Abstract
Cells respond to many signals with a limited number of signaling components. Heterotrimeric G proteins and MAPK cascades are universally used by eukaryotic cells to transduce signals in various developmental processes or stress responses by activating different effectors. MAPK cascade is integrated with G proteins by scaffold protein during plant immunity. However, the molecular relationship between G proteins and MAPK modules in plant development is still unclear. In this study, we demonstrate that Arabidopsis Gβ protein AGB1 interacts with MPK3 and 6, MKK4 and 5, as well as the regulatory domains of YODA (YDA), the upstream MEKK of MKK4/5. Remarkably, YDA interacts with the plasma membrane associated SHORT SUSPENSOR (SSP) through its N- and C-terminal region in vitro and in vivo. Additionally, genetic analysis shows that AGB1 functions together with MPK3/6 signaling cascade during the asymmetric division of the zygote. These data indicate that Gβ may function likely as a scaffold, through direct physical interaction with the components of the MPK signaling module in plant development. Our results provide new insights into the molecular functions of G protein and will advance the understanding of the complex mechanism of kinase signaling cascades.
Similar content being viewed by others
Introduction
Heterotrimeric G proteins transmit numerous and diverse extracellular cues by coupling with the plasma membrane-localized receptors and different effectors in eukaryotic cells. In contrast to metazoans, the repertoire of genes encoding G proteins subunits in plants is small. The Arabidopsis thaliana genome encodes one prototypical Gα (GPA1), one Gβ (AGB1), three Gγ subunits (AGG1, AGG2 and AGG3) and one regulator of G-protein signaling (AtRGS1) protein1. In Arabidopsis and rice, heterotrimeric G proteins play diverse roles in a variety of processes, such as hormone regulation, drought stress, pathogenesis, and development2, and they often serve as a converging point of different signaling pathways triggered by distinct receptors3. Genetic and biochemical evidences implicated that G proteins likely integrate signaling pathways as a variable resistor to control the output of diverse signal information4. However, the molecular basis of G proteins in plant signaling is yet to be determined.
Mitogen-activated protein kinase (MAPK) cascade is evolutionarily conserved and mediates diverse cellular responses to a variety of extra- and intracellular stimuli in eukaryotes5. Phosphorylation activation of MAPKs is executed by MAPK kinases (MAPKKs or MEKs), which are phosphorylated by MAPKK kinases (MAPKKKs or MEKKs). One main question in MPK cascade signaling is how the large signaling diversity is achieved by similar or the same modules. One pivotal way to regulate signaling modules is through scaffold proteins6, 7. In animals and fungi, MPK modules bind different scaffold proteins which promote signaling efficiency and/or specificity6, 8,9,10. In mammals, the WD40 repeat protein Receptor for Activated C Kinase 1 (RACK1) has been established as a scaffold protein by interacting with a range of proteins in global control of gene transcription, translation, and ribosome assembly and activation11,12,13. Recently, it was shown that RACK1 interacts with AGB1 and functions as a dynamic scaffold of MEKK1-MKK5-MPK3/6 cascade during plant immune response14. It has been proposed that the diverse roles of Gβ may be conferred by its binding to distinct WD40 repeat-containing proteins which mediate diverse cellular processes in animals15. Similar to RACK1, Gβ itself contain seven WD40 repeats which adopt a circular β-bladed propeller structure16. This unique ternary structure confers its property to interact with different proteins, while the molecular mechanics of Gβ in diverse signaling is still unclear in plants.
In plants, the zygote elongates largely after fertilization and before the first asymmetric cell division which is essential for the following embryo development. This process is regulated by a kinase-dependent pathway. Loss of YDA, encoding a MAPKKK, suppresses zygote elongation and causes shortened suspensor cells and embryo lethality17. Loss of SSP, which encodes a sperm-derived kinase, causes a similar phenotype to yoda 18. This suggests that SSP likely activates YDA and initiates zygote elongation although the mechanism is unknown. Knock-down of MKK4 and MKK5, which activates MPK3/6, causes developmentally-arrested embryo19. The mpk3 mpk6 double mutant suppresses suspensor formation and ovule development19. YDA has been verified to be an upstream MAPKKK of MKK4/MKK5-MPK3/MPK6 module in stomata development and patterning20. Recently, we found that a leucine-rich repeat receptor-like kinase ZAR1 regulates zygote elongation and asymmetric division through AGB1 and SSP21. In these contexts, the kinases-governed zygote development appears dependent on a ZAR1-SSP-YDA-MKK4/5-MPK3/6 signaling cascade. In this pathway, how the signals are relayed and how G proteins are integrated are still unclear.
In this study, we provide biochemical and genetic evidences that AGB1 directly interacts with MPK3/6. AGB1, but not GPA1, interacts with MKK4/5. AGB1 and SSP interact with the extended N- and C-terminal domains but not the kinase domain of YDA. Further genetics study also confirmed the genetic interaction between MPK3/6 and AGB1 during zygote and fruit development. These data support the model that AGB1 acts as a scaffold for the MAPK signaling cascade during Arabidopsis development.
Results
Heterotrimeric G protein subunits physically interact with MPK3 and MPK6
Since both AGB1 and YDA-MKK4/MKK5-MPK3/MPK6 module play roles in plant architecture, zygote and embryo development19, 21,22,23, we are curious about whether heterotrimeric G proteins and MPK3/6 signaling module interact biochemically and genetically. To answer this question, we first examined the interaction of GPA1, AGB1, AGG1 and AGG2 with MPK3 and MPK6 in vivo by a firefly luciferase complementation imaging assay in tobacco leaves24. As shown in Fig. 1A–J, combinations of MPK6-nLUC or MPK3-nLUC with cLUC-AGB1, cLUC-AGG1, cLUC-AGG2, cLUC-GPA1 and cLUC-GPA1Q222L (the constitutively active form of GPA1) generate strong luciferase activity signals, indicating that GPA1, AGB1, AGG1 and AGG2 could interact with MPK6 and MPK3. Pull-down assay with the purified epitope-tagged proteins confirmed the interaction between MPK6 and different G protein subunits (Fig. 1K). These results suggest that the interaction of GPA1 with MPK3/6 is independent of its GTPase activity. Coimmunoprecipitation (Co-IP) result further shows that GPA1 and AGB1 interact selectively with MPK6, but not with MPK4 in vivo (Fig. 1L,M), indicating that the interaction is specific. In addition, through bimolecular fluorescence complementation (BiFC) assay in tobacco leaf, we further confirmed the interaction between MPK6 with AGB1 (Fig. 2A–C). Together, these results suggest that each component of the heterotrimeric G proteins physically interact with MPK3/6 both in vitro and in vivo.
AGB1, GPA1, AGG1 and AGG2 interact with MPK6 and MPK3. (A–J) Tobacco leaves co-infiltrated with agrobacterium containing 35S-driven split luciferase (LUC) constructs as indicated were photographed with a charge-coupled device camera. Each image is representative of three images in three independent experiments. (A–E) MPK6-nLUC interacts with cLUC-AGB1 (A), cLUC-WtGPA1 (B), cLUC-AGG1(C), cLUC-AGG2 (D), cLUC-GPA1Q222L (E), respectively. (F–J) MPK3-nLUC interacts with cLUC-AGB1 (F), cLUC-WtGPA1 (G), cLUC-AGG1 (H), cLUC-AGG2 (I), cLUC-GPA1Q222L (J), respectively. The pseudocolor bar shows the relative range of luminescence intensity in images. Pull-down assay shows that AGB1, WtGPA1, GPA1Q222L, AGG1 and AGG2 interact with MPK6 (K), respectively. AGB1 and GPA1 interact with MPK6 (L), but not MPK4 (M) by Co-IP assay. Full blots are shown in Supplemental Data.
Bimolecular fluorescence complementation analysis showing the interaction between AGB1, MPK6 and MKK5. Images of tobacco leaf sections expressing CFPC-AGB1 and CFPN-MPK6 (A), CFPN-AGB1 and CFPC-MKK5 (D) exhibit fluorescence, but not CFPC-AGB1 and CFPN (B), CFPC and CFPN-MPK6(C), CFPN-AGB1 and CFPC (E), or CFPN and CFPC-MKK5 (F). Each image is a representative of three images in three independent experiments.
AGB1 but not GPA1 interacts with MKK4 and MKK5
MKK4/5 relay the signal from YDA to MPK3/6 in plant architecture regulation. MKK4/MKK5 knock-down plants phenocopy agb1 in controlling fruit length23. To elucidate whether AGB1 interacts with MKK4/5, we performed biochemical analysis. Direct interaction between MKK4/5 and AGB1 was detected in tobacco leaves using BiFC (Fig. 2D–F) and firefly luciferase complementation imaging (Fig. 3A and B) assay, respectively. Co-IP assay with Arabidopsis protoplasts shows that AGB1 interacts with MKK4 and MKK5, while GPA1 does not (Fig. 3C and D). Furthermore, pull-down assay also demonstrated that AGB1 interacts with MKK4 and MKK5 (Fig. 3E and F), while GPA1 does not (Fig. S1). These results suggest a directly physical interaction between AGB1 and MKK4/5 both in vitro and in vivo.
AGB1 interact with MKK4 and MKK5. (A,B) Tobacco leaves co-infiltrated with agrobacterium containing 35S-driven construct pairs as indicated were photographed with a charge-coupled device camera. AGB1-cLUC interacts with nLUC-MKK4 (A) and nLUC-MKK5 (B). The upper lane in (A) and (B), luminescence images; the lower lane in (A) and (B), intensity quantification of the upper lane. The pseudocolor bar shows the relative range of luminescence intensity in images. (C) Co-IP assay in Arabidopsis protoplasts shows that AGB1 interacts with MKK4 (C) and MKK5 (D), but not GPA1. Pull-down assay shows that AGB1, AGG1 and AGG2 interact with MKK4 (E) and MKK5 (F). Full blots are shown in Supplemental Data.
AGB1 and SSP interact with the regulatory domains of YDA
Then we speculated that if AGB1 functions as a scaffold protein for the MPK cascade, AGB1 might also interact with the MAPKKK. Based on this hypothesis, we examined the interaction between AGB1 and YDA. YDA contains an N-terminal, a kinase-active and a C-terminal domain (Fig. 4A). The N-terminal domain is a negative regulatory domain which inhibits the kinase activity of YDA, while the C-terminal is required for the full activity of the kinase domain17. Firefly luciferase complementation assay shows that AGB1 interacts with both the N- and C-terminal of YDA, but not with the full-length protein (Fig. 4B–F). Consistently, pull-down assay also shows that AGB1 interacts with the N- and C-terminal of YDA, but not the kinase domain (Fig. 4L). Both constitutive activation and loss-of-function of YDA are detrimental to plants17, suggesting the physiological importance of the spatiotemporal modulation of its kinase activity. Furthermore, SSP genetically activates YDA after the zygote formation but the molecular mechanism is unknown. We showed that SSP strongly interacts with the N-terminal and weakly with the C-terminal of YDA, but not with the full-length protein (Fig. 4G–K). In addition, pull-down assay further shows that SSP interacts with the N- and C-terminal of YDA, respectively, but not the kinase domain (Fig. 4M and N). This specific interaction of AGB1 and SSP with the regulatory domains of YDA indicates that AGB1 and SSP may be directly involved in the signaling of YDA. And these results confirm the speculation that AGB1 interacts with different layers of MPK signaling cascade.
AGB1 and SSP physically interact with the N- and C-termial of YODA by luciferase complementation assay and pull-down assay. (A) Protein structure of YODA. N-Tail, kinase, C-Tail and Δ334 (334–883aa) are indicated in the diagram. (B–F) Tobacco leaves co-infiltrated with agrobacterium containing 35S-driven construct pairs as indicated were photographed with a charge-coupled device camera. (B–F) cLUC-AGB1 interacts with nLUC-YDA-N-Tail (C) and nLUC-YDA-C-Tail (F), but not nLUC-YDA (B), nLUC- YDA-Kinase (E) or nLUC-YDA-Δ334 (D), respectively. (G–K) cLUC-SSP interacts with nLUC-YDA-N-Tail (H) and nLUC-YDA-C-Tail (K), but not nLUC-YDA (G), nLUC-YDA-Kinase (J) or nLUC-YDA-Δ334 (I), respectively. The pseudocolor bar shows the relative range of luminescence intensity in images. Pull-down assay shows that AGB1 (L) and SSP (M) interact with the N-Tail and C-Tail of YODA, but not the kinase domain. GST does not interact with the N-Tail, kinase domain or C-Tail of YODA (N). Full blots are shown in Supplemental Data.
AGB1 and MPK6 function together in zygote development
To investigate the genetic relation between mpk6 and agb1, the early embryonic phenotype was studied. In mpk6 homozygotes, two categories of phenotypes based on the length of the daughter cells of the zygote were observed: Category I in which the total length of apical and basal cell is similar or slightly shorter than the wild-type (45%, Fig. 5A and B) and Category II in which the length is significantly shortened (55%, Fig. 5C). Similarly, as previously reported21, agb1 causes shortened basal cell leading to an increased ratio of the apical to basal cell length (Fig. 5D). In the agb1 mpk6 double mutant, 60% embryos show the shortened phenotype (Category II) (Fig. 5E–G). Furthermore, we measured the ratio of the apical/basal cell length of all the sibling embryos in mpk6-4, agb1-2 and agb1-2 mpk6-4. The results show that the average ratio for agb1 and mpk6 is higher than the wild-type, while the average ratio for agb1 mpk6 is of no significance to that of the mpk6 (Fig. 5H). However, 6% of agb1 mpk6 zygotes exhibit severe reduced elongation which is not seen in single mutant, and display almost symmetric cell division compared with that in mpk6 (Fig. 5G). It is not known how such a phenotypic variation takes place. MPK6 appears more important than AGB1 in controlling the zygotic elongation and division since mpk6 exhibits a stronger phenotype than agb1, although they physically interact. It is possible that the scaffold role of AGB1 is dispensable for the MPK cascade which can assemble with less efficiency without AGB1. Alternatively, other scaffold proteins take over AGB1’s role in the absence of AGB1, or other MPKs such as MPK3 takes over when MPK6 is absent. Indeed, MPK6 and MPK3 were reported to function redundantly in suspensor formation19. Furthermore, the suspensor cells of yda are also shortened due to defective zygote elongation and symmetric division21. It is noticeable that the increased ratio of the apical to basal cell length of yda is more pronounced. To determine the genetic relationship between AGB1 and YDA, agb1-2 yda double mutant was also constructed. The result shows that the ratio for agb1-2 yda is the same as yda (Fig. 5I–K), indicating that yda is epistatic to agb1. Together, we conclude that AGB1 genetically acts in the same pathway with the YDA-MPK6/3 signaling cascade during zygote elongation and division.
Zygotic phenotype of mpk6, yda and agb1 mutants. (A–G) Representative images of the two-cell stage zygote in the wild type (A) and mpk6-4 (B and C), agb1-2 (D), yda-8 (I) and m6-4 agb1-2 (E to G), yda-8 agb1-2 (J) mutants. (H and K) Statistical ratio of apical/basal cell length. Averages ( ± SE, n ≥ 30 per genotype) were determined in three independent experiments. ac, apical cell; bc, basal cell; dp, division plane. Students’ t test. p* < 0.05, p* < 0.01. n.s. no significance. Bars = 10 μm.
Genetic relationship between G protein subunits and MPK3/6 in fruit development
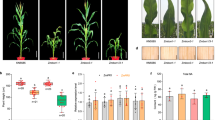
G proteins and the MPK3/6 cascade were reported to regulate fruit length22, 23. Thus, we further examined the genetic interaction between G proteins and MPK3/6 during fruit development. Previous studies showed that null mutant of AGB1 exhibits pleiotropic developmental defects, including shorter siliques25. To confirm the physiological relevance of the direct interaction of AGB1 and GPA1 with the MPK cascade in silique development, we analyzed the silique length of agb1-2 mpk6-4, agb1-2 mpk3-1, gpa1-4 mpk6-4 and gpa1-4 mpk3-1. The agb1-2 mutant exhibited shorter siliques than the wild-type as previously reported22, but double mutant agb1-2 mpk6-4 displayed much shorter siliques than agb1-2, only 60% of the wild-type (Fig. 6A and C). The agb1-2 mpk3-1 exhibits the same silique length as agb1-2. mpk3-1 does not exhibit shorter siliques compared to the wild-type, while gpa1-4 and mpk6 displayed clearly obvious shortened siliques (Fig. 6A–D). This data indicate that MPK6 plays a role in fruit length which is enhanced by AGB1. Intriguingly, the shortened silique phenotype of mpk6 is also exaggerated by gpa1, as gpa1-4 mpk3-1 exhibits the same silique length as gpa1, while gpa1-4 mpk6-4 displays much shorter siliques than mpk6 (Fig. 6B,D). This result suggests that GPA1 also plays a role in MPK signaling cascade during fruit development.
Length of siliques and pedicels of agb1, gpa1, mpk3 and mpk6. (A) Siliques of wild-type plants (Col-0), mpk3-1, mpk6-4, agb1-2 and double mutant agb1-2 mpk3-1, agb1-2 mpk6-4. (B) Siliques of wild-type plants (Col-0), mpk3-1, mpk6-4, gpa1-4 and double mutant gpa1-4 mpk3-1, gpa1-4 mpk6-4. Bar, 1 mm. (C and D) Statistics of silique length of wild-type plants (Col-0), mpk3-1, mpk6-4, agb1-2, gpa1-4 and double mutant agb1-2 mpk3-1, agb1-2 mpk6-4, gpa1-4 mpk3-1, gpa1-4 mpk6-4. (E and F) Statistics of pedicel length of genotype mentioned above. Averages (±SE, n ≥ 30 per genotype) were determined in three independent experiments. Different letters indicate significantly different values (b, p < 0.01; c, p < 0.001; ANOVA, Tukey test). Asterisks indicate a significant difference between a given genotype and the wild type (*p < 0.05, **p < 0.01, ***p < 0.001, Student’s t test). n.s. no significance.
Except for fruit length, pedicel length is also regulated by the MPK3/6 cascade23. Deletion of AGB1 mildly exacerbates the shortened pedicel of mpk6, although agb1 mutant does not show shortened pedicels (Fig. 6E). Pedicels of gpa1 are longer than the wild-type, while gpa1 mpk6 pedicels are comparable to mpk6 (Fig. 6F), implying that in contrast to AGB1, GPA1 likely function in a different pathway in pedicel development.
Together, we conclude that MPK6 and AGB1 play positive roles in controlling zygote and fruit development. The additive effect of mpk6 and agb1 is possibly due to the functional redundancy of MPK3 and MPK6, as well as AGB1 and other WD40-repeat proteins which has been reported previously19, 23, 26. GPA1 also positively promotes silique development, but plays a negative role in pedicel length. GPA1 does not associate with MPKK4/5 as we suggested and dissociates with Gβγ in plants27, implying that GPA1 may not function as a scaffold of MPK cascade as AGB1.
Discussions
In this study, we demonstrated the biochemical and genetic interactions between heterotrimeric G proteins and the MPK signaling components. Our results suggest that AGB1 and the MPK cascade may function together during zygote and fruit development.
Proper development of the zygote is dependent on receptor-like kinases, plasma membrane associated kinases and the MAPK signaling pathway, very similar to the early fertilization process28. Direct hierarchical interaction between components of the ZAR1-SSP-YDA-MKK4/5-MPK3/6 signaling cascade has been genetically and biochemically revealed, but SSP-YDA interaction was only implicated by genetic analysis. AGB1 plays a positive role in regulating the kinase activity of ZAR1 through direct interaction21. In this study, we showed that AGB1 genetically and biochemically interact with YDA, MKK4/5, and MPK3/6. And strikingly, we detected the direct interaction between SSP and YDA. These findings potentiate AGB1 as a fine-tuning scaffold protein in the kinase signaling module.
We show that AGB1 could interact with MPK3/MPK6, MKK4/MKK5 and regulatory domains of YDA. The biological significance of this scaffolding is still unclear. One possible effect is to recruiting different components to promote signal transduction. However, we found that AGB1 does not promote the interaction between MKK4/5 and MPK3/6 when AGB1 was co-expressed with MKK5 and MPK6 in Arabidopsis protoplasts (Fig. S2). One likely reason is that the promoting effect needs upstream extracellular signals from the neighboring cells or the environment. Another possible effect is to localize the signaling cascade at specific cellular compartment. ZAR1 and SSP are localized on the plasma membrane, while the MPK kinases are distributed extensively throughout the cell. AGB1 appears to be membrane-associated by lipid modification which supports it as a signaling component to transduce signal from outside to inside the cell. Third, AGB1 may affect the kinase activity of MPK3/6 to their substrates in the cellular responses. AGB1 has been shown to promote the kinase activity of ZAR123. Forth, the scaffolding of a signal module could insulate related pathways from cross-talk or improve signaling efficiency or fidelity.
In metazoans and yeasts, scaffold proteins not only bring MAPK components together to enhance specificity and accelerate their activation, but also sequester MAPK modules to distinct subcellular locations in response to different types of stimuli29. Interestingly, AGB1 interacts with RACK1 that recruits MPK signaling cascades in plant immunity14. AGB1 has an N-terminal helix motif and a C-terminal WD40-repeat domain that forms a seven-bladed propeller structure30. Combining with our results, AGB1 and other WD40-repeat containing proteins, can function as scaffold proteins by forming dimers or even oligomers to integrate signals from different cellular processes in plants. Such dimerization mechanism of scaffold proteins is also utilized by RACK1 in mammals via interacting with Gβ15. Although RACK1 is not a Gβ as it does not bind GPA1 and lacks the N-terminal Gγ-binding motif, RACK1 shares similar WD40-repeat domains31. Interestingly, rack1 agb1 double mutation causes more severe growth defects than both single mutant, indicating that RACK1 performs overlapping roles with AGB1 during plant development26. Furthermore, single MPK cascade can utilize multiple scaffold proteins which can render a wide-range of signaling potentials and fine-tuning7. In plants, AGB1 and RACK1 may function as a scaffold complex in the MPK signaling cascade, although the genetic relationship between RACK1 and MPK cascade components during development is yet to be determined. Our results support the hypothesis that Gβ, similar to RACK1, also functions as a scaffold protein for multiple protein kinases to enhances the robustness of plants development.
Materials and Methods
Plant materials and growth conditions
Arabidopsis thaliana ecotype Columbia-0 (Col-0), the T-DNA insertion lines CS6536 (agb1-2), SALK_016750 (mpk6-4), SALK_100651 (mpk3-1) and SALK_001846 (gpa1-4), Salk_105078 C (yda-10) were obtained from ABRC stock center (http://www.arabidopsis.org). Plants were grown in an air-conditioned room at 22 °C under a 16-hlight/8-h-dark cycle.
Firefly luciferase complementation imaging assay
To generate MPK6-nLUC, MPK3-nLUC, MKK5- nLUC and MKK4- nLUC, YDA-nLUC, YDA-N-Tail-nLUC, YDA-C-Tail-NLuc, and YDA-Kinase-nLUC, the corresponding coding sequences were subcloned into pCAMBIA-nLUC32. To generate cLUC-AGB1, cLUC-GPA1, cLUC-AGG1, cLUC-AGG2, and cLUC-SSP, the corresponding coding sequences were subcloned into pCAMBIA-cLUC. The constructs were transformed into agrobacterium strain GV3101. Bacterial suspensions in MgCl2 were infiltrated into leaves of 7-week-old Nicotiana benthamiana plants using a needleless syringe. After infiltration, plants were grown in 16 h light/8 h darkness for 3 days at 22 °C. Images were captured by a low-light cooled charge-coupled device imaging apparatus (NightOWL II LB983).
BiFC assay
To generate CFPN-AGB1 and CFPN-MPK6, the corresponding coding sequences were subcloned into pSPYNE33. To produce CFPC-AGB1 and CFPC-MKK5, the corresponding coding sequences were subcloned into pSPYCE. The constructs were then transformed into agrobacterium strain GV3101. Bacterial suspensions were infiltrated into leaves of 7-week-old N. benthamiana plants using a needleless syringe. After infiltration, plants were grown in 16 h light/8 h darkness for 3 days at 22 °C. For microscopic analyses, leaf discs were cut for imaging of BiFC signal by confocal laser scanning microscopy (LSM510META, Zeiss).
Pull-down assay
Fusion proteins were expressed in E. coli and purified using glutathione agarose beads (GE Healthcare). For pull-down assay, 5 mg of each purified proteins were incubated with 30 ml glutathione agarose beads in a buffer containing 25 mM Tris-HCl (pH 7.5), 100 mM NaCl and 1 mM DTT for 1 hr. The beads were washed seven times with the washing buffer containing 25 mM Tris-HCl (pH 7.5), 100 mM NaCl, 1 mM DTT and 0.1% Trition-X 100. The bound protein was eluted with elution buffer containing 25 mM Tris-HCl (pH 7.5), 100 mM NaCl, 1 mM DTT and 15 mM GSH. Immunoblot was performed with the corresponding antibodies.
Co-immunoprecipitation assay
The Arabidopsis protoplasts prepared as reported34 were transformed with the indicated plasmids, and then cultured for 12 hours at 22 °C. Total protein was extracted for Co-IP assay with the extraction buffer containing 50 mM HEPES [pH 7.5], 150 mM KCl, 1 mM EDTA, 0.5% Trition-X 100, 1 mM DTT, proteinase inhibitor cocktail. For anti-FLAG IP, total protein was incubated with 50 μL agarose conjugated anti-FLAG antibody (Sigma) for 4 hr. and washed seven times with washing buffer [50 mM HEPES (pH 7.5), 150 mM KCl, 1 mM EDTA, 0.5% Trition-X 100, 1 mM DTT]. The bound protein was eluted with 60 μL 0.5 mg/mL FLAG peptides for 1 hr. The protein was separated by SDS-PAGE and subjected to immunoblot by anti-HA and anti-FLAG antibodies. For anti-HA IP, the protein was incubated with 50 mL agarose conjugated anti-HA antibody (Thermo) for 4 hr. After washing, the bound protein was eluted with 60 μL 0.5 mg/ml HA peptide for 1 hr. The protein was separated by SDS-PAGE and detected by anti-HA and anti-FLAG immunoblot. For anti-GFP IP, the protein was incubated with 50 μL agarose conjugated anti-GFP antibody (Thermo) for 4 hr. After washing, the protein was separated by SDS-PAGE and detected by anti-GFP, anti-HA and anti-FLAG immunoblot.
Whole- Mount Clearing of Embryos
The method for phenotypic analysis of mutant embryos was described previously35.
Accession numbers
Sequences from this article can be found in the GenBank/EMBL or Arabidopsis Genome Initiative database by the following accession numbers: AGB1 (At4G34460), AGG1 (At3G63420), AGG2 (At3G22942), GPA1 (At2G26300), MPK6 (At2G43790), MPK3 (At3G45640), MPK4 (At4G01370), MKK4 (At1G51660), MKK5 (At3G21220), YDA (At1G63700).
References
Chen, J. G. et al. A seven-transmembrane RGS protein that modulates plant cell proliferation. Science. 301, 1728–1731 (2003).
Perfus-Barbeoch, L. et al. Plant heterotrimeric G protein function: insights from Arabidopsis and rice mutants. Curr Opin Plant Biol. 7, 719–731 (2004).
Liu, J. et al. Heterotrimeric G proteins serve as a converging point in plant defense signaling activated by multiple receptor-like kinases. Plant Physiol. 161, 2146–2158 (2013).
Colaneri, A. C. & Jones, A. M. The wiring diagram for plant G signaling. Curr. Opin. Plant Biol. 22, 56–64 (2014).
Xu, J. & Zhang, S. Mitogen-activated protein kinase cascades in signaling plant growth and development. Trends Plant Sci. 20, 56–64 (2015).
Morrison, D. K. & Davis, R. J. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 19, 91–118 (2003).
Schwartz, M. A. & Madhani, H. D. Principles of MAP kinase signaling specificity in Saccharomyces cerevisiae. Annu Rev Genet. 38, 725–748 (2004).
Zalatan, J. G. et al. Conformational control of the Ste5 scaffold protein insulates against MAP kinase misactivation. Science. 337, 1218–1222 (2012).
Teichert, I. et al. PRO40 is a scaffold protein of the cell wall integrity pathway, linking the MAP kinase module to the upstream activator protein kinase C. PLoS Genet. 10, e1004582 (2014).
McCahill, A. et al. The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol Pharmacol. 62, 1261–1273 (2002).
Dell, E. J. et al. The βγ subunit of heterotrimeric G proteins interacts with RACK1 and two other WD repeat proteins. J Biol Chem. 277, 49888–49895 (2002).
Ceci, M. et al. Release of eIF6 (p27BBP) from the 60S subunit allows 80S ribosome assembly. Nature. 426, 579–584 (2003).
Nilsson, J. et al. Regulation of eukaryotic translation by the RACK1 protein: a platform for signalling molecules on the ribosome. EMBO Rep. 5, 1137–1141 (2004).
Cheng, Z. et al. Pathogen-secreted proteases activate a novel plant immune pathway. Nature. 521, 213–216 (2015).
Chen, S. et al. Interaction of Gbetagamma with RACK1 and other WD40 repeat proteins. J Mol Cell Cardiol. 37, 399–406 (2004).
Ullah, H. et al. Structure of a signal transduction regulator, RACK1, from Arabidopsis thaliana. Protein sci. 17, 1771–1780 (2008).
Lukowitz, W. et al. A MAPKK kinase gene regulates extra-embryonic cell fate in Arabidopsis. Cell. 116, 109–119 (2004).
Bayer, M. et al. Paternal control of embryonic patterning in Arabidopsis thaliana. Science. 323, 1485–1488 (2009).
Wang, H. et al. Haplo-insufficiency of MPK3 in MPK6 mutant background uncovers a novel function of these two MAPKs in Arabidopsis ovule development. Plant Cell. 20, 602–613 (2008).
Wang, H. et al. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell. 19, 63–73 (2007).
Yu, T. Y. et al. The Arabidopsis receptor kinase ZAR1 is required for zygote asymmetric division and its daughter cell fate. PLoS Genet. 12, e1005933 (2016).
Lease, K. A. et al. A mutant Arabidopsis heterotrimeric G-Protein subunit affects leaf, flower, and fruit development. Plant cell. 13, 2631–2641 (2001).
Meng, X. et al. A MAPK cascade downstream of ERECTA receptor-like protein kinase regulates Arabidopsis inflorescence architecture by promoting localized cell proliferation. Plant Cell. 24, 4948–4960 (2012).
Chen, H. et al. Firefly luciferase complementation imaging assay for protein- protein interactions in plants. Plant Physiol. 146, 368–376 (2008).
Jiang, K. et al. Dissecting Arabidopsis Gβ signal transduction on the protein surface. Plant Physiol. 159, 975–983 (2012).
Guo, J. et al. Dissection of the relationship between RACK1 and heterotrimeric G-proteins in Arabidopsis. Plant Cell Physiol. 50, 1681–1694 (2009).
Johnston, C. A. et al. GTPase acceleration as the rate-limiting step in Arabidopsis G protein-coupled sugar signaling. Proc. Natl. Acad. Sci. 104, 17317–17322 (2007).
Li, H. J. & Yang, W. C. RLKs orchestrate the signaling in plant male-female interaction. Sci China Life Sci. 59, 867–77 (2016).
Harding, A. et al. Subcellular localization determines MAP kinase signal output. Curr Biol. 15, 869–873 (2005).
Li, J. H. et al. A signaling pathway linking nitric oxide production to heterotrimeric G protein and hydrogen peroxide regulates extracellular calmodulin induction of stomatal closure in Arabidopsis. Plant Physiol. 150, 114–124 (2009).
Chen, J. G. et al. RACK1 mediates multiple hormone responsiveness and developmental processes in Arabidopsis. J. Exp. Bot. 57, 2697–2708 (2006).
Chen, H. et al. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 146, 368–376 (2008).
Rainer, W. et al. Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J. 56, 505–516 (2008).
Yoo, S. D. et al. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2, 1565–1572 (2007).
Ding, Y. H. et al. Arabidopsis GLUTAMINE-RICH PROTEIN23 is essential for early embryogenesis and encodes a novel nuclear PPR motif protein that interacts with RNA polymerase II subunit III. Plant Cell. 18, 815–830 (2006).
Acknowledgements
We thank Dr. Dongtao Ren (China Agricultural University) for mpk3 and mpk6 seeds and Dr. Hongquan Yang (Fudan University) for yda mutant seeds. This work is supported by the Ministry of Science and Technology of China (2015CB910202 to H.L and 2013CB945103 to W.Y) and National Natural Science Foundation of China (31330053 to W.Y., 31622010 and 31571385 to H.L).
Author information
Authors and Affiliations
Contributions
W.Y. conceived the project. H.L. G.Y. and W.Y. analyzed the data and wrote the manuscript. G.Y. performed all the experiments.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuan, GL., Li, HJ. & Yang, WC. The integration of Gβ and MAPK signaling cascade in zygote development. Sci Rep 7, 8732 (2017). https://doi.org/10.1038/s41598-017-08230-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-08230-4
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.