Abstract
The genetic variants near the Melanocortin-4 receptor gene (MC4R), a key protein regulating energy balance and adiposity, have been related to obesity and glucose metabolism. We aimed to assess whether the MC4R genotype affected longitudinal changes in body weight and glucose metabolism biomarkers among women with prior gestational diabetes mellitus (GDM). The MC4R genotype, postpartum weight reduction, and glycemic changes between after delivery and pregnancy were assessed in a cohort of 1208 Chinese women who had experienced GDM. The adiposity-increasing allele (C) of the MC4R variant rs6567160 was associated with greater postpartum increase of HbA1c (β = 0.08%; P = 0.03) and 2-hour OGTT glucose concentrations (β = 0.25 mmol/L; P = 0.02). In addition, we found an interaction between the MC4R genotype and postpartum weight reduction on changes in fasting plasma glucose (P-interaction = 0.03). We found that the MC4R genotype was associated with postpartum glycemic changes; and the association with fasting glucose were significantly modified by postpartum weight reduction in women who had experienced GDM.
Similar content being viewed by others
Introduction
In women, body weight changes considerably during pregnancy, usually increasing during the gestational period and decreasing at postpartum period1. In epidemiology studies, low postpartum weight reduction has been consistently related to abnormal glucose metabolism and an increased risk of type 2 diabetes in later life2. The positive relationship between postpartum weight reduction and risk of diabetes was found to be even stronger among women with a history of gestational diabetes mellitus (GDM)3,4,5,6.
The melanocortin-4 receptor (MC4R) is a G protein-coupled receptor that plays a pivotal role in regulating food intake, energy expenditure and adiposity, primarily through modulation of sympathetic outflow7,8,9,10,11,12,13. Rare mutations in the MC4R gene have been found to cause morbid obesity in humans12, and common polymorphisms were recently related to higher BMI in various populations in genome-wide association studies (GWAS)14, 15. In addition, the MC4R genotypes have been also related to increased risk of insulin resistance and type 2 diabetes7, 14,15,16,17. However, little is known whether the MC4R genotypes affect longitudinal changes in body weight and glucose metabolism during and after pregnancy among women.
In thus far one of the largest cohorts of women with a history of GDM, we examined the associations of an obesity-associated MC4R variant with postpartum changes in body weight and glucose metabolism. We particularly assessed the interaction between the MC4R genotype and postpartum weight reduction in relation to the changes of glucose metabolism.
Results
The present study included a total of 1208 Chinese women with prior GDM. The frequency of the adiposity-increasing allele of MC4R rs6567160 (C allele) was 23%, and the genotype distribution fit the Hardy-Weinberg equilibrium (P = 0.26). The characteristics of participants during the pregnancy and at the postpartum survey by the MC4R genotype are presented in Table 1. The MC4R genotype was not related to measures of glucose metabolism (fasting glucose, 2-h OGTT glucose, and HbA1c) during pregnancy, but showed significant and positive associations (P < 0.05) with 2-h OGTT glucose, HbA1c, weight and BMI measured at postpartum survey.
MC4R and glycemic changes
We further analyzed the associations of the MC4R genotype with reduction in body weight and measures of glucose metabolism from pregnancy to postpartum survey. Carriers of the adiposity-increasing allele (C) of rs6567160 showed less significant reduction of 2-h OGTT and HbA1c after adjustment for covariates including age at postpartum, pre-pregnancy BMI, follow-up years since delivery, concentrations of its respective biomarker (fasting or 2-hour plasma glucose or HbA1c) during pregnancy, number of children delivered, family history of diabetes (P = 0.03 for change of HbA1c and P = 0.02 for change of 2-h OGTT glucose) (Table 2). There was no significant association between the MC4R genotype and postpartum changes from the time point when women were diagnosed with GDM to postpartum 1–5 years in body weight and fasting glucose levels after adjustments.
Postpartum weight reduction and glycemic changes
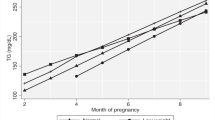
In the study population, postpartum weight reduction showed significant associations with decreased levels of fasting glucose, 2-h OGTT, and HbA1c (all P values < 0.05) from pregnancy to postpartum survey. Adjustment for age at postpartum, pre-pregnancy BMI, follow-up years since delivery, level of the corresponding biomarkers during the pregnancy, number of children delivered, and family history of diabetes did not appreciably change the results. Women who were in the highest tertile of postpartum weight reduction presented −0.13 mmol/L, −2.79 mmol/L, and −0.30% changes in fasting glucose, 2-h OGTT, and HbA1c, respectively; while the corresponding changes were 0.28 mmol/L, −1.28 mmol/L, and −0.03%, respectively among women in the lowest tertile of postpartum weight reduction. The mean changes of glucose metabolism biomarkers stratified by MC4R genotype and postpartum weight reduction were shown in Fig. 1. In sensitivity analyses, we did not observe difference in longitudinal changes of these glucose metabolism markers according to the length of follow-up.
Mean change of glucose metabolism biomarkers by MC4R and tertiles of postpartum weight reduction. Values are mean change of fasting glucose (a), change of 2-h OGTT (b), change of HbA1c (c) ± SD. From the lowest to highest weight reduction; Tertile 1 mean: −2.8 kg/y (min −4.8 kg; max: 4.8 kg); Tertile 2 mean: −6.4 kg/y (min −7.9 kg; max: −4.8 kg); Tertile 3 mean: −11.5 kg/y (min −29.6 kg; max: −7.9 kg). Associations between levels of glucose biomarkers and MC4R by tertiles of postpartum weight reduction were analyzed by general linear regression models adjusted for age at postpartum, pre-pregnancy BMI, follow-up years since delivery, level of the corresponding biomarkers during the pregnancy, number of children delivered, and family history of diabetes. HbA1c, glycated hemoglobin; OGTT, 2-h 75-g oral glucose tolerance test.
Interaction analysis
We then tested the interaction between the MC4R genotype and postpartum weight reduction in relation to changes in biomarkers of glucose metabolism. Significant interactions were observed between MC4R SNP rs6567160 and postpartum weight reduction on changes of fasting glucose levels (P-interaction = 0.03). The adiposity-increasing allele (C) was related to increased fasting glucose concentrations among women in the lowest tertile of postpartum weight reduction; whereas an opposite-directional association was observed among women in the highest tertile (Fig. 1). The MC4R genotype did not interact with postpartum weight reduction on changes of 2-h OGTT and HbA1c levels.
Discussion
In this study of a large cohort of Chinese women with a history of GDM, we found that the obesity-associated MC4R genotype was significantly related to greater reduction in HbA1c and 2-h OGTT levels during postpartum. In addition, we found that the MC4R genotype significantly interacted with postpartum weight reduction in relation to changes in fasting glucose levels.
Women usually gain around 20% body weight during pregnancy1, 2 and lose weight during postpartum period. Compelling evidence suggests that postpartum weight reduction plays a pivotal role in determining glucose metabolism after delivery and affects long-term risk of type 2 diabetes in later life, especially among those with a history of GDM2,3,4,5,6, 18. Consistent with previous studies2, in our cohort, a greater postpartum weight reduction was found to be related to significant decreases in fasting glucose, HbA1c and 2-h OGTT concentrations. Even though the MC4R genotype was significantly related to greater body weight, consistent with previous studies7, 15, it was not associated with postpartum weight reduction in our study.
The MC4R genotype was significantly related to changes in biomarkers of glucose metabolism including HbA1c and 2-h OGTT from pregnancy to postpartum survey, independent of pre-pregnancy BMI. Melanocortin-4 receptor, which is coded by the MC4R gene, is a key protein in regulation of energy balance, adiposity, and glucose metabolism10, 16, 17. The common variants in the MC4R gene have been previously related to measures of glucose metabolism such as insulin resistance16, insulin sensitivity11, and HbA1c19. Our data suggest that the MC4R genotype may also play a role in regulating postpartum change patterns of glycemic profiles caused by pregnancy.
Intriguingly, we found that the MC4R genotype was differently associated with changes in fasting glucose according to levels of postpartum weight reduction. The adiposity-predisposing allele (C) was associated with increased fasting glucose concentrations among women in the lowest tertile of postpartum weight reduction, but was related to decreased fasting glucose concentrations among women in the highest tertile. Such opposite genetic effects could be partly explained by the “differential susceptibility hypothesis”20, 21, which suggests that genes may be conceptualized as “plastic”, because genetic risk can be modified by environmental factors20, 21, such as change in body weight. We assume that the magnitude of postpartum weight reduction may differently affect expression or activity of the MC4R gene associated with the variant, and subsequently affect glucose metabolism during postpartum period.
To the best of our knowledge, the present study is among the first to show the effects of the MC4R genotype on postpartum changes in glucose metabolism among women with a history of GDM. The major strengths of our study include a large sample size, and longitudinally measured markers of glucose metabolism including fasting glucose, 2-h OGTT, and HbA1c at two time points (during and after pregnancy). We acknowledged some limitations of this study. For example, few lifestyle covariates were collected during the pregnancy and at the postpartum period; therefore, these potential confounders such as breast-feeding status and the duration of breast-feeding status could not be adjusted for in our analyses. In addition, we acknowledged that the participation rate was relatively low, however there were no differences between the women with GDM at 26–30 gestational weeks who returned and those who did not return, with regard to age, fasting glucose, 2-h glucose concentrations, and the prevalence of impaired glucose tolerance and diabetes. We excluded women with diagnosed diabetes, and such exclusion might affect the effect size of the associations. However, the number of women with diabetes was small (n = 15); therefore the influence would be moderate. We acknowledged that multiple outcomes were analyzed; however, these measures were correlated, and correction for multiple testing might increase type 2 error. We recognized that measurement errors might bias the associations, especially when the sample size is relatively small22, however in our study, we have a good sample size and the clinical phenotypes were objectively measured. Even though, scientific replications of our findings are essentially important. Our study was focused on a GWAS-identified gene, even though, we acknowledged that the ratio of false-positive to false-negative findings might be higher as compared with genome-wide analysis23. Therefore, the results from our study would be interpreted with caution and may not be generalizable to other populations and women without GDM.
In conclusion, our results for the first time indicate that the MC4R genotype is associated with postpartum changes in glucose metabolism among women with a history of GDM; and the genetic effects on glycemic changes might be modified by postpartum weight reduction.
Methods
Study population
The Tianjin Gestational Diabetes Mellitus Prevention Program is a retrospective cohort study in women with a history of GDM at 1–5 y after delivery. Detailed information of the study has been described elsewhere24,25,26,27. Briefly, all pregnant women in the urban areas of Tianjin, China, who had diagnosed with GDM at 26–30 gestational weeks (according to World Health Organization criteria)28 between 2005 and 2009 (n = 4,644) were invited to participate in a postpartum survey from August 2009 to July 201125. A total of 3381 women were excluded due particularly to the impossibility of being contacted, to have refused, and to not meet study criteria24. The exclusion criteria were: age <20 or ≥50 y; had a diagnosis of diabetes (2-h 75-g oral glucose tolerance test (OGTT) glucose concentration ≥11.1 mmol/L or fasting glucose ≥7.0 mmol/L); presence of any chronic diseases that could reduce the life expectancy or the ability to participate in the study, such as cancer and cardiovascular diseases; taking medicines that alter 2-h OGTT glucose; pregnant during the follow-up period; and unable to give informed consent. Therefore 1,263 GDM women completed the postpartum survey (participation rate 27%). We excluded 55 women without genotype information due to lack of DNA samples and these women were not different in clinical characteristics and biochemical markers from those included in the final analysis. Thus, a total of 1,208 participants were analyzed in the present study. A previous study showed there were no differences in 2-h OGTT glucose concentration, fasting glucose concentration, and the prevalence of impaired glucose tolerance and diabetes at 26–30 gestational weeks between women who returned postpartum survey and those who did not27. In the postpartum survey, each eligible participant had a physical examination and answered a self-administered questionnaire24. The questionnaire contained information about participants’ pregnancy outcomes (pre-pregnancy weight, gestational weight gain, and number of children delivered), medical records of GDM history, family history of diseases (diabetes, hypertension, stroke, and cancer), medical history (pregnancy hypertension, diabetes, hypertension and hypercholesterolemia), and socio-demographic information25. The study protocols were guided by the Ethical Principles and Guidelines for the Protection of Human Subjects of Research and the study was approved by the Human Subjects Committee of the Tianjin Women’s and Children’s Health Center. A informed consent was obtained from all participants24.
Assessment of anthropometrics
In the postpartum survey, body weight and height were measured using the standardized protocol. Pre-pregnancy and current BMI were calculated by dividing pre-pregnancy or current weight (in kilograms) by the square of height (in meters).
The postpartum weight retention (weight change between pre-pregnancy and 1–5 y postpartum) was divided in two parts: the gestational weight gain and postpartum weight reduction. For the present study, we used only the postpartum weight reduction divided by the number of follow-up years since delivery.
Assessment of biomarkers of glucose metabolism and covariates
The biomarker concentrations were measured in plasma both during the pregnancy (26–30 gestational weeks, the time point when the women were diagnosed with GDM) and at the postpartum survey. Blood samples were collected from all participants after an overnight fast of at least 12 hours. Fasting glucose and 2-h 75 g OGTT glucose were measured using an automatic analyzer (TBA-120FR; Toshiba, Japan). Glycated hemoglobin (HbA1c) was measured using Automatic Glycohaemoglobin Analyzer (ADAMS A1c HA-8160; Arkray, Japan). Changes in biomarkers were calculated as the difference in biomarker concentrations between postpartum 1–5 y (at postpartum survey) and pregnancy.
DNA extraction, SNP selection, and genotyping
Genomic DNA was extracted from the buffy coat fraction of centrifuged blood using a QIAamp Blood Maxi Kit (Qiagen, Chatsworth, CA). MC4R single nucleotide polymorphism (SNP) rs6567160 was determined by quantitative real-time TaqMan polymerase chain reaction (Applied Biosystems, Foster City, CA). The success rate of genotyping was over 98%. For quality control, 10% of the samples were re-genotyped with more than 99% concordance.
Statistical analysis
The Hardy-Weinberg equilibrium of the genotype was examined by a χ2 test (P > 0.05). The normal distribution of the variables was evaluated using the Kolmogorov-Smirnov test. Data were expressed as the mean and standard deviation (SD). The associations of the MC4R genotype and postpartum weight reduction with changes in fasting glucose, 2-h OGTT glucose, and HbA1c were analyzed using general linear regression models adjusted for age at postpartum, pre-pregnancy BMI, follow-up years since delivery, number of children delivered, respective biomarker concentrations measured when women were diagnosed with GDM at 26–30 gestational weeks, and family history of diabetes. The MC4R genotype was analyzed as a continuous variable (additive model), which indicates that the associations between MC4R genotype and glucose metabolism biomarkers are increased γ-fold for genotype T/C and by 2γ-fold for genotype C/C. The interaction between the MC4R genotype and postpartum weight reduction was tested by introducing a product term for these variables in the models. We also analyzed the associations between the MC4R genotypes and glucose metabolism biomarkers by the tertiles of postpartum weight reduction (from the lowest to the highest: Tertile 1, −4.8 to 4.8; Tertile 2, −7.9 to −4.8; and Tertile 3, −29.6 to −7.9 kg/y), using linear regression model, adjusted for age at postpartum, pre-pregnancy BMI, follow-up years, level of the corresponding biomarkers during the pregnancy, number of children delivered, and family history of diabetes. In addition, sensitivity analyses were performed to check whether the number of follow-up years could influence the relationship between changes in biomarker concentrations and postpartum weight reduction. All statistical analyses were performed using SAS statistical software (version 9.4; SAS Institute, Inc., Cary, NC, USA). P < 0.05 was considered statistically significant.
References
Rasmussen, K. M. & Yaktine, A. L. Weight Gain During Pregnancy: Reexamining the Guidelines. Nutrition 1, (National Academies Press, 2009).
Kew, S. et al. Cardiometabolic implications of postpartum weight changes in the first year after delivery. Diabetes Care 37, 1998–2006 (2014).
Huopio, H. et al. Long-term changes in glucose metabolism after gestational diabetes: a double cohort study. BMC Pregnancy Childbirth 14, 2393 (2014).
Ehrlich, S. F. et al. Post-partum weight loss and glucose metabolism in women with gestational diabetes: The DEBI Study. Diabet. Med. 31, 862–867 (2014).
Baptiste-Roberts, K. et al. Risk factors for type 2 diabetes among women with gestational diabetes: a systematic review. Am. J. Med. 122, 207–214.e4 (2009).
Kim, C. Gestational Diabetes Mellitus in Korean Women: Similarities and Differences from Other Racial/Ethnic Groups. Diabetes Metab J. 38, 1–12 (2014).
Loos, R. J. et al. Common variants near MC4R are associated with fat mass, weight and risk of obesity. Nat Genet 40, 768–775 (2008).
Loos, R. J. The genetic epidemiology of melanocortin 4 receptor variants. Eur J Pharmacol 660, 156–164 (2011).
Berglund, E. D. et al. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nat Neurosci 17, 911–913 (2014).
Rossi, J. et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab 13, 195–204 (2011).
Kring, S. I. I. et al. Common variants near MC4R in relation to body fat, body fat distribution, metabolic traits and energy expenditure. Int. J. Obes. (Lond). 34, 182–189 (2010).
Kievit, P. et al. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes 62, 490–497 (2013).
Koegler, F. H., Grove, K. L., Schiffmacher, A., Smith, M. S. & Cameron, J. L. Central melanocortin receptors mediate changes in food intake in the rhesus macaque. Endocrinology 142, 2586–2592 (2001).
Pei, Y. F. et al. Meta-analysis of genome-wide association data identifies novel susceptibility loci for obesity. Hum Mol Genet 23, 820–830 (2014).
Wen, W. et al. Meta-analysis identifies common variants associated with body mass index in east Asians. Nat Genet 44, 307–311 (2012).
Chambers, J. C. et al. Common genetic variation near MC4R is associated with waist circumference and insulin resistance. Nat Genet 40, 716–718 (2008).
Kong, X. et al. Obesity-related genomic Loci are associated with type 2 diabetes in a han chinese population. PLoS One 9, e104486 (2014).
Peters, R. K., Kjos, S. L., Xiang, A. & Buchanan, T. A. Long-term diabetogenic effect of single pregnancy in women with previous gestational diabetes mellitus. Lancet 347, 227–230 (1996).
Mutombo, P. et al. MC4R rs17782313 gene polymorphism was associated with glycated hemoglobin independently of its effect on BMI in Japanese: the Shimane COHRE study. Endocr Res 39, 115–119 (2014).
Belsky, J. et al. Vulnerability genes or plasticity genes? Mol. Psychiatry 14, 746–754 (2009).
Belsky, J. & Pluess, M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol. Bull. 135, 885–908 (2009).
Loken, E. & Gelman, A. Measurement error and the replication crisis: The assumption that measurement error always reduces effect sizes is false. Science 355, 584–585 (2017).
Ioannidis, J. P. A., Tarone, R. & McLaughlin, J. K. The False-positive to False-negative Ratio in Epidemiologic Studies. Epidemiology 22, 450–456 (2011).
Hu, G. et al. Tianjin Gestational Diabetes Mellitus Prevention Program: study design, methods, and 1-year interim report on the feasibility of lifestyle intervention program. Diabetes Res Clin Pr. 98, 508–517 (2012).
Liu, H. et al. Prepregnancy body mass index and weight change on postpartum diabetes risk among gestational diabetes women. Obes. (Silver Spring) 22, 1560–1567 (2014).
Wang, L. et al. Obesity index and the risk of diabetes among Chinese women with prior gestational diabetes. Diabet. Med. 31, 1368–1377 (2014).
Li, W. et al. Different associations of diabetes with β-cell dysfunction and insulin resistance among obese and non-obese Chinese women with prior gestational diabetes mellitus. Diabetes Care 37, 2533–2539 (2014).
Consultation WHO. Definition, diagnosis and classificationof diabetes mellitus and its complications. Part 1: diagnosisand classification of diabetes mellitus (Geneva World Heal. Organ, 1999).
Acknowledgements
The study was supported by grants from the European Foundation for the Study of Diabetes (EFSD)/Chinese Diabetes Society (CDS)/Lilly Program for Collaborative Research between China and Europe, and Tianjin Public Health Bureau. Dr Qi is supported by grants from the National Heart, Lung, and Blood Institute (HL071981, HL034594, HL126024), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718, DK100383, DK078616), the Boston Obesity Nutrition Research Center (DK46200), and United States – Israel Binational Science Foundation Grant2011036. Dr. Hu is supported by grant from the National Institute of Diabetes and Digestive and Kidney Diseases under Award Number R01DK100790.
Author information
Authors and Affiliations
Contributions
A.M.C. wrote manuscript and data analysis. P.S. design, data collection, and wrote manuscript. H.L. design, data collection, and wrote manuscript. H.C. wrote manuscript and data analysis. Y.Z. wrote manuscript and data analysis. J.L design, data collection, and wrote manuscript. W.L. design, data collection, and wrote manuscript. T.H. wrote manuscript and data analysis. T.W. wrote manuscript and data analysis. L.W. design, data collection, and wrote manuscript. S.Z. design, data collection, and wrote manuscript. G.H. design, data collection, and wrote manuscript. L.Q. design, data collection, and wrote manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Carvalho, A.M., Shao, P., Liu, H. et al. The MC4R genotype is associated with postpartum weight reduction and glycemic changes among women with prior gestational diabetes: longitudinal analysis. Sci Rep 7, 9654 (2017). https://doi.org/10.1038/s41598-017-10101-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-10101-x
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.