Abstract
Pulmonary CT angiography (CTPA) is regarded as the preferred imaging method in diagnosing pulmonary embolism (PE). Considering the harm of radiation exposure and the side effect of iodinated contrast agent, CTPA protocol with low tube voltage and low dose of contrast agent became research hotspot in last decade. The present study evaluates the image quality, radiation dose, positive rate of PE and the location of PE with a CTPA protocol using low tube voltage (80 kVp) and low-iodine-concentration contrast agent (270 mg I/ml) in patients suspected of PE compared to a conventional CTPA protocol (120 kVp, 350 mg I/ml). The results showed that 80 kVp CTPA protocol with 40 ml 270 mg I/ml achieved equally subjective image quality and a positive rate for diagnosing PE, though the quantitative image quality was reduced compared to the 120 kVp CTPA protocol with 40 ml 350 mg I/ml administered, with a 63.6% decrease in radiation dose and a 22.9% reduction in iodine content of contrast agent. Our results document that CTPA protocol with low tube voltage and low iodine concentration of contrast agent is satisfied to the clinical application.
Similar content being viewed by others
Introduction
Pulmonary embolism (PE) is a common disorder that is accompanied by high morbidity (estimated 600,000 patients annually in the United States) and mortality (50,000–200,000 deaths)1. Currently, it is the third most common cause of cardiovascular death, following coronary artery disease and stroke world widely2. The rapid diagnosis and treatment is beneficial to patients with PE. The diagnosis of PE is mainly based on clinical signs and symptoms, D-dimer measurements and contrast-enhanced pulmonary CT angiography3,4,5. Contrast-enhanced pulmonary CT angiography allows the pulmonary arteries to be viewed to at least the sub-segmental level and is currently considered the preferred imaging method for diagnosing PE5,6. Therefore, CTPA is widely used in patients suspected of PE, which can lead to the overuse of CTPA and unnecessary radiation exposure to patients.
Many strategies are being developed to limit the radiation dose, including minimizing tube current, reducing tube voltage, the automatic tube current technique, and iterative reconstruction algorithms7,8,9. Low tube voltage technique achieves increased attenuation in the vessel by exploiting the k-edge of iodine, and therefore improves the enhancement in vessels. Thus, a CTPA protocol with low tube voltage and low iodine may improve or maintain image quality10,11. ASIR (the adaptive statistical iterative reconstruction technique, ASIR) is a way to improve image noise using “a reconstructed image through modeling fluctuations in projection data due to photon statistics and electronic system noise”12, as a useful technique to improve SNR (signal to noise ratio, SNR) and CNR(contrast to noise ratio, CNR) whilst reducing contrast and radiation dose. Recent studies10,13,14 have shown that CT angiography protocol with low tube voltage, low iodine and ASIR results in an effective reduction in the radiation dose and iodine content of contrast agent whilst maintaining image quality.
To acquire high iodine concentrations in pulmonary arteries as well as good image quality, the current CTPA protocols mainly adopt high concentrations and large doses of iodine contrast agent15,16. It was previously shown17 that the incidence of contrast-induced nephropathy increased as the contrast agent’s dose increased. And the viscosity of the contrast agent increased along with the concentration of contrast agent. Therefore, it is very important to use low concentration of contrast agents and use doses of contrast agent that are as low as possible without affecting CTA imaging. In recent decades, low concentration of contrast agent is advanced into the clinic, such as 240 mg I/ml18 and 270 mg I/ml17. Low concentration of contrast agent is proved with a lower viscosity and gets an acceleration of contrast agent distribution within blood. Furthermore, the peak injection pressure of contrast agent with low concentration is lower with fewer complications (such as extravasation) compared to contrast agent with high concentration19.
The objective of this study was to compare the image quality, radiation dose, positive rate of detecting PE and the location of PE between CTPA protocols using low tube voltage (80 KVp) and low-iodine-concentration contrast agent (270 mg I/ml) in patients suspected of having PE with those of a conventional CTPA protocol (120 kVp, 350 mg I/ml).
Materials and Methods
Patients
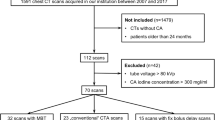
Present study was approved by the Ethics Committee of Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology. All participants provided written informed consent and all methods were performed in accordance with the relevant guidelines and regulations. Between November 2013 and June 2016, outpatients and inpatients that were clinically suspected of PE were enrolled consecutively in this study and required CTPA examinations. Inclusion criteria: 1, Patients suspected of PE based on clinical signs and symptoms(acute chest pain and dyspnea), abnormal plasmatic D-dimer level and/or deep vein thrombosis in the lower extremity; 2, Patients aged >18 years old; 3 patients with a body mass index (BMI) <30 kg/m2; 4, patients with a glomerular filtration rate >60 ml/min/1.73 m2. Exclusion criteria: 1, Patients with history of allergy to iodinated contrast agent; 2, patients with comorbidities such as severe pneumonia and atelectasis. A total of 382 patients were randomly divided into two groups. One hundred ninety-two patients (group A, double low group) were examined with the 80 kVp/40 ml 270 mg I/ml (iodixanol, GE Healthcare, Carrigtohill, Co:Cork, Ireland) protocol, and 190 patients (group B, contrast group) were examined with the 120 kVp/40 ml 350 mg I/ml (Ioversol, tyco Healthcare, Pointe-Claire, Quebec Canada) protocol. The demographic data are shown in Table 1. There were no statistically significant differences in age, gender or BMI between two groups.
CTPA examination
All CTPA examinations were performed using a commercially available 64-MDCT scanner (Discovery CT750 HD, GE healthcare). Patients were examined in a supine position, and both arms were extended above the head. All CTPA data were acquired in the craniocaudal direction from the lung apex to the costophrenic angles during a single breath-hold (inspiration). The bolus-tracking technique was used in the main pulmonary artery with a trigger attenuation threshold of 50 HU. Total 40 ml of contrast agent was injected. First, 12 ml contrast agent and 18 ml saline was injected simultaneously at a flow rate of 4 ml/s using a dual-head power injector in order to compensate for a delay time of 7 s when the table moved from the bolus-tracking level to the lung apex. Then, the rest 28 ml contrast agent injected followed by a constant saline flush of 30 ml at a flow rate of 5 ml/s. The automatic tube current technique was employed with a noise index of 18 HU. The data reconstruction of group A (80 kVp/40 ml 270 mg I/ml) used 40% ASIR and 60% FBP(filter back projection, FBP), however, the data of group B (120 kVp/40 ml 350 mg I/ml) was reconstructed only using FBP. The technical parameters of CTPA for two groups were shown in Table 2.
Image analysis and radiation exposure dose
The CTPA data were transferred to an external workstation (Advantage Windows 4.5, GE Healthcare). The quantitative analysis was performed by a radiologist with more than 5 years of experience. The CT attenuation in the nine arteries was measured using a ROI that was slightly less than the selected artery: the pulmonary trunk, left pulmonary artery, right pulmonary artery, both upper lobe arteries, both lower lobe arteries, right middle lobe artery and left lingual artery. The reader may adjust the window level to avoid the pulmonary emboli. Then, the average value was calculated as the mean CT attenuation. The average standard deviation in the three regions (left, middle and right) with a ROI area of 20 mm2 in front of the chest at the right pulmonary artery slice was measured as the background noise. The CT value of the bilateral paravertebral muscle was measured and the mean value was calculated as the background CT attenuation. The signal-to-noise ratio (SNR) was calculated as the mean CT attenuation/the background noise. The contrast-to-noise ratio (CNR) was calculated as follows: (mean CT attenuation-background CT attenuation)/background noise.
The subjective image quality analysis and the diagnosis of PE were performed by two radiologists with more than 4 years of experience who were blinded to the clinical features and the CTPA protocols. The displayed pulmonary branch level and the severity of iodine contrast agent beam hardening artifacts in the superior vena cava were recorded. The pulmonary arteries were graded as follows: the first pulmonary arterial branch (left and right pulmonary arteries), the second pulmonary branch (lobe arteries), the third pulmonary branch (segmental arteries), the fourth pulmonary branch (sub-segmental arteries), the fifth pulmonary branch and the sixth pulmonary branch. For assessment of the displayed level of pulmonary branches, a coronal MIP-reconstruction image standard with 40mm-slice thickness, 800 Hu-window width and 80 Hu-window center was used. The CTPA images needed to display at least the fourth pulmonary branch to satisfy the requirement of diagnosis. The severity of the iodine contrast agent beam hardening artifacts in the superior vena cava was graded as follows: mild, gentle artifacts that did not affect the measurement; moderate, a few artifacts affected the measurement in the right pulmonary artery; and severe, severe artifacts affected the measurement in the right pulmonary artery and the upper lobe artery. The two radiologists discussed and agreed on diagnosis of PE and the location of pulmonary emboli was recorded. The readers may adjust the window level for diagnosis of PE.
The dose length product (DLP) was available from the CTPA protocols recorded automatically. The effective dose (ED) was calculated using DLP, ED = k * DLP.
The K value adopted was 0.017, as shown by the European Commission (CEC)20.
Data analysis
Statistical analysis was performed using the SPSS 19.0 software package. All quantitative data were described as the means ± standard deviation. The independent samples t-test was used to compare age, BMI, mean CT value, noise, SNR, CNR and ED between the groups. Gender, the severity of iodine contrast agent beam hardening artifacts in the superior vena cava, the displayed pulmonary branch level, the positive rate for detecting PE and the location of pulmonary emboli were analyzed between the groups using the chi-square test. A p value < 0.05 was considered to represent statistical significance. The inter-observer agreement for the subjective image was assessed using Kappa analysis.
Results
Both the mean CT value in the pulmonary arteries and the background noise were significantly higher in group A than in group B, whereas the SNR and CNR were lower in group A than group B (Table 3). There was no significant difference in the displayed pulmonary arterial branch level and severity of iodine contrast agent beam hardening artifacts in the superior vena cava between the groups, with good inter-observer agreement (Tables 4 and 5, Figs 1 and 2). There was no statistically significant difference between the groups in terms of the positive rate for detecting PE and the location of pulmonary emboli (Tables 3 and 6, Figs 3 and 4). The ED in group A was significantly lower than that in group B (Table 3). The radiation exposure in group A was reduced by nearly 63.6% compared to that in group B, and the iodine content administered in group A was decreased by 22.9% relative to group B.
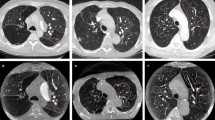
(a) 64-year-old man with pulmonary embolism. The CTPA performed with 80 kVp/40 ml 270 mg I/ml. Mild iodine contrast agent beam hardening artifacts in the superior vena cava (a, axial thin slice image) were observed, and 5–6 pulmonary arterial branches (b, coronal MIP image with 800 Hu-window width and 80 Hu-window center) were displayed.
(a) 40-year-old man with pulmonary embolism. The CTPA performed with 120 kVp/40 ml 350 mg I/ml. Severe iodine contrast agent beam hardening artifacts in the superior vena cava (a, axial thin slice image) were observed, and 5–6 pulmonary arterial branches (b, coronal MIP image with 800 Hu-window width and 80 Hu-window center) were displayed.
(a) 40-year-old man with pulmonary embolism. The CTPA performed with 120 kVp/40 ml 350 mg I/ml. Filling defects were seen in the right inferior lobe artery (c, axial thin slice image; d, sagital thin slice image; e, coronal MIP image; red arrow) and in the dorsal segmental artery (c, d and e, green arrow).
Discussion
The study demonstrated that CTPA protocol with 80 kVp and 40 ml 270 mg I/ml acquired equally subjective image quality and a positive rate for diagnosing PE, though the quantitative image quality was reduced compared to the conventional CTPA protocol (120 kVp, 40 ml 350 mg I/ml). It also achieved a 63.6% decrease in radiation dose and a 22.9% reduction in iodine content of contrast agent.
With respect to patient health, reducing the radiation exposure during CT examinations is one of the most important goals of CT scientists and clinical operators. A decrease in radiation dose can be accomplished by reducing the tube voltage and the tube current. In theory, the radiation dose is proportional to the square of tube voltage21. Therefore, the low tube voltage scanning technique is an effective method for decreasing the radiation dose and has become a hotspot of research over the last decade. Several studies6,21,22 have demonstrated that the CTPA protocol with 80 kVp achieved a 50–70% reduction in the radiation dose compared to the 120 kVp CTPA protocol when the other parameters remained unchanged. The automatic tube current modulation technique can adjust the tube current according the individual size and anatomy to acquire stable image quality and an 18% decrease of radiation exposure dose compared to fixed tube current technique8. In our study, we employed low tube voltage combined with the automatic tube current modulation technique to minimize radiation exposure dose and achieved a nearly 63.6% reduction in the radiation exposure dose, as expected.
An advantage of the low tube voltage technique is that the attenuation in the vessel increases because the energy level moves closer to the K-edge of iodine contrast agent, thus potentially decreasing the dose of contrast agent10. In the present study, although the iodine content administered to patients in the 80 kVp group was lower, the mean CT value in the pulmonary arterial branches was still higher than that observed in the 120 kVp group. The results of our study were in line with those of other researchers10,21,22,23.
A disadvantage of the low tube voltage technique is increased image noise due to the decreased X-ray energy level. An article by Zamboni22 demonstrated that the noise in the segmental pulmonary arteries or above was higher in the 80-kv protocol than in the 120-kv protocol. In our study, the mean standard deviation of the air in front of the chest was chosen as the background noise to avoid the influence of other tissues, referencing the article by Szucs-Farkas24. The CT scanner used in our study was equipped with ASIR, which has a satisfactory effect on noise elimination. A number of researchers9,25,26,27 have found that ASIR was a useful tool to decrease the radiation dose while maintaining the image quality; furthermore, the noise was decreasing along with the percentage of ASIR increasing. But 30–50% ASIR for chest27,28,29 and 40–60% ASIR for coronary CTA30 was recommended, based on the subjective image quality assessment. So we used a 40%ASIR for our low voltage protocol. The results of our study showed that the background noise in the low tube voltage group was still higher than that in the conventional tube voltage group, though ASIR was employed. One possible reason is that decreased background noise by 40% ASIR + 60% FBP cannot compensate for the increased background noise by reducing tube voltage from 120 kVp to 80 kVp. A higher percentage of ASIR may be need for CTPA protocol with low tube voltage.
Recently, large doses of a high-iodine-concentration contrast agent, such as 400 mg I/ml and 370 mg I/ml, were administered to achieve high enhancement in the vessels1,22. Considering the harm that iodine contrast agent inflicts on the kidney and cardiovascular system, contrast agents with low iodine concentrations are clinically well accepted. Iodixanol (270 mg I/ml) has a low viscosity and is easy to mix with blood after injected into a blood vessel. The pulmonary circulation is faster, and pulmonary artery reinforcement would reach a peak at 7–13 s after the contrast agent is injected. If the scan time is calculated exactly and the optimum dose of contrast agent is administered, we can obtain high-quality images with reduced superior vena cava iodine contrast agent beam hardening artifacts. In our study, only 40 ml of contrast agent was used, which was less than the doses reported by other studies15,31. However, the results were not quite satisfactory. Though the CTPA protocol using 40 ml of contrast agent could display the pulmonary arteries to at least the sub-segmental level, the iodine contrast agent beam hardening artifacts in vena cava were equal between the groups, and severe iodine contrast agent beam hardening artifacts in the vena cava were observed in many cases. This means that there is probably room to further minimize the dose of contrast agent. Some literatures demonstrated that it was feasible for contrast agent reduced to 20–30 ml for CTPA protocols without compromising diagnostic image quality23,32. But different injection and scanning protocols were needed, such as bolus testing technique and different trigger position (the superior vena cava). The advantage of our study is that a larger number of samples and lower concentration of contrast agent was used.
In recent decade, the dual-energy CT scanner was introduced to clinic, which could obtain conventional CT images, virtual monenergistic images and accurate material decomposition images (such as iodine-based material decomposition images)33. Previous studies have confirmed that the optical monenergisteic images (range of 65–70 keV)34 could improve image quality and diagnostic confidence for detection of PE and the iodine maps correlated to SPECT/CT35 and MR perfusion36 could detect the peripheral pulmonary emboli missing in CTPA images. That means it is feasible to minimize the dose of contrast agent to less than 40 ml with maintaining even improving image quality and the diagnostic performance for PE using dual energy CT scanner. It may be one of the research orientations in the future.
In our study, both the mean CT value and the background noise were increasing in low tube voltage group, but the SNR and CNR were decreasing compared to regular tube voltage group. This result indicates that the reduced quantitative image quality using low tube voltage and low concentration contrast agent was not in line with expectations. One probable reason is that reducing tube voltage from 120 kVp to 80 kVp generated much background noise. A 22.9% reduction of iodine concentration may be another reason. Though the quantitative image quality of CTPA protocol using low tube voltage and low iodine concentration of contrast agent was decreased, the displayed pulmonary arterial level and the positive rate for detecting PE were equal as conventional CTPA protocol with high iodine concentration of contrast agent. That means CTPA protocol with low tube voltage and low iodine concentration of contrast agent is satisfied to the clinical application.
Limitations
There are some limitations to our study. First, individual doses of contrast agent were not used. A dose of 40 ml contrast agent for all patients may have affected image quality. Second, pulmonary arterial branches of grades 5–6 were not displayed in a minority of cases. Thus, the pulmonary emboli in such pulmonary arteries may be missed, and false negative results will appear in these cases, although the positive rate of detecting PE is not the focus of this article. Additionally, multiple percentages of ASIR were not used in this study to find optical percentage of ASIR for CTPA protocol with low voltage tube and low dose of contrast agent. Finally, we limited the use of CTPA protocol with low tube voltage and low iodine concentration to patients with BMI <30 kg/m2.
Conclusion
Compared to a conventional CTPA protocol, the CTPA protocol with low tube voltage and low-iodine-concentration contrast agent achieved at least equally subjective image quality and the positive rate of diagnosing PE, with an approximately 63.6% decrease in radiation dose and a 22.9% reduction in the iodine content of the contrast agent.
Change history
12 March 2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has not been fixed in the paper.
References
Faggioni, L. et al. 80-kV pulmonary CT angiography with 40 mL of iodinated contrast material in lean patients: comparison of vascular enhancement with iodixanol (320 mg I/mL)and iomeprol (400 mg I/mL). AJR Am J Roentgenol 199, 1220–5 (2012).
Zhang, L. J. et al. Pulmonary embolism and renal vein thrombosis in patients with nephrotic syndrome: prospective evaluation of prevalence and risk factors with CT. Radiology 273, 897–906 (2014).
Mayo, J. & Thakur, Y. Pulmonary CT angiography as first-line imaging for PE: image quality and radiation dose considerations. AJR Am J Roentgenol 200, 522–8 (2013).
Konstantinides, S.V. Trends in incidence versus case fatality rates of pulmonary embolism: Good news or bad news? Thromb Haemost 115 (2015).
Righini, M., Robert-Ebadi, H. & Le Gal, G. Diagnosis of pulmonary embolism. Presse Med 44, e385–91 (2015).
Szucs-Farkas, Z. et al. Diagnostic accuracy of pulmonary CT angiography at low tube voltage: intraindividual comparison of a normal-dose protocol at 120 kVp and a low-dose protocol at 80 kVp using reduced amount of contrast medium in a simulation study. AJR Am J Roentgenol 197, W852–9 (2011).
Dong, F., Davros, W., Pozzuto, J. & Reid, J. Optimization of kilovoltage and tube current-exposure time product based on abdominal circumference: an oval phantom study for pediatric abdominal CT. AJR Am J Roentgenol 199, 670–6 (2012).
Lee, E. J. et al. Comparison of image quality and radiation dose between fixed tube current and combined automatic tube current modulation in craniocervical CT angiography. AJNR Am J Neuroradiol 30, 1754–9 (2009).
Leipsic, J. et al. Estimated radiation dose reduction using adaptive statistical iterative reconstruction in coronary CT angiography: the ERASIR study. AJR Am J Roentgenol 195, 655–60 (2010).
Li, X. et al. 70-kVp High-pitch Computed Tomography Pulmonary Angiography with 40 mL Contrast Agent: Initial Experience. Acad Radiol 22, 1562–70 (2015).
Shen, Y. et al. Did low tube voltage CT combined with low contrast media burden protocols accomplish the goal of “double low” for patients? An overview of applications in vessels and abdominal parenchymal organs over the past 5 years. Int J Clin Pract 70 (Suppl 9B), B5–B15 (2016).
Brady, S. L., Moore, B. M., Yee, B. S. & Kaufman, R. A. Pediatric CT: implementation of ASIR for substantial radiation dose reduction while maintaining pre-ASIR image noise. Radiology 270, 223–31 (2014).
Shen, Y. et al. High-pitch, low-voltage and low-iodine-concentration CT angiography of aorta: assessment of image quality and radiation dose with iterative reconstruction. PLoS One 10, e0117469 (2015).
Li, Z. et al. Adrenal and nephrogenic hypertension: an image quality study of low tube voltage, low-concentration contrast media combined with adaptive statistical iterative reconstruction. Int J Clin Pract 70 (Suppl 9B), B29–36 (2016).
Roggenland, D. et al. CT angiography in suspected pulmonary embolism: impact of patient characteristics and different venous lines on vessel enhancement and image quality. AJR Am J Roentgenol 190, W351–9 (2008).
Wu, C. C., Lee, E. W., Suh, R. D., Levine, B. S. & Barack, B. M. Pulmonary 64-MDCT angiography with 30 mL of IV contrast material: vascular enhancement and image quality. AJR Am J Roentgenol 199, 1247–51 (2012).
Seeliger, E., Sendeski, M., Rihal, C. S. & Persson, P. B. Contrast-induced kidney injury: mechanisms, risk factors, and prevention. Eur Heart J 33, 2007–15 (2012).
Mihl, C. et al. Coronary CT angiography using low concentrated contrast media injected with high flow rates: Feasible in clinical practice. European Journal Of Radiology 84, 2155–2160 (2015).
Bae, K. T. Intravenous Contrast Medium Administration and Scan Timing at CT: Considerations and Approaches. Radiology 256, 32–61 (2010).
Viteri-Ramirez, G. et al. Low radiation and low-contrast dose pulmonary CT angiography: Comparison of 80 kVp/60 ml and 100 kVp/80 ml protocols. Clin Radiol 67, 833–9 (2012).
Szucs-Farkas, Z. et al. Detection of pulmonary emboli with CT angiography at reduced radiation exposure and contrast material volume: comparison of 80 kVp and 120 kVp protocols in a matched cohort. Invest Radiol 44, 793–9 (2009).
Zamboni, G. A. et al. Low voltage CTPA for patients with suspected pulmonary embolism. Eur J Radiol 81, e580–4 (2012).
Lu, G. M. et al. High-pitch computed tomography pulmonary angiography with iterative reconstruction at 80 kVp and 20 mL contrast agent volume. European Radiology 24, 3260–3268 (2014).
Szucs-Farkas, Z. et al. Patient exposure and image quality of low-dose pulmonary computed tomography angiography: comparison of 100- and 80-kVp protocols. Invest Radiol 43, 871–6 (2008).
Martillotti, J. et al. Dose of reduced z-axis length of computed tomography angiography (CTA) of the chest for pulmonary embolism using 64-detector rows and adaptive iterative reconstruction techniques. Emerg Radiol 20, 39–44 (2013).
Patino, M. et al. A quantitative comparison of noise reduction across five commercial (hybrid and model-based) iterative reconstruction techniques: an anthropomorphic phantom study. AJR Am J Roentgenol 204, W176–83 (2015).
Qi, L. P. et al. Evaluation of dose reduction and image quality in chest CT using adaptive statistical iterative reconstruction with the same group of patients. Br J Radiol 85, e906–11 (2012).
Singh, S. et al. Adaptive statistical iterative reconstruction technique for radiation dose reduction in chest CT: a pilot study. Radiology 259, 565–73 (2011).
Leipsic, J., Nguyen, G., Brown, J., Sin, D. & Mayo, J. R. A prospective evaluation of dose reduction and image quality in chest CT using adaptive statistical iterative reconstruction. AJR Am J Roentgenol 195, 1095–9 (2010).
Leipsic, J. et al. Adaptive statistical iterative reconstruction: assessment of image noise and image quality in coronary CT angiography. AJR Am J Roentgenol 195, 649–54 (2010).
Bedayat, A. et al. Association Between Confidence Level of Acute Pulmonary Embolism Diagnosis on CTPA images and Clinical Outcomes. Acad Radiol 22, 1555–61 (2015).
Wu, C. C., Lee, E. W., Suh, R. D., Levine, B. S. & Barack, B. M. Pulmonary 64-MDCT Angiography With 30 mL of IV Contrast Material: Vascular Enhancement and Image Quality. American Journal Of Roentgenology 199, 1247–1251 (2012).
McCollough, C. H., Leng, S. A., Yu, L. F. & Fletcher, J. G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 276, 637–653 (2015).
Cheng, J. J. et al. Optimal Monochromatic Energy Levels in Spectral CT Pulmonary Angiography for the Evaluation of Pulmonary Embolism. Plos One 8 (2013).
Hansmann, J. et al. Correlation analysis of dual-energy CT iodine maps with quantitative pulmonary perfusion MRI. World J Radiol 5, 202–7 (2013).
Thieme, S. F. et al. Dual Energy CT lung perfusion imaging–correlation with SPECT/CT. Eur J Radiol 81, 360–5 (2012).
Acknowledgements
This study was supported by GE health care (VIS-12-19).
Author information
Authors and Affiliations
Contributions
Z.L. and D.H. contributed to the study design. J.Z. provided technical guidance for CT. X.H. wrote the main manuscript and prepared all the references. Y.S. and L.M. contributed to data analysis and revision of the manuscript. All authors reviewed the manuscript and approved the final vision to be submitted.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A correction to this article is available online at https://doi.org/10.1038/s41598-018-22948-9.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hu, X., Ma, L., Zhang, J. et al. Use of pulmonary CT angiography with low tube voltage and low-iodine-concentration contrast agent to diagnose pulmonary embolism. Sci Rep 7, 12741 (2017). https://doi.org/10.1038/s41598-017-13077-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-13077-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.