Abstract
The formate pathway and NADH pathway as two common hydrogen-producing metabolic pathways have been well characterized to understand and improve biohydrogen production. These two pathways have been thought to be separate and have been independently investigated. However, in this study, perturbation of genes (hycA, fdhF, fhlA, ldhA, nuoB, hybO, fdh1, narP, and ppk) in Enterobacter aerogenes related to the formate pathway or NADH pathway revealed that these two pathways affected each other. Further metabolic analysis suggested that a linear relationship existed between the relative change of hydrogen yield in the formate pathway or NADH pathway and the relative change of NADH yield or ATP yield. Thus, this finding provides new insight into the role of cellular reducing power and energy level in the hydrogen metabolism. It also establishes a rationale for improving hydrogen production from a global perspective.
Similar content being viewed by others
Introduction
Fossil fuels have been the conventional and principal sources to satisfy the world’s demand of energy. However, their intensive use has caused environmental problems1. In such a context, hydrogen can be a promising future energy carrier, which is pollution free for its combustion only generating water without any byproduct2. At present, 40% of hydrogen is produced from natural gases, 30% from heavy oil and naphtha, 18% from coal, and 4% from electrolysis3. However, these production methods require highly intensive energy sources. It is worthless to produce clean energy by consuming much energy and still cause environmental problems. In the present scenario, biohydrogen production is of great importance to be an alternative2, 4.
Compared to conventional production methods, biological hydrogen production can use renewable resources as the reactant and be operated at ambient temperature and pressure, which means much less energy input and lower cost4. There are three basic production methods: direct biophotolysis, photofermentation, and dark fermentation. Compared to the first two methods, dark fermentation seems to be the most practical and promising method on account of having no need for direct solar energy input, while having a wide substrate range from energy crops to waste streams, and requiring only simple reactor technology. However, the dark fermentation method is currently limited by low hydrogen yield5, 6. Therefore, many studies focus on how to improve the hydrogen yield of dark fermentation by metabolic engineering.
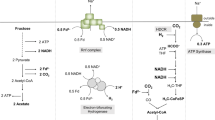
To improve the hydrogen yields, many attempts have been made to understand the hydrogen producing mechanism of dark fermentation in depth. Various microorganisms, typically the anaerobes, have been used for dark fermentation, such as Escherichia coli, Clostridium sp.7, Enterobacter sp.8, and Rhodobacter sp.9. Although each strain has its different metabolic features, there are two main hydrogen-producing pathways in common: the formate pathway and NADH pathway (Fig. 1). To enhance hydrogen yields, many attempts focusing on the formate pathway or the NADH pathway have been made to redistribute the metabolic fluxes by genetic engineering.
The key element of the formate pathway is the formate hydrogen lyase (FHL) complex10. The FHL catalyzes the formate oxidation and the proton reduction, and ultimately produces molecular hydrogen and carbon dioxide at a 1:1 molar ratio. The core FHL complex comprises of formate dehydrogenase (FDH) and hydrogenase (H2ase) (Fig. 1). Many genetic engineering studies about the FHL-related genes have been done to regulate the metabolism of the formate pathway to enhance the hydrogen production10,11,12,13. The NADH pathway was first reported in the 1980s7, with NADH as the precursor involved in the hydrogen production through ferredoxin(Fd)-NAD+ reductase and ferredoxin hydrogenase. Many steps have been made to regulate the metabolism of NADH to enhance the H2 production7, 14,15,16.
The formate pathway and NADH pathway have been thought to be independent, but recent studies demonstrated that there might be a link between them (Fig. 1). In Niu’s research on Klebsiella pneumoniae, a higher temperature can enhance the hydrogen-producing NADH pathway, while the flux in the formate pathway reach their maximum at pH 7.0–7.517. Ma et al. found that the hydrogen production fluxes in the formate pathway and the NADH pathway both increased when NADH dehydrogenase was impaired in Enterobacter aerogenes 18. It seems that regulation of the NADH pathway is accompanied by the perturbation of the formate pathway.
To figure out the puzzle of the interaction between the formate pathway and the NADH pathway, further research on metabolic regulation of fermentative hydrogen production is needed. In this study, a representative hydrogen-producing bacterium, facultatively anaerobic E. aerogenes, was used. Two hydrogen-producing pathways, the formate pathway and the NADH pathway, coexist in E. aerogenes. Genes related to the formate pathway or NADH pathway were perturbed, and then the hydrogen production was investigated. Cellular energy metabolism was further explored by metabolic analysis. Findings obtained in this study could help to thoroughly understand how perturbation of formate pathway and NADH pathway act on the hydrogen metabolism.
Results
Perturbation of the formate pathway and NADH pathway in wild-type E. aerogenes
To explore the perturbation of the formate pathway and NADH pathway in wild-type E. aerogenes, a fourfold strategy was adopted (Table 1). First, mutations directly related to the formate pathway were evaluated. In the wild-type strain, knockout of hycA (FHL repressive regulon gene), overexpression of fdhF (formate dehydrogenase H gene), or overexpression of fhlA (FHL activator protein FHLA gene) made the hydrogen yields increase from the formate pathway but decrease from the NADH pathway. Second, mutations directly related to the NADH pathway were then evaluated. The most NADH-consuming pathway is lactate production pathway. The knockout of ldhA (lactate dehydrogenase gene) greatly improved the hydrogen yield from the NADH pathway, but the hydrogen yield from the formate pathway had no change. The nuoB gene encoding the NADH dehydrogenase/NADH-quinone oxidoreductase was impaired, which made the hydrogen yields from the formate pathway and NADH pathway both increase. The third task was directly altering both the formate pathway and NADH pathway at the same time. Knockout of hybO (uptake hydrogenase gene) improved the hydrogen yields from both the formate pathway and NADH pathway. An enzyme called NAD+-dependent formate dehydrogenase can work on both formate and NADH, which uses formate to regenerate NADH from NAD+ 19. The gene fdh1 encoding NAD+-dependent formate dehydrogenase from Candida boidinii was expressed into wild-type E. aerogenes. It resulted in the increase of hydrogen yields from the formate pathway but the decrease of hydrogen yields from the NADH pathway. The fourth strategy was alternating the whole cellular metabolism that might indirectly affect the hydrogen production. A reported global regulator NarP encoded by narP is a transcriptional regulator of many anaerobic electron transport and fermentation-related genes20. The polyphosphate kinase (PPK) encoded by ppk regulates cellular energy level21, 22. Overexpression of narP or ppk improved the hydrogen yields from both formate pathway and NADH pathway. The relative change of hydrogen yield in the NADH pathway was bigger than that in the formate pathway. Further metabolite analysis (Fig. 2) showed that cellular metabolism was greatly regulated. The knockout of ldhA changed the metabolism the most.
Perturbation of the formate pathway and NADH pathway in E. aerogenes mutants
Furthermore, in different E. aerogenes mutants, fdhF, fhlA or fdh1 were overexpressed to investigate the perturbation of the formate pathway and NADH pathway (Table 2). In the hycA-deficient strain, overexpression of fdhF, fhlA or fdh1 greatly disturbed the hydrogen production in the NADH pathway. However, in ldhA-deficient strain, overexpression of fhlA or fdh1 greatly disturbed the hydrogen production in the formate pathway. In contrast,, in the nuoB-deficient or hybO-deficient strain, overexpression of fdhF, fhlA or fdh1 almost did not affect the hydrogen production in the formate pathway or the NADH pathway. Besides hydrogen, other key cellular metabolites were measured (Fig. 3). In the ldhA-deficient strain, overexpression of fdhF, fhlA or fdh1 affected cellular metabolism more obviously than other gene perturbations. Overexpression of fdhF regulated cellular levels of lactate, acetate, and 2,3-butanediol better than the overexpression of fhlA or fdh1. Overexpression of fdh1 regulated cellular ethanol level better than overexpression of fdhF or fhlA.
Quantitative relationship of hydrogen production with cellular energy metabolism
Perturbation of the formate pathway and NADH pathway inevitably led to the change of the whole anaerobic metabolism. Besides H2 and CO2, other main final metabolites of E. aerogenes are lactate, ethanol, acetate, succinate, and 2,3-butanediol (Fig. 4a). Based on metabolic analysis, the relationships between hydrogen productivity and NADH level or ATP level were quantitatively evaluated. By perturbing genes (hycA, fdhF, fhlA, ldhA, nuoB, hybO, fdh1, narP, and ppk) related to the formate pathway or NADH pathway (Tables 1 and 2), cellular metabolites (Fig. 4a) were measured and analyzed to evaluate the change of cellular NADH and ATP level. Metabolic analysis suggested that there were linear relationships between the relative change of hydrogen yields (total yields, yields in the formate pathway, or yields in the NADH pathway) and the relative change of NADH yields or ATP yields (Fig. 4b–d).
In cellular anaerobic metabolism (a), quantitative relationships between NADH yield or ATP yield and the total hydrogen yield (b), the hydrogen yield in the formate pathway (c), or the hydrogen yield in the NADH pathway (d). Hydrogen yield values were in moles of hydrogen produced per mol of consumed glucose. The NADH yield values were in moles of consumed NADH (2 × ethanol + 2 × succinate + lactate + butanediol + NADH for hydrogen production) per mol of consumed glucose. ATP yield values were in moles of ATP produced (lactate + acetate + formate + CO2 + CO2 consumed by succinate production) per mol of consumed glucose. Relative change (RC) of yield = (Yield after the overexpression of genes - Yield of the blank control)/Yield of the blank control.
Discussion
To study the perturbation of the formate pathway and NADH pathway acting on the hydrogen metabolism, choosing a proper model strain is particularly important. Among the fermentative hydrogen producers, the facultative anaerobe E. aerogenes with excellent hydrogen-producing ability can be applicable for this work. Two hydrogen-producing pathways, the formate pathway and the NADH pathway, coexist in E. aerogenes, which provides a stable and balanced cellular environment for these two pathways. Therefore, investigating the interaction of both the formate pathway and NADH pathway can be quite convenient and easy in E. aerogenes. Furthermore, E. aerogenes shows a high hydrogen-producing rate and a high growth rate with no inhibited growth in 100% H2 atmosphere. A wide range of carbon sources can be used by E. aerogenes, which implies the great potential for industrial applications23, 24.
In the formate pathway, the structure and the mechanism of the FHL at the gene level is already clear, such as the sequence of its operons and regulons. Therefore, many genetic engineering studies about the FHL-related genes have been done to regulate the metabolism of the formate pathway to enhance the hydrogen production (Supplementary Table S2). These operations include inactivation of hycA (a negative regulator)25 and focA (formate transmembrane protein)26, as well as overexpression of fhlA (a transcriptional activator)27, 28, fdhF (formate dehydrogenase H)29, modE (a transcriptional activator)26 and selC (tRNA related to formate dehydrogenase)26.
The regulation of the NADH pathway is different from the formate pathway. Since the metabolic mechanism and genetic information of the NADH pathway are unclear, it is difficult to regulate the expression of enzymes on the NADH pathway. Fortunately, NADH participates in a wide range of metabolic reactions, in which the NAD(H) pool keeps a dynamic balance. Therefore, many steps have been made to regulate the metabolism of NADH to enhance the H2 production (Supplementary Table S2), for example, usually reducing the consumption of NADH of other metabolic pathways. In anaerobic metabolism, NADH generated from glycolysis is mainly consumed by the lactate production pathway and the alcohol production pathway, so the disruption of the lactate dehydrogenase gene ldhA and the alcohol dehydrogenase gene adh to increase H2 production is feasible30. Some other genes or gene clusters related to the NADH metabolism such as frdBC 16, nuoB 29, nadE 15 and hoxEFUYH 31 have also been regulated to improve the hydrogen yields.
In this work, genes (hycA, fdhF, fhlA, ldhA, nuoB, hybO, fdh1, narP, and ppk) directly or indirectly related to the formate pathway or NADH pathway were perturbed. The genes hycA, fdhF and fhlA are directly related to the formate pathway, and the genes ldhA and nuoB are directly related to the NADH pathway. Furthermore, the genes hybO and fdh1 are directly related to both the formate pathway and NADH pathway. The genes narP and ppk alternate cellular global metabolism, which are indirectly related to hydrogen-producing pathways. The exploration of the perturbation of those genes further confirmed that these two pathways mutually impacted each other or were regulated together. The increased ratios of total hydrogen yield by the knockout of the nuoB gene or the hybO gene were much higher than that by the knockout of other genes, as shown in Table 1. In most case, as shown in Table 1, the NADH pathway for the hydrogen production was more easily perturbed than the formate pathway. Additionally, it is proposed that the increase of hydrogen yields might have a limit in E. aerogenes, which may be the reason why overexpression of fdhF, fhlA or fdh1 in nuoB-deficient or hybO-deficient strains almost did not affect the hydrogen production, as shown in Table 2. Besides the hydrogen, analysis of other metabolites further demonstrated that cellular metabolism has been greatly regulated by perturbations of those genes (Figs 2 and 3). Main final organic metabolites of E. aerogenes are lactate, ethanol, acetate, succinate, and 2,3-butanediol.Unfortunately, because of the complexity of cellular metabolism, it was hard to describe the interaction between the formate pathway and the NADH pathway for the hydrogen production in a quantitative way. The two substrates, formate and NADH, are both important metabolites in the complicated cellular metabolic network. It might be more feasible to understand the hydrogen metabolism not only from the local point of view but also from the global point of view.
In essence, biohydrogen production is a routine of releasing excess electrons or energies in anaerobic metabolism. It is proposed that the global regulation of anaerobic metabolism might alter the electron or energy flow to affect the hydrogen yield. Our findings demonstrated that the formate pathway and the NADH pathway might interact with each other indirectly through the global regulation. The global regulation of anaerobic metabolism redistributed the hydrogen metabolism into the formate pathway and the NADH pathway. However, how to redistribute is still unclear and will need further investigation.
Since global regulation of anaerobic metabolism on the reducing power or energy level can alter the hydrogen production, the hydrogen yield may have tight interactions with cellular reducing power or energy level. Intracellular reducing power is typically stored in the form of NADH. The major cellular energy currency molecule is ATP. This study quantitatively showed the relationships of hydrogen production with cellular reducing power and energy level. The important roles of cellular NADH and ATP in hydrogen metabolism have been demonstrated, thus providing a basis for deciphering the essence of the hydrogen production. Based on this finding, metabolic regulations could be better rationalized to allow for improving biohydrogen production.
In conclusion, this work has presented how the perturbation of the formate pathway and NADH pathway affected the biohydrogen production. To enhance the hydrogen yields, many genetic engineering attempts have been focusing on two dominant metabolic pathways: formate pathway and NADH pathway. These two pathways have been studied independently, but this perturbation study exhibited that these two pathways interact with each other indirectly through the global regulation of reducing power and energy level. There are linear relationships between the relative change of hydrogen yield and the relative change of NADH yield or ATP yield. To further improve the hydrogen yield, an effective strategy would be to introduce intracellular NADH or ATP regeneration pathways to improve the NADH or ATP yields. This finding delineates a rationale for improving the hydrogen productivity from a global point of view that may contribute to our understanding of hydrogen metabolism.
Methods
Strains and plasmids
E. aerogenes IAM1183 was purchased from the Institute of Applied Microorganisms of the University of Tokyo, Japan. A list of bacterial strains, mutants, plasmids and recombinant plasmids used in the study are shown in Supplementary Table S1. The genes hycA, ldhA, nuoB, and hybO in E. aerogenes IAM1183 were knocked out, forming the mutant E. aerogenes-ΔhycA, E. aerogenes-ΔldhA, E. aerogenes-ΔnuoB, and E. aerogenes-ΔhybO 30. The genes fdhF, fhlA, fdh1, narP, and ppk were inserted into the multiple cloning sites of the plasmid pMCL to form recombinant plasmids, including pMCL-fdhF, pMCL-fhlA, pMCL-narP, pMCL-fdh1, and pMCL-ppk 6. The genetic analysis has been performed in previous studies (Supplementary Table S1). The recombinant plasmids were chemically transformed into E. aerogenes strains, and the empty plasmid pMCL was used as the control.
Medium preparation
The culture medium (1 liter) consisted of glucose 15.0 g, tryptone 5.0 g, (NH4)2SO4 2.0 g, KH2PO4 14.0 g, K2HPO4.3H2O 6.0 g, and MgSO4 0.2 g6. All chemicals were of analytical grade. The glucose was sterilized individually by autoclave. The initial pH value of the medium was controlled at 6.0 by the addition of NaOH or HCl.
Cell cultivation
Twenty mL of medium was put into the serum bottle with a total volume of 70 mL. The serum bottle was air-sealed with a butyl rubber stopper and degassed with 100% of aseptic nitrogen for 5 minutes. The cultivation was performed at 37 °C on a 170 rpm shaker for 15 h. When the bacterial cells cultivated in a 100 mL Erlenmeyer flask containing 20 mL medium reached 1.0 in terms of optical density at 660 nm (OD660), 1 mL of the culture as the inoculum was transferred into the serum bottle. The whole operation was done under anaerobic conditions. The plasmids were induced by 0.5 mM IPTG. The inducers were added into the culture at the beginning.
Analysis of cell density and glucose concentration
Cell densities at 600 nm (OD600) were measured with an ultraviolet-visible spectrophotometer (UV757CRT, Shanghai Precision & Scientific Instrument CO., Ltd., China). The dry cell weight of E. aerogenes was then calculated by the equation of dry cell weight (g/L) = 0.132 × OD600. The glucose concentration was measured by 3,5-dinitrosalicylic acid (DNS) colorimetry32.
Measurement of hydrogen gas
Hydrogen was analyzed by a gas chromatograph (GC112A, Shanghai Precision & Scientific Instrument Co., Ltd., China) equipped with a thermal conductivity detector (TCD) and a 2 m × 3 mm (i.d.) stainless-steel column packed with TDX-01 (80~100 mesh). The temperatures of the injector, detector, and column were kept at 120, 120, and 80 °C, respectively. Nitrogen was used as a carrier gas at a flow rate of 10 mL/min.
Measurement of organic metabolites
The concentrations of organic acids and alcohols in metabolites were analyzed by high-performance liquid chromatography (HPLC) (Shimadzu 10 A) equipped with a refractive index detector and a Shimadzu SCR-102H column after pretreatment with a 0.45 μm membrane filter. Five mM HClO4 was used as a mobile phase at a flow rate of 1 mL/min. The retention times of succinate, lactate, formate, acetate, 2,3-butanediol, and ethanol were 6.681 min, 8.666 min, 9.404 min, 10.088 min, 11.746 min, and 13.670 min, respectively.
Analysis of the hydrogen yields
The hydrogen yield produced by the formate pathway was calculated from the sum of final acetate and ethanol minus the residual formate (H2 yield produced by formate pathway = (acetate + ethanol-formate)/consumed glucose). The hydrogen yield produced by the NADH pathway was calculated from the sum of the pathways producing NADH minus those consuming NADH (H2 produced by NADH pathway = ((2 × glucose + CO2 + CO2 consumed by succinate production-2 × 2,3-butanediol)-(2 × ethanol + 2 × succinate + lactate + 2,3-butanediol + H2-CO2))/consumed glucose).
References
Sinha, P., Roy, S. & Das, D. Genomic and proteomic approaches for dark fermentative biohydrogen production. Renew. Sust. Energ. Rev. 56, 1308–1321, doi:10.1016/j.rser.2015.12.035 (2016).
Rosales-Colunga, L. M. & Rodriguez, A. D. Escherichia coli and its application to biohydrogen production. Rev. Environ. Sci. Bio-Technol. 14, 123–135, doi:10.1007/s11157-014-9354-2 (2015).
Balat, M. Hydrogen-Rich Gas Production from Biomass via Pyrolysis and Gasification Processes and Effects of Catalyst on Hydrogen Yield. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 30, 552–564, doi:10.1080/15567030600817191 (2008).
Kotay, S. M. & Das, D. Biohydrogen as a renewable energy resource - Prospects and potentials. Int. J. Hydrog. Energy 33, 258–263, doi:10.1016/j.ijhydene.2007.07.031 (2008).
Hallenbeck, P. C. & Ghosh, D. Advances in fermentative biohydrogen production: the way forward? Trends Biotechnol. 27, 287–297, doi:10.1016/j.tibtech.2009.02.004 (2009).
Lu, Y., Zhao, H. X., Zhang, C. & Xing, X. H. Insights into the global regulation of anaerobic metabolism for improved biohydrogen production. Bioresour. Technol. 200, 35–41, doi:10.1016/j.biortech.2015.10.007 (2016).
Tanisho, S., Kamiya, N. & Wakao, N. Hydrogen evolution of Enterobacter aerogenes depending on culture pH: mechanism of hydrogen evolution from NADH by means of membrane-bound hydrogenase. Biochimica Et Biophysica Acta 973, 1–6, doi:10.1016/s0005-2728(89)80393-7 (1989).
Zhang, C., Lv, F.-X. & Xing, X.-H. Bioengineering of the Enterobacter aerogenes strain for biohydrogen production. Bioresour. Technol. 102, 8344–8349, doi:10.1016/j.biortech.2011.06.018 (2011).
Rachman, M. A., Furutani, Y., Nakashimada, Y., Kakizono, T. & Nishio, N. Enhanced hydrogen production in altered mixed acid fermentation of glucose by Enterobacter aerogenes. Journal of Fermentation and Bioengineering 83, 358–363, doi:10.1016/s0922-338x(97)80142-0 (1997).
McDowall, J. S. et al. Bacterial formate hydrogenlyase complex. Proceedings of the National Academy of Sciences of the United States of America 111, E3948–E3956, doi:10.1073/pnas.1407927111 (2014).
Leonhartsberger, S., Korsa, I. & Bock, A. The molecular biology of formate metabolism in enterobacteria. Journal of Molecular Microbiology and Biotechnology 4, 269–276 (2002).
Maeda, T., Sanchez-Torres, V. & Wood, T. K. Hydrogen production by recombinant Escherichia coli strains. Microb. Biotechnol. 5, 214–225, doi:10.1111/j.1751-7915.2011.00282.x (2012).
Yoshida, A., Nishimura, T., Kawaguchi, H., Inui, M. & Yukawa, H. Enhanced hydrogen production from formic acid by formate hydrogen lyase-overexpressing Escherichia coli strains. Applied and Environmental Microbiology 71, 6762–6768, doi:10.1128/aem.71.11.6762-6768.2005 (2005).
Maeda, T., Sanchez-Torres, V. & Wood, T. K. Enhanced hydrogen production from glucose by metabolically engineered Escherichia coli. Appl. Microbiol. Biotechnol. 77, 879–890, doi:10.1007/s00253-007-1217-0 (2007).
Wang, J., Yu, W. Y., Xu, L., Wang, S. Y. & Yan, Y. J. Effects of increasing the NAD(H) pool on hydrogen production and metabolic flux distribution in Enterobacter aerogenes mutants. Int. J. Hydrog. Energy 38, 13204–13215, doi:10.1016/j.ijhydene.2013.07.121 (2013).
Yoshida, A., Nishimura, T., Kawaguchi, H., Inui, M. & Yukawa, H. Enhanced hydrogen production from glucose using ldh- and frd-inactivated Escherichia coli strains. Appl. Microbiol. Biotechnol. 73, 67–72, doi:10.1007/s00253-006-0456-9 (2006).
Niu, K., Zhang, X., Tan, W.-S. & Zhu, M.-L. Effect of culture conditions on producing and uptake hydrogen flux of biohydrogen fermentation by metabolic flux analysis method. Bioresour. Technol. 102, 7294–7300, doi:10.1016/j.biortech.2011.05.001 (2011).
Ma, K., Zhao, H. X., Zhang, C., Lu, Y. & Xing, X. H. Impairment of NADH dehydrogenase for increased hydrogen production and its effect on metabolic flux redistribution in wild strain and mutants of Enterobacter aerogenes. Int. J. Hydrog. Energy 37, 15875–15885, doi:10.1016/j.ijhydene.2012.08.017 (2012).
Lu, Y. et al. Expression of NAD(+)-dependent formate dehydrogenase in Enterobacter aerogenes and its involvement in anaerobic metabolism and H-2 production. Biotechnology Letters 31, 1525–1530, doi:10.1007/s10529-009-0036-z (2009).
Kang, Y. S., Weber, K. D., Yu, Q., Kiley, P. J. & Blattner, F. R. Genome-wide expression analysis indicates that FNR of Escherichia coli K-12 regulates a large number of genes of unknown function. J Bacteriol 187, 1135–1160, doi:10.1128/Jb.187.3.1135-1160.2005 (2005).
Haeusler, P. A. et al. Catalytic properties of Escherichia coli polyphosphate kinase: an enzyme for ATP regeneration. Biotechnol Appl Biochem 15, 125–133 (1992).
Li, H. C. & Brown, G. G. Orthophosphate and histone dependent polyphosphate kinase from E. coli. Biochem Biophys Res Commun 53, 875–881 (1973).
Ito, T., Nakashimada, Y., Kakizono, T. & Nishio, N. High-yield production of hydrogen by Enterobacter aerogenes mutants with decreased alpha-acetolactate synthase activity. Journal of Bioscience and Bioengineering 97, 227–232, doi:10.1016/s1389-1723(04)70196-6 (2004).
Nakashimada, Y., Rachman, M. A., Kakizono, T. & Nishio, N. Hydrogen production of Enterobacter aerogenes altered by extracellular and intracellular redox states. Int. J. Hydrog. Energy 27, 1399–1405, doi:10.1016/s0360-3199(02)00128-3 (2002).
Zhao, H. X. et al. Cloning and knockout of formate hydrogen lyase and H-2-uptake hydrogenase genes in Enterobacter aerogenes for enhanced hydrogen production. Int. J. Hydrog. Energy 34, 186–194, doi:10.1016/j.ijhydene.2008.10.025 (2009).
Fan, Z. M., Yuan, L. & Chatterjee, R. Increased Hydrogen Production by Genetic Engineering of Escherichia coli. PLoS One 4, 8, doi:10.1371/journal.pone.0004432 (2009).
Maeda, T., Sanchez-Torres, V. & Wood, T. K. Escherichia coli hydrogenase 3 is a reversible enzyme possessing hydrogen uptake and synthesis activities. Appl Microbiol Biotechnol 76, 1035–1042, doi:10.1007/s00253-007-1086-6 (2007).
Cordonnier, C. et al. Enterohemorrhagic Escherichia coli pathogenesis: role Long polar fimbriae in Peyer’s patches interactions. Sci Rep 7, 44655, doi:10.1038/srep44655 (2017).
Arias-Cartin, R. et al. Redox cofactors insertion in prokaryotic molybdoenzymes occurs via a conserved folding mechanism. Sci Rep 6, 37743, doi:10.1038/srep37743 (2016).
Zhao, H. X. et al. Disruption of lactate dehydrogenase and alcohol dehydrogenase for increased hydrogen production and its effect on metabolic flux in Enterobacter aerogenes. Bioresour. Technol. 194, 99–107, doi:10.1016/j.biortech.2015.06.149 (2015).
Eckert, C. et al. Genetic analysis of the Hox hydrogenase in the cyanobacterium Synechocystis sp. PCC 6803 reveals subunit roles in association, assembly, maturation, and function. J Biol Chem 287, 43502–43515, doi:10.1074/jbc.M112.392407 (2012).
Miller, G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428, doi:10.1021/ac60147a030 (1959).
Author information
Authors and Affiliations
Contributions
D.L., Y.S., and Y.L. designed and performed the research, analyzed the data, and wrote the manuscript. Y.L. supervised all of the research and edited the manuscript, which was approved by all co-authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, D., Sun, Y., Li, Y. et al. Perturbation of formate pathway and NADH pathway acting on the biohydrogen production. Sci Rep 7, 9587 (2017). https://doi.org/10.1038/s41598-017-10191-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10191-7
This article is cited by
-
Optimization of hydrogen production in Enterobacter aerogenes by Complex I peripheral fragments destruction and maeA overexpression
Microbial Cell Factories (2023)
-
Description of Sporanaerobium hydrogeniformans gen. nov., sp. nov., an obligately anaerobic, hydrogen-producing bacterium isolated from Aravali hot spring in India
Archives of Microbiology (2023)
-
A review on potential of biohydrogen generation through waste decomposition technologies
Biomass Conversion and Biorefinery (2023)
-
Novel strategies towards efficient molecular biohydrogen production by dark fermentative mechanism: present progress and future perspective
Bioprocess and Biosystems Engineering (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.