Abstract
In-stent restenosis (ISR) remains the leading problem encountered after percutaneous coronary intervention (PCI). Thiazolidinediones (TZDs) has been shown to be associated with reduced ISR and target lesion revascularization (TLR); however, the results are inconsistent, especially between rosiglitazone and pioglitazone. In this study, fourteen RCTs with a total of 1350 patients were finally included through a systematical literature search of Embase, Pubmed, the Cochrane Library, and ClinicalTrials.gov from inception to January 31, 2017. The follow-up duration of the included trials ranged from 6 months to 18 months. The results demonstrated that TZDs treatment is associated with significantly reduced risk of TLR (RR:0.45, 95%CI 0.30 to 0.67 for pioglitazone, RR:0.68, 95%CI 0.46 to 1.00 for rosiglitazone). Pioglitazone is associated with significantly reduced risks of ISR (RR:0.47, 95%CI 0.27 to 0.81), major adverse cardiac events (MACE) (RR:0.44, 95%CI 0.30 to 0.64) and neointimal area (SMD: −0.585, 95%CI −0.910 to −0.261). No significant relationship was observed between rosiglitazone and ISR (RR:0.91, 95%CI 0.39 to 2.12), MACE (RR:0.73, 95%CI 0.53 to 1.00) and neointimal area (SMD: −0.164, 95%CI −1.146 to 0.818). This meta-analysis demonstrated that TZDs treatment is associated with significant reduction in ISR, TLR and MACE for patients after PCI. Pioglitazone treatment seems to have more beneficial effects than rosiglitazone and no significantly increased cardiovascular risk was detected for both agents.
Similar content being viewed by others
Introduction
In-stent restenosis (ISR) remains a significant problem after percutaneous coronary intervention (PCI), both for the bare-metal stent (BMS) and drug-eluting stent (DES)1,2. Besides antiplatelet therapy, no additional drugs are routinely used to prevent ISR.
Thiazolidinediones (TZDs), as a class of agonists of the peroxisome proliferation-activated receptor-γ (PPAR-γ), have widely been used since 1990 as insulin sensitizers in the treatment of diabetes3.
This class of agonists can also modulate several biological processes to inhibit cellular proliferation and reduce inflammation after vascular injury4,5,6,7, adding to cardiovascular interest and promise. Rosiglitazone and pioglitazone currently are commercially available. Previous meta-analysis have demonstrated benefits of both TZDs and pioglitazone in prevention of ISR and target lesion revascularization (TLR)8,9,10,11,12. Few of these analyses are based on RCT data; the results of these studies are also inconsistent.
Because of the potential cardiac risk, as reported by several studies for rosiglitazone13, most studies focused only on pioglitazone; the different effects on ISR and TLR between rosiglitazone and pioglitazone have not been discussed and clearly demonstrated. However, reevaluation of the RECORD trial data demonstrated that rosiglitazone did not increase any risk of heart attack; therefore, its clinical restrictions eventually have been removed14.
This present meta-analysis, based on the updated information, was performed to examine further the role of TZDs in the prevention of ISR and TLR after PCI in diabetic and non-diabetic patients. Additionally, potential differences between rosiglitazone and pioglitazone were investigated.
Materials and Methods
No ethical approval was required as all the data were acquired from previously published studies.
Search Strategy and Selection Criteria
We systematically searched articles on effects of TZDs after PCI with Embase, Pubmed, the Cochrane Library, and ClinicalTrials.gov from inception to January 31, 2017. The following terms and variants thereof were used: “stent”, “restenosis”, “thiazolidinediones”, “rosiglitazone”, “pioglitazone.” Additionally, the references of the selected articles, relevant reviews and previous meta-analyses were manually searched for potentially relevant citations. Only RCTs in the English language with full article text were included.
Studies were required to meet the following criteria to be included in the research: (1) randomized controlled trial (RCT), (2) original data showing the effects of TZDs after PCI, (3) TZDs therapy compared with placebo, without TZDs, or other anti-diabetic therapy, (4) the outcomes of interest were reported, and (5) the length of follow-up was at least 6 months. RCTs concerned with troglitazone or without a full article were excluded.
Data Collection and Quality Assessment
Two reviewers performed the data extraction and quality assessment independently, and disagreements were resolved by consensus. The following data were extracted: number of patients assigned to each group, participant characteristics, TZDs type, duration of follow-up, and outcomes of interest. The quality of the RCTs included was assessed with the Cochrane Collaboration tool15.
Outcomes
The primary outcomes of interest were the number of patients with angiographic ISR by quantified coronary angiography (QCA) and the patients required to have TLR during follow-up. Secondary outcomes included major adverse cardiac events (MACE) and other QCA results including late lumen loss (LLL), minimum lumen diameter (MLD) and percentage stenosis (PS). The most frequently used intravascular ultrasound (IVUS) measurements, average in-stent neointimal area (neointimal volume/stent length) and neointimal index (neointimal volume/stent volume or neointimal area/stent area) were also analysed if IVUS procedure was performed.
Statistical Analysis
STATA version 12.0 (STATA Corporation, TX, USA) was used to perform statistical analysis. Relative risk (RR) or standard mean difference (SMD) and their 95% confidence intervals (CIs) were calculated to demonstrate the overall result. Heterogeneity across studies was assessed with the chi-square test, and I2 > 50% was considered indicative of significant heterogeneity. The causes were investigated and a random effects model was applied when a significant heterogeneity was present; otherwise, a fixed effects model was used. Publication bias was analysed graphically with funnel plots and statistically with Egger’s and Begg’s tests. A two-sided P value of < 0.05 was considered to be statistically significant.
Results
Eligible Studies and Characteristics
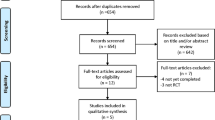
A total of 226 studies were identified in the initial search, of which 37 studies were further assessed. Ultimately, 14 RCTs with a total of 1350 patients were included in the analysis, with follow-up ranging from 6 months to 18 months after intervention16,17,18,19,20,21,22,23,24,25,26,27,28,29. No additional studies were identified when we manually searched the references of the included articles and relevant reviews (Fig. 1). The baseline characteristics of the included studies are outlined in Table 1. Briefly, 8 trials were treated with pioglitazone20,21,22,23,24,25,27,28, and 6 trials were treated with rosiglitazone16,17,18,19,26,29.
All 14 RCTs included for pooling analysis had high qualities and relatively low risks of bias according to the Cochrane Collaboration tool. No significant publication bias was found by a funnel plot (Fig. 2) or revealed by the Egger’s and Begg’s tests based on the outcome of ISR (Egger’s: p = 0.163, Begg’s: p = 0.161).
Primary End Points
The ISR rate was 15.7% in the TZDs group compared with 26.8% in the control group (RR:0.58, 95% CI:0.38 to 0.90, p = 0.016) (Fig. 3). However, moderate heterogeneity for this analysis was detected (I2 = 54.8%) and was further addressed by subgroup analysis according to the different TZDs type used. The results showed that the ISR was 14.4% in studies treated with pioglitazone and 30.9% in the control group (RR:0.47, 95% CI:0.27 to 0.81, p = 0.006), whereas analysis of studies treated with rosiglitazone showed an ISR rate of 17.8% and 20.3% in rosiglitazone and the control group, respectively (RR:0.91, 95% CI:0.39 to 2.12, p = 0.823) (Fig. 3). Heterogeneity was still observed for both subgroups.
TLR events occurred in 9.7% patients treated with TZDs compared to 17.8% of patients in the control group. TZDs treatment was associated with a significant reduction in TLR events (RR:0.55, 95%CI 0.42 to 0.73, P < 0.05) (Fig. 4). Additionally, in the subgroup analysis, both pioglitazone and rosiglitazone treatment resulted in significant reduction in TLR (RR:0.45, P < 0.05, RR:0.68, P < 0.05, respectively). No significant heterogeneity was found both for overall analysis (I2 = 33.5%) and subgroup analysis according to the TZDs type used (I2 = 49.5% for pioglitazone and I2 = 0% for rosiglitazone) (Fig. 4).
Secondary End Points
No significant heterogeneity was detected for the analysis of the incidence of MACE across the studies (I2 = 44.6%). The results demonstrated that the treatment with TZDs was associated with a significant reduction of MACE (RR:0.58, 95% CI:0.46 to 0.74, P < 0.05) (Fig. 5). Further subgroup analysis showed that pioglitazone treatment resulted in significant MACE reduction (RR:0.44, P < 0.05), whereas no significant association was observed between rosiglitazone treatment and MACE (RR:0.73, P = 0.053) (Fig. 5).
Comparison of other QCA results, including late lumen loss, minimum lumen diameter and percentage stenosis during follow-up, are exhibited in Table 2. Results demonstrated that treatment with TZDs resulted in less late lumen loss (SMD: −0.42, P < 0.05), greater minimum lumen diameter (SMD:0.24, P < 0.05) and lower percentage stenosis (SMD: −0.39, P < 0.05). The heterogeneity was large for all three analysis (I2 > 50%); thus, further subgroup analysis was performed. Heterogeneity remained moderate and results showed that pioglitazone treatment exhibited significant influence on LLL, MLD and PS (P < 0.05 for all), whereas no relationship between rosiglitazone treatment and these three targets was determined(p > 0.05 for all).
IVUS data were provided in eight studies19,21,22,23,24,26,27,28, and the results demonstrated that TZDs treatment was associated with significant reduction in neointimal area (SMD: −0.552, 95%CI −0.853 to −0.250, P < 0.05) and neointimal index (SMD: −0.550, 95%CI −0.990 to −0.111, P < 0.05). Moderate to large heterogeneity was detected for both analysis. Further subgroup analysis exhibited significantly lower neointimal area (SMD: −0.585, 95%CI −0.910 to −0.261, P < 0.05) and neointimal index (SMD: −0.704, 95%CI −1.071 to −0.337, P < 0.05) in pioglitazone-treated patients, while no significant influences on both outcomes were observed in rosiglitazone-treated patients((p > 0.05 for both).
Discussion
Results indicated a significant clinical benefit for patients after stent implantation with the addition treatment of TZDs in reducing events of ISR, TLR and MACE. The results of the QCA examinations, including LLL, MLD and PS, and IVUS results, including neointimal area and neointimal index during follow-up also further support this conclusion.
The precise mechanisms of restenosis have not been thoroughly elucidated to date. The development of intimal hyperplasia after stent-implantation induced by vascular injury and inflammation response plays a crucial role in the progression of ISR.
As insulin-sensitizing agents, TZDs have been used widely for diabetes patients and have been demonstrated to have the effects of both anti-inflammation and anti-proliferation mediated by binding to PPAR-γ30,31,32,33 and eventually attenuate the development of intimal hyperplasia after PCI to reduce the rates of ISR and TLR. The significant reduced neointimal area and neointimal index in TZDs-treated patients from IVUS procedure further confirmed these effects, which may also be independent of glycaemic and lipid control34.
Results showed that both rosiglitazone and pioglitazone treatments led to a significant reduction in the events of TLR, which was in consistent with results reported by previous studies35. However, there were significant differences between rosiglitazone and pioglitazone treatment as for ISR and MACE events; the QCA results including LLL, MLD and PS; and the IVUS results. Pioglitazone showed significant benefits whereas no significant relationship was detected between rosiglitazone and all those results.
The difference may be partly explained by the different gene modulation patterns and biological effects36 between the two agents. According to several studies, pioglitazone increases low-density lipoprotein (LDL), high-density lipoprotein (HDL) and triglycerides (TG) levels, whereas rosiglitazone mainly affects LDL37,38. Pioglitazone also has properties of stabilizing plaque39, enhancing apoptosis40 and suppressing fibrin formation41, which might contribute to its cardiovascular benefits.
However, previous studies showed that PPAR-γ could prevent arteriosclerosis through its anti-inflammatory effects42; in-stent restenosis was also demonstrated to be associated with insulin resistance but not lipids34.
The fact that few studies investigated rosiglitazone to investigate its effects beyond anti-diabetes after its restrictions were imposed may also partly contribute to the difference. It is interesting to see the imbalances between the cardiovascular effects of rosiglitazone and pioglitazone, and further studies are warranted to make this difference clear and definite.
The present analysis demonstrated that TZDs use was associated with a significant reduction of MACE events, especially for pioglitazone treatment. It should be noted that, two previous published meta-analyses indicated that rosiglitazone treatment resulted in a significant increased risk of myocardial infarction13,43, leading to the imposition of strict restrictions on its clinical use by the U.S. Food and Drug Administration (FDA) and the China Food and Drug Administration (CFDA). Conversely, pioglitazone has been shown in many studies to be associated with decreased risk of mortality and myocardial infarction44,45.
Reevaluation of the RECORD trial data in 2013 revealed that rosiglitazone did not associated with significant negative cardiovascular outcomes14, and its clinical restrictions were removed. Ten-year results of the PROactive trial showed that pioglitazone failed to significantly reduce cardiovascular events46. In addition, no relationship was found between rosiglitazone and MACE events in the present studies, and favourable benefits were even seen for rosiglitazone.
According to the evidences available, TZDs treatment, including rosiglitazone and pioglitazone, has significant benefits for patients after PCI without remarkably increased cardiovascular risks. The results should still be interpreted with caution due to the moderate heterogeneity and the inconsistency among studies.
Study limitations
The present meta-analysis was performed based on 14 high-quality RCTs with 1350 patients; however, several limitations should still be noted. First, potential publication biases were inevitable to a certain extent, as considerable heterogeneities were detected for ISR, the QCA and IVUS results. Only half of the included studies provided IVUS results and IVUS procedure was not performed for every patient as routine in each study; thus, it may could not represent overall patients included in this study. Second, as only subgroup analysis of different TZDs type was performed, the lack of subgroup analysis, including stents type implanted and the dosage of TZDs, might contribute to the bias for this study. Finally, the follow-up lengths were abbreviated, as the longest was 18 months, which may be insufficient to measure the rates of ISR, TLR and MACE. The findings in our study might be lack of sufficient power and should be interpreted with caution. Further large-scale RCTs are needed to confirm the findings of this study.
Conclusions
TZDs treatment for patients after PCI is associated with significant reduction in ISR, TLR and MACE. Subgroup analysis demonstrated that pioglitazone treatment showed more benefits than rosiglitazone. No significantly increased cardiovascular risk was detected for TZDs, especially for rosiglitazone. More large-scale RCTs are warranted to confirm these results further.
References
Dangas, G. D. et al. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol 56, 1897–907 (2010).
Roiron, C., Sanchez, P., Bouzamondo, A., Lechat, P. & Montalescot, G. Drug eluting stents: an updated meta-analysis of randomised controlled trials. Heart 92, 641–9 (2006).
Yasmin, S. & Jayaprakash, V. Thiazolidinediones and PPAR orchestra as antidiabetic agents: From past to present. Eur J Med Chem 126, 879–893 (2017).
Joner, M. et al. Pioglitazone inhibits in-stent restenosis in atherosclerotic rabbits by targeting transforming growth factor-beta and MCP-1. Arterioscler Thromb Vasc Biol 27, 182–9 (2007).
Kasai, T. et al. Pioglitazone attenuates neointimal thickening via suppression of the early inflammatory response in a porcine coronary after stenting. Atherosclerosis 197, 612–9 (2008).
Okura, H., Takagi, T. & Yoshida, K. Therapies targeting inflammation after stent implantation. Curr Vasc Pharmacol 11, 399–406 (2013).
Yki-Jarvinen, H. Thiazolidinediones. N Engl J Med 351, 1106–18 (2004).
Geng, D. F., Jin, D. M., Wu, W., Wang, Z. & Wang, J. F. Effect of thiazolidinediones on in-stent restenosis in patients after coronary stenting: a meta-analysis of randomized controlled trials. Atherosclerosis 202, 521–8 (2009).
Patel, D., Walitt, B., Lindsay, J. & Wilensky, R. L. Role of pioglitazone in the prevention of restenosis and need for revascularization after bare-metal stent implantation: a meta-analysis. JACC Cardiovasc Interv 4, 353–60 (2011).
Rosmarakis, E. S. & Falagas, M. E. Effect of thiazolidinedione therapy on restenosis after coronary stent implantation: a meta-analysis of randomized controlled trials. Am Heart J 154, 144–50 (2007).
Zhang, M. D. et al. Effect of pioglitazone on in-stent restenosis after coronary drug-eluting stent implantation: a meta-analysis of randomized controlled trials. PLoS One 9, e109614 (2014).
Zhao, S. J. et al. Effect of Pioglitazone in Preventing In-Stent Restenosis after Percutaneous Coronary Intervention in Patients with Type 2 Diabetes: A Meta-Analysis. PLoS One 11, e0155273 (2016).
Nissen, S. E. & Wolski, K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356, 2457–71 (2007).
Mahaffey, K. W. et al. Results of a reevaluation of cardiovascular outcomes in the RECORD trial. Am Heart J 166, 240–249.e1 (2013).
Higgins, J., Green, S. (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. http://handbook.cochrane.org (2011).
Cao, Z. et al. Rosiglitazone could improve clinical outcomes after coronary stent implantation in nondiabetic patients with metabolic syndrome. Chin Med J (Engl) 119, 1171–5 (2006).
Choi, D. et al. Preventative effects of rosiglitazone on restenosis after coronary stent implantation in patients with type 2 diabetes. Diabetes Care 27, 2654–60 (2004).
Finn, A. V. et al. Predictive factors for in-stent late loss and coronary lesion progression in patients with type 2 diabetes mellitus randomized to rosiglitazone or placebo. Am Heart J 157, 383.e1–8 (2009).
Garcia-Garcia, H. M. et al. Evaluation of in-stent restenosis in the APPROACH trial (Assessment on the Prevention of Progression by Rosiglitazone On Atherosclerosis in diabetes patients with Cardiovascular History). Int J Cardiovasc Imaging 28, 455–65 (2012).
Hong, S. J. et al. Cellular and molecular changes associated with inhibitory effect of pioglitazone on neointimal growth in patients with type 2 diabetes after zotarolimus-eluting stent implantation. Arterioscler Thromb Vasc Biol 30, 2655–65 (2010).
Kaneda, H. et al. Efficacy and safety of pioglitazone in patients with ST elevation myocardial infarction treated with primary stent implantation. Heart 95, 1079–84 (2009).
Katayama, T. et al. Reduction of neointimal hyperplasia after coronary stenting by pioglitazone in nondiabetic patients with metabolic syndrome. American Heart Journal 153, 762.e1–762.e7 (2007).
Lee, H. W. et al. Effects of low dose pioglitazone on restenosis and coronary atherosclerosis in diabetic patients undergoing drug eluting stent implantation. Yonsei Med J 54, 1313–20 (2013).
Marx, N. et al. Pioglitazone reduces neointima volume after coronary stent implantation: A randomized, placebo-controlled, double-blind trial in nondiabetic patients. Circulation 112, 2792–2798 (2005).
Nishio, K. et al. A randomized comparison of pioglitazone to inhibit restenosis after coronary stenting in patients with type 2 diabetes. Diabetes Care 29, 101–6 (2006).
Osman, A. et al. Effect of rosiglitazone on restenosis after coronary stenting in patients with type 2 diabetes. American Heart Journal 147, e23 (2004).
Takagi, T. et al. A prospective, multicenter, randomized trial to assess efficacy of pioglitazone on in-stent neointimal suppression in type 2 diabetes: POPPS (Prevention of In-Stent Neointimal Proliferation by Pioglitazone Study). JACC Cardiovascular Interventions 2, 524–31 (2009).
Takagi, T. et al. Pioglitazone reduces neointimal tissue proliferation after coronary stent implantation in patients with type 2 diabetes mellitus: an intravascular ultrasound scanning study. American Heart Journal 146, E5 (2003).
Wang, G. et al. Peroxisome proliferator-activated receptor-γ agonist rosiglitazone reduces clinical inflammatory responses in type 2 diabetes with coronary artery disease after coronary angioplasty. Metabolism: Clinical and Experimental 54, 590–597 (2005).
Desouza, C. V., Gerety, M. & Hamel, F. G. Long-term effects of a PPAR-gamma agonist, pioglitazone, on neointimal hyperplasia and endothelial regrowth in insulin resistant rats. Vascul Pharmacol 46, 188–94 (2007).
Goetze, S. et al. PPAR gamma-ligands inhibit migration mediated by multiple chemoattractants in vascular smooth muscle cells. J Cardiovasc Pharmacol 33, 798–806 (1999).
Igarashi, M. et al. Characterization of an inhibitory effect of pioglitazone on balloon-injured vascular smooth muscle cell growth. Metabolism 50, 955–62 (2001).
Little, P. J. et al. Anti-proliferative activity of oral anti-hyperglycemic agents on human vascular smooth muscle cells: thiazolidinediones (glitazones) have enhanced activity under high glucose conditions. Cardiovasc Diabetol 6, 33 (2007).
Nishio, K. et al. Insulin resistance as a predictor for restenosis after coronary stenting. Int J Cardiol 103, 128–34 (2005).
Riche, D. M., Valderrama, R. & Henyan, N. N. Thiazolidinediones and risk of repeat target vessel revascularization following percutaneous coronary intervention: a meta-analysis. Diabetes Care 30, 384–8 (2007).
Takagi, T. et al. Pioglitazone reduces neointimal tissue proliferation after coronary stent implantation in patients with type 2 diabetes mellitus: an intravascular ultrasound scanning study. Am Heart J 146, E5 (2003).
Deeg, M. A. et al. Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care 30, 2458–64 (2007).
Goldberg, R. B. et al. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 28, 1547–54 (2005).
Nissen, S. E. et al. Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. Jama 299, 1561–73 (2008).
Aizawa, Y., Kawabe, J., Hasebe, N., Takehara, N. & Kikuchi, K. Pioglitazone enhances cytokine-induced apoptosis in vascular smooth muscle cells and reduces intimal hyperplasia. Circulation 104, 455–60 (2001).
Li, D. et al. The effects of PPAR-gamma ligand pioglitazone on platelet aggregation and arterial thrombus formation. Cardiovasc Res 65, 907–12 (2005).
Ishibashi, M. et al. Antiinflammatory and antiarteriosclerotic effects of pioglitazone. Hypertension 40, 687–93 (2002).
Singh, S., Loke, Y. K. & Furberg, C. D. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. Jama 298, 1189–95 (2007).
Dormandy, J. A. et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 366, 1279–89 (2005).
Lincoff, A. M., Wolski, K., Nicholls, S. J. & Nissen, S. E. Pioglitazone and risk of cardiovascular events in patients with type 2 diabetes mellitus: a meta-analysis of randomized trials. Jama 298, 1180–8 (2007).
Erdmann, E., Harding, S., Lam, H. & Perez, A. Ten-year observational follow-up of PROactive: a randomized cardiovascular outcomes trial evaluating pioglitazone in type 2 diabetes. Diabetes Obes Metab 18, 266–73 (2016).
Author information
Authors and Affiliations
Contributions
X.Z. and S.C. designed the research, performed the statistical analysis and wrote the main manuscript text, M.Z. and J.H. performed the literature search and data collation, J.D. and X.X. prepared figures and tables, Y.Q. and W.M. have jointly supervised the work and revised the article critically. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, X., Chen, S., Zhu, M. et al. Different Effects of Thiazolidinediones on In-Stent Restenosis and Target Lesion Revascularization after PCI: A Meta-Analysis of Randomized Controlled Trials. Sci Rep 7, 14464 (2017). https://doi.org/10.1038/s41598-017-14873-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-14873-0
This article is cited by
-
Aim to normalize glucose levels and reduce cardiovascular mortality when managing type 2 diabetes in the elderly
Drugs & Therapy Perspectives (2019)
-
Cardiovascular Safety of Antihyperglycemic Agents: “Do Good or Do No Harm”
Drugs (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.