apoptotic cells was similar between DSS and DSS+SM groups. Moreover, myeloperoxidase activity was not affected by SM treatment (Supplementary Figure S3).
Morphological aspect of the mucosa in the four experimental groups. Representative macroscopic (A) and histological (B,C) appearance of the colon of the four experimental groups. The first 2 groups, Control and SM, displayed a normal colon morphology. The colon of the DSS group had numerous frank tumors. Treatment with 145425 reduced the number of tumors. Histological sections were stained with the proliferation marker anti Phospho-Histone H3 (PH3) antibody (B), and the TUNEL technique (C) to determine levels of proliferation and apoptosis, respectively. The number of proliferating cells was low in control animals whereas this number greatly increased in the DSS group. The number of proliferating cells was similar between DSS and DSS+SM groups (B). The TUNEL technique detected few apoptotic cells in the colon of animals belonging to control groups but the number increased in animals treated with DSS but did not vary after treatment with 145425 (C). Scale bar for A = 10 mm; for B and C = 100 µm.
Metagenomic analysis of gut bacterial populations
Okayasu et al.33, among others, suggested a contribution of colonic bacteria or their products to the development of DSS-induced colitis. They observed increased numbers of Enterobacteriaceae, Bacteroidaceae, and Clostridium spp. in the colons of DSS-treated mice33. Using the same model we have studied the effects of AM modulator, 145425, on gut microbiota. Supplementary Figure S4 shows the relative abundance of the major bacterial phyla present in the gut in the four experimental groups. As expected, around 90% of the bacteria detected (93.4 and 94.4% for control and 145425 animals respectively) belong to the Bacteroidetes and Firmicutes phyla (Table 2). No significant differences were observed in major phyla elicited by either DSS treatement or the SM (Table 2). However, significant changes were observed in less represented phyla (Table 2). Thus, a significant reduction in the abundance of Cyanobacteria and Tenericutes was observed in DSS-treated mice (DSS and DSS+145425 groups) when compared with Control groups (Control and 145425). Surprisingly, the abundance of Verrucomicrobia was decreased in the 145425 group when compared to the other groups (Table 2). At the family level, DSS treatment resulted in a significant increase in the abundance of Bacteroidaceae, Turicibacteraceae, Peptostreptococcaceae, Erysipelotrichaceae, and Alcaligenaceae in comparison to control groups (Supplementary Table S2). In contrast, DSS treatment resulted in a significant decrease in the abundance of Prevotellaceae, Paraprevotellaceae, Cyanobacteria;c__4C0d-2;o__YS2, Alphaproteobacteria, and Anaeroplasmataceae (Supplementary Table S2). However, after 145425-treatment this decrease in the abundance of the Prevotellaceae, and Paraprevotellaceae was normalized (Supplementary Table S2,S3,S4). Interestingly, Erysipelotrichaceae was significantly increased and Paraprevotellaceae decreased by 145425. Verrucomicrobiaceae was significantly reduced by 145425 in untreated mice but that difference did not persist after DSS treatment (Supplementary Table S4). Concerning lower taxonomic levels (genus), DSS-treated mice exhibited a significant increase in the abundance of Bacteroidales, Bacteroides, Turicibacter (Fig. 6A), Peptostreptococcaceae, Erysipelotrichaceae, Allobaculum and Sutterella and a significant decrease in the presence of Prevotella (Fig. 6C), Cyanobacteria;c__4C0d-2;o__YS2, Dorea, Alphaproteobacteria;o__RF32, and Anaeroplasma. In some genera, SM 145425 was able to influence bacterial abundance in control animals. For instance, Akkermansia was significantly reduced by 145425 in untreated mice but that difference did not persist after DSS treatment (Fig. 6B). The case of Prevotella was particularly interesting: DSS greatly reduced Prevotella abundance but after 145425-treatment the presence of Prevotella was completely normalized (Fig. 6C). The ratio Bacteroides/Prevotella has been shown to have clinical relevance34,35. DSS induced a large increase of this ratio whereas 145425-treatment prevented this elevation completely (Fig. 6D). Supplementary Figure S5 shows the alpha and beta-diversity analysis.
Relative abundance of particular microbiota genera in the four experimental groups. Genus Turicibacter increased significantly with DSS treatment (A). Akkermansia was significantly reduced by 145425 in untreated mice but that difference did not persist after DSS treatment (B). Interestingly, Prevotella decreased with DSS treatment but this decrease was completely prevented by 145425 (C). Besides, 145425 prevented the large increase in the Bacteroides/Prevotella ratio elicited by DSS (D). Data are shown as mean ± SEM. Kruskal‐Wallis test followed by Mann Whitney. Asterisks represent statistically significant differences with control; *p < 0.05; **p < 0.01; ***p < 0.001. Ampersands indicate statistically significant differences between DSS treated mice; &p < 0.05; &&p < 0.01.
Discussion
In this study we have shown that AM positive modulation by 145425 treatment reduces tumor incidence in a mouse model of colitis-associated colon cancer and results in gut microbiota changes, thus linking AM with colorectal cancer initiation and progression. Previous studies have shown a strong positive effect using AM as treatment for colitis symptoms in rodents22 and humans15,23. Besides, this study shows that AM negative modulation by 16311 exacerbates colitis symptoms suggesting that AM prevents the symptoms of the disease in line with previous experiments20,36. Microscopic analysis of colonic sections confirmed data from macroscopic observations reflecting a tumor reduction in 145425-treated mice. These results demonstrated that AM exerts a protective action in DSS-induced experimental colitis, and are in line with previous papers22. As previously described37, three cycles of DSS result in the development of colitis-associated dysplasias and adenocarcinomas in approximately 15%–20% of mice which is in agreement with our data. No differences were found among mice treated with PAMP modulators (87877 and 106221), indicating that PAMP may not play a major role in colon cancer or that the PAMP-related SMs were not very effective.
Our data suggest that AM is a beneficial factor since this peptide significantly delays CRC development. This observation seems to be in contrast with previous studies where inhibition of AM or its receptors produced tumor reduction17,18. Previous experimental studies consisted on xenograft models whereas our working paradigm uses a long-term orthotopic carcinogen-induced model of CRC, which therefore is closer to the natural occurrence in humans and its results may be more relevant. In addition, our experimental model is very dependent on colitis as the trigger of CRC. Since AM has been shown repeatedly to be very efficient in reducing colitis symptoms19,20,21, the observed effects on CRC may be just a consequence of the milder colitis suffered by SM treated animals. Future studies should address whether AM is also protective in other models of colon cancer.
The DSS-induced colitis model induces high amounts of Th1 cytokines (TNF-α, IL-6)22. These cytokines exert potent proinflammatory effects that, when uncontrolled, can lead to tissue injury. Our results demonstrated that DSS administration induces an inflammatory response in the colon. However, SM treatment did not modify this response suggesting that these SMs play no role in immunoregulation.
Although the mechanism of action of the SMs implicates their direct binding to the mature peptide and a consequent modification of the peptide’s functions30,32, we also looked at the expression of AM and AM2 genes and their potential modulation by the SMs. As expected, there was no significant modification of AM levels in untreated animals but, in DSS-treated mice, there was a downregulation by the inhibitory SM (16311) and a significant upregulation by the positive modulator (145425), which would help to explain the beneficial effects of SM 145425 and the increase of colitis symptoms by SM 16311. The expression of AM2 was not modified by SM 16311 but SM 145425 reduced the expression of AM2 on untreated animals. This effect could be explained by an increase on the physiological actions of AM that, through an inhibitory feedback loop, may result in a reduction of AM2 expression.
The stem cell marker Lgr5 significantly increased with DSS treatment, indicating extensive proliferation. This marker’s increase was significantly prevented by treatment with 145425. Lgr5 is a marker of adult stem cells involved in self-renewal and cancer38. On the other hand, Erbb2 had the reverse behavior, decreasing by DSS and being modulated by 145425. Erbb2 regulates recovery from DSS-induced colitis by promoting mouse colon epithelial cell survival39. These results suggest that AM plays important cytoprotective and reparative roles in the colonic epithelium following injury, by promoting colon epithelial cell survival.
Another possible mechanism by which AM protects from colon cancer development is microbiota modulation. In a previous study we showed that mice lacking AM had an altered gut microbiota20. In this study, Turicibacter and Bacteroidaceae increased significantly with DSS treatment, in agreement with previous reports by Berry40 and Okayasu33, respectively. Interestingly, we observed that the positive modulator of AM (145425) induced a decrease in the Bacteroides/Prevotella ratio. This ratio is higher in colon cancer patients than in healthy controls34,35 and a greater abundance of Prevotella has been found in healthy rats than in CRC rats41. Furthermore, individuals consuming healthy diets have a lower Bacteroides/Prevotella ratio than people on high-calory diets42,43. Thus, we can hypothesize that the beneficial effects of 145425 may be partially mediated through this mechanism. Moreover, 145425 modulates some bacteria by itself, for example Akkermansia was significantly reduced by 145425 in untreated mice but that difference did not persists after DSS treatment. Akkermansia was more abundant in CRC samples35 which can represent an advantage for the 145425 treatment. Therefore, AM positive modulation is clearly associated with healthy changes in microbiota composition. The dysbiosis produced by DSS predisposes mice to worse colitis symptoms and 145425 prevents such microbiota modifications.
In conclusion, AM may have a protective role during the progression phase of colon cancer, and treatment with AM or with positive modulator SMs may represent a novel treatment for colon cancer.
Material and Methods
Colitis-associated cancer induction
The protocol was performed as previously described44. Briefly, treated animals received a single intraperitoneal (i.p) injection (10 mg/Kg) of the carcinogen azoxymethane (AOM) (Sigma-Aldrich, Madrid, Spain). One week later, animals were given 2.5% dextran sulfate sodium (DSS) (Sigma-Aldrich) in the drinking water for 1 week followed by 2 weeks of tap water. The DSS treatment was repeated for 2 additional cycles and tumorigenesis was examined 2 weeks after the last cycle. Untreated control mice received a saline injection instead of AOM and drank tap water only. Four SM were tested: 16311 (a negative modulator of AM), 145425 (a positive modulator of AM), 87877 (a negative modulator of PAMP), and 106221 (a positive modulator of PAMP). Sixty 8-week old male C57BL/6 mice were used in this study for each SM. Experimental groups were formed as follows: (i) Control group (injected i.p. with vehicle and drank regular water, n = 10); (ii) SM group (vehicle, regular water, and injected i.p. with the SM 3 times a week at a concentration of 20 nm/Kg, n = 10); (iii) DSS group (injected i.p. with AOM and drank DSS, n = 20); and (iv) DSS + SM group (treated with AOM, DSS, and the SM, n = 20). Small molecules were generously provided by the NCI Developmental Therapeutic Program (Frederick, MD), and their selection and characterization has been previously published30,32. All procedures involving animals were carried out in accordance with the European Communities Council Directive (2010/63/UE) and Spanish legislation (RD53/2013) on animal experiments and with approval from the ethical committee on animal welfare of our institution (Órgano Encargado del Bienestar Animal del Centro de Investigación Biomédica de La Rioja, OEBA-CIBIR).
Clinical assessment of colitis
Mice were observed and weighed weekly. Assessments of rectal bleeding, diarrhea, prolapse, inactivity, and percent weight loss relative to baseline were scored according to the system described by Gommeaux et al.45 and used as a surrogate measure of colitis severity.
Mouse sacrifice, macroscopic analysis, and tissue harvesting
All mice were sacrificed by an overdose of anesthesia (ketamine-xylazine) 70 days after AOM injection. Entire colons were dissected, rinsed with ice-cold phosphate buffer solution (PBS) to remove fecal residues, and weighed. Photographs of colon samples were taken using an EOS50D camera (Canon, Tokyo, Japan). Colon fragments were snap-frozen in liquid N2 and stored at −80 °C for further analysis. Central portions of colonic tissue were fixed in 10% buffered formalin
Hematoxylin-eosin staining
Following fixation, tissues were dehydrated and paraffin embedded. Tissue sections (3 µm-thick) were rehydrated and stained with hematoxylin-eosin. Three sections from different colon pieces were analyzed for each animal and 3 random pictures were taken from each section with the 4x objective. At least 7 animals per group were included in the analysis.
TUNEL staining
Colonic cells undergoing apoptosis were identified by means of a TUNEL assay kit (Promega, Madison, WI), following manufacturer’s instructions. Random pictures were taken from each section with the 10x objective.
Immunohistochemical staining
Paraffin-embedded sections were rehydrated, and antigen retrieval was performed by heating in citrate buffer (pH 6.0) for 20 min at 96 °C. After blocking with normal donkey serum, sections were incubated overnight with Phospho-Histone H3 primary antibody (Cell Signaling, Danvers, MA) at 1:100. The next day, following several washes in PBS, a biotinylated donkey anti-rabbit (Jackson Immunoresearch, Suffolk, UK) at 1:500 was added for 60 min, followed by the ABC complex (Vector, Burlingame, CA) and developed with diaminobenzidine (Dako, Carpinteria, CA). Slides were counterstained with hematoxylin. Pictures were taken from each section with the 10x objective.
RNA isolation and quantitative real-time PCR
RNA isolation, cDNA synthesis, and qRT-PCR were performed as described46. Briefly, total RNA was isolated from distal colon fragments using Qiagen RNeasy MiniKit (Qiagen, Hilden, Germany) with DNAse digestion step performed (Qiagen) according to manufacturer’s instructions. Total RNA (1 µg) of each sample was reverse transcribed using the SuperScriptR III Reverse Transcriptase Kit (Thermo Fisher Scientific, Waltham, MA). The synthesized cDNA was amplified by qRT-PCR with a 7300 real-time PCR System (Applied Biosystems, Foster City, CA) and gene expression was calculated using relative quantification by interpolation into a standard curve using RQ software (Applied Biosystems), as described47. All values were divided by the expression of the house keeping gene, GAPDH, to avoid potential loading errors. Target genes (IFN-γ, TNF-α, IL-6, IL-10, Lgr5, and Erbb2) and primers are described in Table S1 of Supplementary Material.
Feces collection and DNA extraction
Fresh fecal contents were collected from each animal and weighed. DNA was subsequently extracted from fecal microbiota using the DNeasy Blood & Tissue Kit (Qiagen, Venlo, Netherlands). DNA purity and concentration were determined by a Nanodrop spectrophotometer (ND-1000; Thermo Fisher Scientific).
Bacterial 16S rDNA massive sequencing and sequence postprocessing
Samples were amplified for the 16 S rRNA hypervariable sequences V3-V4 using Illumina recommended primers in a MiSeq Instrument (2 × 300 bp reads) (Illumina, INC, SanDiego, CA). Quality of sequenced reads was assessed by FastQC software (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Raw reads were quality trimmed with Trimmomatic48. Reads were assigned into OTU categories with Qiime software49 by following the “pick open reference otus” methodology with Usearch61 clustering algorithm (http://www.ddrive5.com/usearch/). Taxonomic classification was performed by using the GreenGenes database50 at 97% of nucleotide identity. OTUs that were present at less than 0,01% of the total read counts on a per-sample basis were removed (spurious sequences). Raw normalization counts in the OTU table were normalized by the Cumulative Sum Scalling (CSS) methodology with metagenomeSeq R package (http://bioconductor.jp/packages/2.14/bioc/vignettes/metagenomeSeq/inst/doc/metagenomeSeq.pdf).
Statistical analysis
All data sets were analyzed for normality and homoscedasticity. Normal data were analyzed by Unpaired Student’s t test or by 2-way ANOVA followed by Bonferroni post-hoc test. Data that did not follow a normal distribution were compared by Kruskal-Wallis test followed by Mann Whitney post-hoc test. For tumor grade comparisons the Fisher’s exact test was used. Analyses were performed with GraphPad Prism version 5.02 (GraphPad Software, Inc. La Jolla, CA). A p value < 0.05 was considered statistically significant.
References
Ait Ouakrim, D. et al. Trends in colorectal cancer mortality in Europe: retrospective analysis of the WHO mortality database. BMJ 351, h4970 (2015).
Siegel, R. L. et al. Colorectal cancer statistics, 2017. CA. Cancer J. Clin. 67, 177–193 (2017).
Schmoll, H. J. et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 23, 2479–516 (2012).
López, J. & Martínez, A. Cell and molecular biology of the multifunctional peptide, adrenomedullin. Int. Rev. Cytol. 221, 1–92 (2002).
Hay, D. L., Garelja, M. L., Poyner, D. R. & Walker, C. S. Update on the pharmacology of calcitonin/CGRP family of peptides: IUPHAR Review:"X". Br. J. Pharmacol. https://doi.org/10.1111/bph.14075 (2017).
Martínez-Herrero, S. & Martínez, A. Adrenomedullin regulates intestinal physiology and pathophysiology. Domest. Anim. Endocrinol. 56(Suppl), S66–83 (2016).
Zudaire, E., Martínez, A. & Cuttitta, F. Adrenomedullin and cancer. Regul. Pept. 112, 175–83 (2003).
Portal-Nuñez, S. et al. Aryl hydrocarbon receptor-induced adrenomedullin mediates cigarette smoke carcinogenicity in humans and mice. Cancer Res. 72, 5790–800 (2012).
Hikosaka, T. et al. Adrenomedullin production is increased in colorectal adenocarcinomas; its relation to matrix metalloproteinase-9. Peptides 32, 1825–31 (2011).
Kim, J.-Y., Park, W.-D., Lee, S. & Park, J.-H. Adrenomedullin is involved in the progression of colonic adenocarcinoma. Mol. Med. Rep. 6, 1030–4 (2012).
Kitani, M., Sakata, J., Asada, Y., Kitamura, K. & Eto, T. Distribution and Expression of Adrenomedullin in Human Gastrointestinal Tissue. Ann. Clin. Biochem. An Int. J. Biochem. Lab. Med. 35, 643–648 (1998).
Marutsuka, K. et al. Adrenomedullin and proadrenomudullin N-terminal 20 peptide (PAMP) are present in human colonic epithelia and exert an antimicrobial effect. Exp. Physiol. 86, 543–5 (2001).
Miller, M. J. et al. Adrenomedullin expression in human tumor cell lines. Its potential role as an autocrine growth factor. J. Biol. Chem. 271, 23345–51 (1996).
Wang, L. et al. Adrenomedullin is a therapeutic target in colorectal cancer. Int. J. cancer 134, 2041–50 (2014).
Ashizuka, S., Inatsu, H., Kita, T. & Kitamura, K. Adrenomedullin Therapy in Patients with Refractory Ulcerative Colitis: A Case Series. Dig Dis Sci 61, 872–880 (2016).
Uemura, M. et al. Hypoxia-inducible adrenomedullin in colorectal cancer. Anticancer Res. 31, 507–14 (2011).
Kaafarani, I. et al. Targeting adrenomedullin receptors with systemic delivery of neutralizing antibodies inhibits tumor angiogenesis and suppresses growth of human tumor xenografts in mice. FASEB J. 23, 3424–35 (2009).
Nouguerède, E. et al. Expression of adrenomedullin in human colorectal tumors and its role in cell growth and invasion in vitro and in xenograft growth in vivo. Cancer Med. 2, 196–207 (2013).
Hayashi, Y. et al. Impact of adrenomedullin on dextran sulfate sodium-induced inflammatory colitis in mice: insights from in vitro and in vivo experimental studies. Int. J. Colorectal Dis. 26, 1453–62 (2011).
Martínez-Herrero, S. et al. Lack of Adrenomedullin Results in Microbiota Changes and Aggravates Azoxymethane and Dextran Sulfate Sodium-Induced Colitis in Mice. Front. Physiol. 7, 595 (2016).
Nagata, S., Yamasaki, M. & Kitamura, K. Anti-Inflammatory Effects of PEGylated Human Adrenomedullin in a Mouse DSS-Induced Colitis Model. Drug Dev. Res. 78, 129–134 (2017).
Ashizuka, S. et al. Adrenomedullin treatment reduces intestinal inflammation and maintains epithelial barrier function in mice administered dextran sulphate sodium. Microbiol Immunol 53, 573–581 (2009).
Ashizuka, S., Inatsu, H., Inagaki-Ohara, K., Kita, T. & Kitamura, K. Adrenomedullin as a potential therapeutic agent for inflammatory bowel disease. Curr Protein Pept Sci 14, 246–255 (2013).
Zudaire, E., Portal-Núñez, S. & Cuttitta, F. The central role of adrenomedullin in host defense. J. Leukoc. Biol. 80, 237–44 (2006).
Allaker, R. P., Zihni, C. & Kapas, S. An investigation into the antimicrobial effects of adrenomedullin on members of the skin, oral, respiratory tract and gut microflora. FEMS Immunol Med Microbiol 23, 289–293 (1999).
Martínez, A. et al. Regulation of insulin secretion and blood glucose metabolism by adrenomedullin. Endocrinology 137, 2626–2632 (1996).
Martinez, A., Miller, M. J., Unsworth, E. J., Siegfried, J. M. & Cuttitta, F. Expression of adrenomedullin in normal human lung and in pulmonary tumors. Endocrinology 136, 4099–4105 (1995).
Ouafik, L. et al. Neutralization of Adrenomedullin Inhibits the Growth of Human Glioblastoma Cell Lines in Vitro and Suppresses Tumor Xenograft Growth in Vivo. Am. J. Pathol. 160, 1279–1292 (2002).
Ishikawa, T. et al. Adrenomedullin antagonist suppresses in vivo growth of human pancreatic cancer cells in SCID mice by suppressing angiogenesis. Oncogene 22, 1238–42 (2003).
Martínez, A. et al. Identification of vasoactive nonpeptidic positive and negative modulators of adrenomedullin using a neutralizing antibody-based screening strategy. Endocrinology 145, 3858–65 (2004).
Ramachandran, V. et al. Adrenomedullin is expressed in pancreatic cancer and stimulates cell proliferation and invasion in an autocrine manner via the adrenomedullin receptor, ADMR. Cancer Res. 67, 2666–75 (2007).
Roldós, V. et al. Identification of first proadrenomedullin N-terminal 20 peptide (PAMP) modulator by means of virtual screening and NMR interaction experiments. Eur. J. Med. Chem. 55, 262–272 (2012).
Okayasu, I. et al. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology 98, 694–702 (1990).
Sobhani, I. et al. Microbial Dysbiosis in Colorectal Cancer (CRC) Patients. PLoS One 6, (e16393 (2011).
Weir, T. L. et al. Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS One 8, e70803 (2013).
Talero, E. et al. Acute and chronic responses associated with adrenomedullin administration in experimental colitis. Peptides 29, 2001–12 (2008).
Cooper, H. S., Murthy, S., Kido, K., Yoshitake, H. & Flanigan, A. Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis 21, 757–768 (2000).
Leushacke, M. & Barker, N. Lgr5 and Lgr6 as markers to study adult stem cell roles in self-renewal and cancer. Oncogene 31, 3009–22 (2012).
Zhang, Y., Dubé, P. E., Washington, M. K., Yan, F. & Polk, D. B. ErbB2 and ErbB3 regulate recovery from dextran sulfate sodium-induced colitis by promoting mouse colon epithelial cell survival. Lab. Invest. 92, 437–50 (2012).
Berry, D. et al. Intestinal Microbiota Signatures Associated with Inflammation History in Mice Experiencing Recurring Colitis. Front Microbiol 6, 1408 (2015).
Zhu, Q. et al. Analysis of the intestinal lumen microbiota in an animal model of colorectal cancer. PLoS One 9, e90849 (2014).
Ou, J. et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am. J. Clin. Nutr. 98, 111–20 (2013).
Greiner, A. K., Papineni, R. V. L. & Umar, S. Chemoprevention in gastrointestinal physiology and disease. Natural products and microbiome. Am. J. Physiol. Gastrointest. Liver Physiol. 307, G1–15 (2014).
Thaker, A. I., Shaker, A., Rao, M. S. & Ciorba, M. A. Modeling colitis-associated cancer with azoxymethane (AOM) and dextran sulfate sodium (DSS). J. Vis. Exp. 67, 4100 (2012).
Gommeaux, J. et al. Colitis and colitis-associated cancer are exacerbated in mice deficient for tumor protein 53-induced nuclear protein 1. Mol. Cell. Biol. 27, 2215–28 (2007).
Ochoa-Callejero, L. et al. Maraviroc, a CCR5 antagonist, prevents development of hepatocellular carcinoma in a mouse model. PLoS One 8, e53992 (2013).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–8 (2008).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7, 335–336 (2010).
DeSantis, T. Z. et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Env. Microbiol 72, 5069–5072 (2006).
Acknowledgements
Small molecules were generously provided by the NCI Developmental Therapeutic Program (Frederick, MD). Authors thank Dr. María de Toro (Genomic Platform, CIBIR, Logroño, Spain) for metagenomic analysis and Dr. Enrique Ramalle-Gómara (Epidemiology Health Prevention Service, Logroño, Spain) for his help with statistical analysis. This study was funded by Instituto de Salud Carlos III (PI13/02166) and FEDER. SM-H was supported by a predoctoral fellowship from the Junta Provincial de La Rioja de la Asociación Española Contra el Cáncer (AECC).
Author information
Authors and Affiliations
Contributions
L.O.-C., S.M.-H., S.R.-M., J.N.-I. and J.G.-S.: Performed experiments. L.O.-C. and A.M.: Interpreted data, wrote the manuscript, and designed the study. A.M.: Provided funding. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ochoa-Callejero, L., García-Sanmartín, J., Martínez-Herrero, S. et al. Small molecules related to adrenomedullin reduce tumor burden in a mouse model of colitis-associated colon cancer. Sci Rep 7, 17488 (2017). https://doi.org/10.1038/s41598-017-17573-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-17573-x
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.