Abstract
Tungsten-doped VO2 thin films have been synthesized by a modified sol–gel process and followed by a post annealing. Vanadium pentoxide and tungstic acid as raw materials with the addition of hydrogen peroxide, concentrated hydrochloric acid (catalyst) and oxalic acid used as reducing agent were reacted in isobutanol. Finally, the uniform sol of vanadyl oxalate in isobutanol solvent was obtained as precursor. Detailed study suggested that W doped in VO2 introduces additional electron carriers and induces the formation of V3+. Post annealing under vacuum promotes the releasing of chemical stress and generates oxygen vacancies in the samples. Temperature dependent transmittance study revealed that the releasing of chemical stress and deliberately introducing oxygen vacancies in W-doped VO2 films have positive effects on enhancing its switching ability in the infrared transmittance as the metal-insulator transition (MIT) occurs. The largest switching of transmittance was obtained about 48% in the infrared range at 43 °C in 1.5%W doped VO2 films, which is significantly larger than the reported ones. The findings in this work open a new way to synthesize the novel and thermochromic W doped VO2 films with facility and low cost. Therefore, it has extensive application to construct smart windows and electronic devices.
Similar content being viewed by others
Introduction
Vanadium dioxide has attracted widespread interest since the discovering of its metal-insulator transition (MIT) in 1959s1. It is well-known that the MIT in VO2 is a first-order reversible transformation from its insulating monoclinic phase (P21/c) with an energy gap of 0.7 eV at low temperature to metallic tetragonal phase (P42/mnm) at high temperature with the critical temperature (Tc) of 68 °C1,2,3. The theoretical explanation of the MIT was presented by Goodenough4 that the t2g band of VO2 is splitting into dII band and the π* anti-bond in the framework of crystal field theory which was verified by structural simulation calculations and experimental results5,6,7. There are significant changes in the electrical resistivity and infrared transmittance along with the occurring the MIT. Infrared light would go through VO2 films mostly whereas it would be reflected above 68 °C with the same transmittance of visible light. So VO2 has potential applications in sensors, optical storage device, infrared modulators and intelligent window coating8, 9.
In view of applications, the Tc of VO2 should be reduced, while the switching ability of its transmittance in infrared should be kept or enhanced during the MIT. In spite of extensive efforts contributed, these problems are still unsolved, which are crucial for the application of VO2 8, 10, 11. Transition metal elements with large atomic radius and rich valences introduced into VO2 could trigger the distortion of its lattice and modify its properties related to MIT. Reducing Tc of the MIT could be achieved efficiently by the incorporation of some metal ions, such as W6+, Mo6+, and Nb5+, into the crystal lattices of VO2 3, 10,11,12. W has been proved to be most effective dopant in reducing the Tc by 21–28 °C/at.%13, 14, which has been confirmed in the study on VO2 films prepared by various methods including CVD, sputtering and sol-gel13,14,15,16. However, W doping in VO2 results in the increase of its electron intensity, which would lead to weakening of its revisable optical switching properties during the MIT13, 14. On the other hand, holes introduced into VO2 could improve its revisable switching properties both in resistivity and transmittance in infrared efficiently17. Therefore, elaborately introducing holes into W-doped VO2 could realize the reducing of its Tc and improve its revisable switching properties during its MIT effectively. Holes can be introduced by deliberately controlling the oxygen vacancies in VO2 films, which can be achieved by post annealing samples in high vacuum. Oxygen vacancies in W-doped VO2 films could be an unexpected route to approach VO2 the sample with low MIT temperature and strong ability to switch both conductivity and infrared transmittance18.
As for the preparation of VO2 films, sol-gel method has been demonstrated to be an effective skill and it is relatively cheap and can be scaled to large areas. In the case of the fabrication of VO2 films by sol-gel, oxalic acid and alcohols are commonly used as reducing agents to prepare the precursor in the aqueous or methanol solvent19, 20. The interaction between sol and precursor has a significant effect on the morphology of the pre-deposited film. Therefore, it is required that the surface of the substrate, for example, silicon substrate, should be subjected to hydrophilic treatment essentially (Details can be seen in the Supplementary Information). For the alcohol reducing agent such as isobutanol21, vanadium pentoxide could be reduced to vanadium isobutoxide (VO(i-OC4H9)2, which needs to be carried out at more than 100 °C due to their weak reducing property. These factors would lead to the complexity of the thin film preparation process. Therefore, new skills and approaches for the synthesis of precursor sols and fabrication of pre-deposition thin films are needed.
In this work, a modified sol-gel method was developed to fabricate VO2 thin films. In brief, a certain ratio of V2O5 and oxalic acid were added to amounts of isobutanol solvent, and uniform sol of vanadyl oxalate was obtained at 70 °C. To introduce W ions into VO2 in this case, tungstic acid was selected and solved in excessed hydrogen peroxide to form a complex solution firstly, and then injected into isobutanol solution that contains oxalic acid and vanadium pentoxide to prepare W-doped precursor. The remaining hydrogen peroxide in the W-doped precursor could promote the reduction of vanadium pentoxide owing that O2 2− bonds as ligand coordinated with V5+ to obtain (VO(O2)2)− 22. Finally, transparent precipitation-free sols with W doping would be obtained and the whole process is more efficient and economic as compared with the direct use of vanadium acetylacetonate and tungsten chloride23.
After then, pre-deposited thin films with high uniformity were formed by spin-coating and drying. Compared to aqueous, methanol and isopropanol sols, isobutanol sols of vanadium oxalate can be spin-coated on the surface of sapphire substrate to form uniform and smooth thin films without hydrophilic treatment. Followed by annealing under high vacuum conditions (~10−3 Pa), the undoped and W-doped VO2 films were fabricated. For the undoped films, the phase transition temperature is significantly reduced below 68 °C with decent infrared performance. According to previous reports, W doping in VO2 induces its MIT temperature decreasing, however, its infrared characteristics faded12, 13, 23. In this paper, the infrared performance of W-doped VO2 films annealed under vacuum conditions have been significantly improved while the phase transition temperature is reduced, which has not been reported in the previous work. These properties make W-doped VO2 films studied in this work meet the requirements in realizing the practical application.
Experimental
All the chemicals used in this work, i.e., V2O5 (99.9%), H2WO4 (99.3%), H2O2 (30 wt%), HCl (37.5 wt%) and H2C2O4 · 2H2O (99%), were utilized without any further purification. The c-cut (0001) Al2O3 was used as substrate to grow VO2 films.
Preparation of precursor solution
First, 28 mg, 42 mg, and 56 mg H2WO4 were dissolved in 3 mL 30 wt% H2O2 by ultrasound respectively and the three transparent solutions were obtained. Next, these H2WO4 solutions were poured into three 100 mL beakers including 40 mL isobutanol solvent under stirring for several minutes at room temperature. Then, 1 g V2O5, 3 mL 36 wt% HCl and 1.26 g H2C2O4 · 2H2O (with the mole ratio of V2O5: H2C2O4 · 2H2O = 1:2) were added into the three systems, and then followed a stirring thermostatically in the water bath at 70 °C for around 9 h. Finally, the three blue-green isobutanol sols of VOC2O4 with 1 at.%, 1.5 at.%, and 2 at.% W-doping were obtained.
Preparation of VO2 film
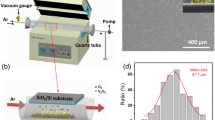
The c-cut Al2O3 substrates were cleaned ultrasonically consequently in ethanol, acetone and No.II cleaning fluid (NH3 · H2O:H2O2:H2O = 2:2:5, molar ratio)24 to remove organic contaminations on the surface. The deposition was carried out by spin coating method, and precursor films were formed on the cleared c-sapphire substrates with a spin speed of 600 rpm/30 s and 4000 rpm/40 s. Then, the films were dried at 60 °C for 20 min. This process was repeated several times to obtain expected thickness. Finally, the pre-deposited films were annealed at 450 °C, 470 °C and 500 °C for 4 hours respectively in a furnace under the vacuum of 10−3 Pa, with the heating rate of 5 °C/min. As a comparison, VO2 films were also fabricated by aqueous and alcohol Sol-Gel, and the morphology of the corresponding samples was recorded by SEM. The detailed results are shown in Figure S1 in the Supplementary Information. Figure S1a–c are the morphology of VO2 films synthesized by aqueous Sol, alcohol Sol and isobutanol Sol, respectively. It is clear to see from Figure S1a that the morphology of VO2 produced from aqueous Sol is discontinued or isolated islands, the morphology of VO2 film produced from alcohol Sol is quite rough with some isolated larger grains distributed on the surface, and the morphology of VO2 film produced from isobutanol Sol is much uniform and smooth. These results demonstrated that employing isobutanol Sol to produce VO2 films has prominent advantages.
Characterizations
The crystalline structure of the films was determined by X-ray diffraction (XRD, Bruker-AXS diffractometer, Model D8 ANVANCE) with Cu-Kα radiation source. 2θ mode was used to scan all the samples in the range of 15°~50° with the step of 0.02° and the stay time is 2~3 s. Morphology was characterized by atomic force microscope (AFM, Bruker-Nano scope Multimode IIIa) by tapping mode. Raman spectrum was measured to determine the lattice vibration of the films by Microscopic confocal Raman Spectrometer (HR800, excitation wavelength: 633 nm, laser power: 1 mW) and the integration time is 80 s. The chemical valences of constituents were measured by X-ray Photoelectron Spectroscopy (XPS) (PHI QUANTERA-II SXM) with Al-Kα radiation source (1486.6 eV). The optical properties of the films were investigated by spectrophotometer (Nicolet iS50 Fourier Transform Infrared Spectrometer) to analyze the transmittance of the films in the wavenumber range of 400–4000 cm−1 on heating and cooling. The device provides a solid heating accessory that can test the infrared transmittance of the sample under variable temperature conditions. The light source is ETC Everglo. Hysteresis loops were obtained by collecting the transmittance of films at a fixed wavelength (2500 nm) at approximately 2 °C intervals in the temperature range of 24–90 °C.
Results and Discussions
Figure 1a~c show the XRD patterns of the VO2 films with 1%W doping and sintered at 450, 470, and 500 °C, respectively. As can be seen from Fig. 1a, in addition to the peak of Al2O3 (0006), only one peak at 39.9° was observed and can be indexed to (020) crystallographic plane of VO2, similar with previous reports18, 24.
For the samples annealed at 470 and 500 °C, however, two dominant diffraction peaks at 27.8° and 39.9° appeared, which can be indexed to VO2 (011) and (020) planes. The intensity of (011) diffraction peaks gradually increased as the annealing temperature increased, which is in well agreement with the reported results25. Therefore, Fig. 1a–c reveal that the orientation of the 1% W-doped VO2 grown on Al2O3 (0006) is strongly dependent on the annealing temperature. The relative low-annealing temperature favors the growth of (020) orientation for VO2 film, while relative high-annealing temperature induces the dominant orientation of (011) of corresponding samples.
Figure 1d~f show the XRD patterns of the VO2 films with undoped, nominal 1.5% and 2% W-doped samples annealed at 470 °C/4 h, two prominent peaks at 27.8° and 39.8° were observed and can be indexed to (011) and (020) planes. However, one additional diffraction pattern at 37.4° appeared in the 2% W-doped samples, which can be assigned to VO2-δ, suggesting second phase was formed in this sample, agreed with previous report18. Therefore, Fig. 1 indicated that the samples were grown with (011) and (020) orientation on the c-Al2O3 substrate, no epitaxial growth behavior observed26.
Figure 2 shows AFM images of VO2 films with nominal 1%, 1.5% and 2% W doping. a)~c) annealed at 470 °C/4 h; d)~f) annealed at 500 °C/4 h. The morphologies of the films are granular structures with grain size in range of 60–80 and 70–110 nm for 470 and 500 °C annealed samples, respectively.
Compared samples annealed at 470 °C, it is clear to see that with increase in W contents, the surfaces of the corresponding samples become more impacted with average grain size reduced gradually, but the grain size distribution broadened. Meanwhile, for the samples annealed at 500 °C, the grain size of 1% W doped sample is significantly larger than that annealed at 470 °C. Moreover, with increase in W doping content, the grain sizes of the samples reduced with a similar tendency of these annealed at 470 °C. However, the agglomeration of grains is distinct, which induces the grain size distribution widened drastically. Another characteristic of the surface morphologies result is that increasing the doped W content or increasing annealing temperature induces the surface of the samples became more softened, which suggested that some downy or porous structures were formed on the surface of the grains, consistent with previous study by SEM27.
To determine the thickness of samples, the cross section image was performed by SEM and it was found that all the samples are about 300 nm. As evidence, the cross section image of 1% W-doped VO2 films annealed at 470 °C/4 h is presented in the Figure S2 in the Supplementary Information.
Raman microscopy is considered to be an effective skill to calibrate the bond vibrations in the lattice of VO2 11, 27, 28. The peaks at 192 (ωV1) and 223 (ωV2) cm−1 in Raman spectrum of M1 phase VO2 are connected with the V-V bond vibrations, while the peak at 614 cm−1 is involved with the vibration of V-O bonds in VO2 11, 29. The characteristics of these Raman peaks are very sensitive to the chemical states of the ions bonded in VO2 lattices30,31,32. Consequently, the substitution of V to W in VO2 lattice should affect the V-V(W) and V(W)-O bonds states, thus, the corresponding Raman vibration states should be modified, as well11, 28, 32. Figure 3a shows room temperature Raman spectra for nominal 1%, 1.5% and 2% W-doped samples annealed at 450, 470 and 500 °C, respectively. In addition to the weak peaks of substrate at 417 and 751 cm−1, the peaks at 192, 223, 264, 307, 338, 387, 440, 498 and 611 cm−1 in Fig. 3a can be assigned to the monoclinic VO2 8, 28,29,30,31. Fig. 3b is the magnified Raman spectra in the range of 350~520 cm−1 for 2%W-doped samples annealed at different temperatures. Compared with the Raman spectra of different samples, an interesting result is that peak located at 438 cm−1 was observed in the sample annealed at 450 °C, which can be assigned to T-phase VO2 and can be a critical evidence for the existing of stress in the studied sample28, 31. However, this peak disappeared in samples annealed at 470 and 500 °C, which suggests that T-phase VO2 or stress was reduced in the corresponding samples.
(a) Raman spectra of the films annealed at 450, 470 and 500 °C; (b) The magnified Raman spectra in the range of 350~520 cm−1 for 2% W-doped samples annealed at different temperatures; (c,d) the annealing temperature dependent FWHMs of the peaks at 192 and 223 cm−1; (e~g) the V-O related vibration mode positions of films with nominal 2 at.% W annealed at 450, 470 and 500 °C; (h) the annealing temperature dependent integrated intensity ratio of peaks at 580 and 614 cm−1.
Figure 3c,d are annealing temperature dependent full width at half maximum (FWHM) of peaks at 192 and 223 cm−1 for samples with different W doping. Figure 3c,d reveal that higher W content sample has larger FWHM of its Raman peaks; meanwhile, with increase of annealing temperature, the FWHMs of 192 and 223 cm−1 peak gradually decreased. These results illustrate that W introduced into VO2 induces its V-V involved Raman peaks broaden, which further suggests W doped in VO2 gave rise to additional stress randomly in the corresponding samples11, 28. However, samples annealed at higher temperature have narrowed FWHMs of the Raman peaks at 192 and 223 cm−1, which suggests that distortion or stress in the VO2 lattice degenerated, consistent with previous reports11, 28, 31.
Figure 3e,f are the magnified Raman spectra in range of 530~730 cm−1 with fitting results also shown in detail. Figure 3e,f suggest that one main peak located at 614 cm−1 and a weak shoulder located at 580 cm−1, the former one is belong to M1 phase VO2, and the later one is considered as an evidence for the existing of T-phase VO2 or the appearance of stress29,30,31,32. The fitted integrated intensity of these peaks for samples with nominal 1.5 and 2% W doping contents and annealed at different temperature are graphed in Fig. 3h.
Figure 3h indicates that increasing in doped W content, the stress of the produced samples enhanced remarkably. While the integrated intensity ratio of peaks at 580 and 614 cm−1 are drastically reduced with annealing temperature increasing, which suggests that the stress is reduced intensely in the corresponding samples32.
Therefore, the results in Fig. 3 demonstrate that increasing in annealing temperature leads to the growth of crystal grains, promotes W diffusion and eliminates its disordered distribution in VO2 lattice. Finally, the crystal quality of the corresponding samples improved with reduced doping related stress, consistent with our XRD study.
The XPS was performed to determine the constituents and element chemical states. Figure 4 is the XPS spectra of films with 1%, 1.5% and 2%W doping and annealed at 470 °C. Figure 4a suggests that O1s peak is located at 530.3 eV, and two distinct peaks of V2p3 at 517.3 eV and 515.9 eV. In the light of the results, the ΔE difference between O1s and V2p3 is 13 eV a little lower than 13.4 eV on previous studies33,34,35, which could be attributed to Vx+(x defines as the mixture of valence between V4+ and V5+ with dominating V4+) and V3+, respectively. Considering the characterization of XPS skill, the presence of fully oxidized vanadium could be originated from the partially oxidized surface36, 37 and also confirmed by surface etching23.
Figure 4b shows the XPS spectra of V and W ions, the insert to Fig. 4b is the details of the XPS spectra of W(4f5/2) and W(4f7/2). It is clear to see that binding energies of W(4f5/2) and W(4f7/2) are located 37.3 eV and 35.3 eV, conforming that the chemical valences of W ions are + 6. These results are consistent with the previous reports38. Compared with XPS spectra of different films, one can see that with increase of W content, the integrated intensity of V3+ gradually increased compared with that of Vx+. This result implies that W doping in VO2 denoted additional electrons that could have reduction effects on the neighbored V ions, which is the intrinsic reason for the increasing of the density of V3+, and finally, will induce the formation of VO2-δ, as demonstrated by our XRD results and previous studies39, 40.
Table 1 lists the integrated intensity ratios for V2p/W4f and Vx+/V3+, which are extracted from XPS result in Fig. 4. Table 1 indicates that as the nominal doped W content increased, the integrated intensity ratio of W to V enhanced, suggesting W ions doped in the lattice VO2.
However, the intensity of VO2-δ related peak observation in Fig. 1b disagrees with the measured XPS results in Fig. 4, which means that large domains with much reduced V formed in samples annealed at 500 °C. Previous studies revealed that inject high energy electrons into VO2 results in the reducing of V4+ to lower chemical states and generates oxygen vacancies25. Specially, the appearance of oxygen vacancies in VO2 could give raise to the relaxation of the chemical stress32. The results of Raman and XPS spectra study in this work support this idea indeed. Therefore, it is reasonable to postulate that there are plentiful oxygen vacancies in the studied samples, which results in some V4+ ions were reduced to V3+, consistent with previous temperature dependent resistance studies41, 42. Another reason for the generation of oxygen vacancies could be the escaping of oxygen from samples owing to annealing at high temperature under high vacuum. This postulation agrees with the Raman study results shown in Fig. 3, and is similar with the report by R. A. Aliev et al., where the authors demonstrated that the vanadium dioxide was reduced to V3+ as the oxygens escaped into a vacuum and a nonstoichiometric oxygen-poor vanadium dioxide films formed18.
Figure 5a,b are the temperature dependent transmittance (Tr) curves of 1.5%W doped sample recorded in 1000~4000 cm−1. The transmittance curves were measured in range of 24~90 °C with interval 2 °C. A series of infrared transmittance curves were obtained throughout the process upon heating and cooling, respectively. Figure 5a indicates that with temperature increasing from 24 to 90 °C, the transmittance of the studied sample gradually reduces in the measured infrared range, vice versa as indicated in Fig. 5b. These results demonstrate that a reversible MIT occurred, consisting with previous studies43. The temperature dependent transmittance of undoped, 1%, and 2% W-doped VO2 films annealed at 500 °C/4 h samples upon heating and cooling branches can be seen in the Figure S3 in the Supplementary Information. From the Figure S3, we can see that all the samples have the similar trends with that of 1.5% W-doped VO2. Fig. 5c~f are hysteresis loops of temperature dependent transmittance at 4000 cm−1(~2500 nm) obtained by extracting the transmittance at a fixed wavelength (2500 nm) at approximately 2 °C intervals for samples with undoped, nominal 1 at.%, 1.5 at.% and 2 at.% W-doped VO2 films annealed at 470 and 500 °C, respectively. The obvious switching in IR transmittance was observed in all samples. W-doped films annealed at 470 °C give low IR transmission switching ability (ΔTr) of 29.2%, 24.5% and 16.4% (defined as ΔTr = Tr24 °C-Tr90 °C) in spite of low transition temperatures (Tc) that is determined by (Tc-heating + Tc-cooling)/2, in which Tc-heating and Tc-cooling are the maximums of the dTr/dT in the heating and cooling cycle, consistent with Nb doped VO2 studies44. The ΔTr was increased drastically after increasing the annealing temperature to 500 °C, which are 42.5%, 48%, and 43.1% for 1%, 1.5% and 3% W doped samples, with slightly increasing transition temperatures, respectively. The details of W content dependent Tc and Twidth are graphed in Fig. 6, which indicates that with increase of W doping content, the Tc and Twidth of the corresponding samples decreased; meanwhile, increasing annealing temperature induces the Tc and Twidth of the corresponding samples increased. The Tc transforms 50.6 °C for undoped VO2 films to 36 °C for 2% W-doped films annealed at 470 °C/4 h (from 55.6 °C to 39.1 °C for films annealed at 500 °C/4 h) and decreases linearly with doping concentration. The solid lines are a linear fitting. The changed ratio of Tc is estimated to be about −7.5~−8.5 °C per 1 at.% of W doping and this change is lower than 21~28 °C/ W at.% but comparable to the value by another group45, 46. The lower rate of change in the critical temperature ΔTc as a function of W-doping concentration is a weakness in the studied samples in this work; however, Sol-Gel processing is simple, low-cost and productive. The much interesting result is that attenuated infrared characteristics induced by W-doping were enhanced remarkably, which were not achieved by conventional sputtering or pulsed laser deposition to date.
The infrared transmittance curves of the 1.5%W doped samples in the (a) heating and (b) cooling in the range of 1000–4000 cm−1. Hysteresis loops for the temperature dependent transmittance of (d) Undoped; (d) nominal 1 at.% W doped; (e) nominal 1.5 at.% W doped; (f) nominal 2 at.% W-doped VO2 films annealed at 470 and 500 °C.
In this work, all samples were annealed under the vacuum of 10−3 Pa and the resulting oxygen vacancies could weaken the decreasing trend induced from W-doping. The vacancies may act as the heterogeneous nucleation sites for the phase transition of undoped and W-doped VO2 films. Furthermore the phase change activation energy of MIT for undoped VO2 films could be lowered so the MIT temperature of undoped VO2 films was decreased far below 68 °C18, 42.
According to our Raman spectra study, with increasing in the content of W, Raman vibration peaks of the produced samples broadening, which implies its chemical stress enhanced due to the differences of ion radius between W6+(0.062 nm) and V4+(0.058 nm). The enhanced stress has been clarified of negative effects on the IR switching ability, which also confirmed by the variation of transmittance during the MIT11. However, with increasing annealing temperature, the chemical stress in samples with different W contents is reduced in spite of its grain size enhanced. This result could be attributed to compensative effect of the additional electrons carriers and generated oxygen vacancies as proved by our Raman and XPS spectra studies, which is consistent with previous reports18, 44, 47, 48.
Previous studies suggested that introduction of W would break V4+-V4+ bonds and the new V3+-W4+ and V3+-V4+ bonds formed and result in nonstoichiometric vanadium oxides, which destabilizes the semiconducting phase of VO2 and lowers the transition temperature of VO2 films from monoclinic phase to tetragonal phase14, 16. Recently, studies evidenced that W replaces V in VO2 lattice leading to local stress in VO2, which results in the decrease of the activation energy for the M-R phase transition, thus, reduce the MIT temperature39, 40. Furthermore, the existing of the internal or external stress on M phase VO2 causes its switching ability of infrared wave band deteriorating13, 23, 49. Accordingly, W doping in VO2 forms some W-rich domains, which results in the MIT temperature of different domains is different, thus, the global MIT temperature of samples much widened. As the temperature of samples rising, these W doped domains will gradually transfer to R Phase VO2, and finally percolation structures formed, consequentially, the width of the MIT broadened. Likewise, rising annealing temperature promotes the diffusion of W in VO2, which will smear the rich W domains and unify the distribution of W in VO2. In this way, the energy barrier for MIT enhanced, which will lead to the increase of the phase transition temperature of MIT with narrowed temperature width of its relaxation. This postulation indeed agrees the characteristics of the temperature dependent transmittance loops as shown in Figs 5 and 6. The optimized MIT properties was realized at nominal 1.5% W doped VO2 films annealed at 500 °C with MIT temperature about 43 °C and the variation of infrared transmittance about 48% in this work, and this result is the reported highest switching ability in IR transmittance as the MIT occurred. Another interesting result in Fig. 5 is that as the doped W is about 2%, plentiful second phase, namely VO2-δ, was formed as corroborated by the XRD and XPS studies in this work. The formation of second phase drastically gives rise to the degeneration of its switching ability about the infrared transmittance in the produced samples.
Based up the above results and analysis, modification of the electron carrier density by doping W and introducing oxygen vacancies would effectively realize the controlling of the MIT temperature and the switching ability of IR transmittance. Therefore, the results in this work supplies a clue to synthesize oxygen-deficient VO2 related functional materials that have potentials applications in fabricating electronic devices and smart windows.
Conclusion
In conclusion, W doped VO2 thin films with controlled oxygen vacancies were prepared by a modified sol-gel related mothed and followed by a post annealing under high vacuum. The films show much enhanced regulation performance in infrared transmittance as compared with conventional W-doping VO2 films. The enhanced infrared transmittance switching ability occurred at low temperature makes it as a suitable material for the applications to smart window coatings and novel electronic devices based on the thermochromic effects.
References
Morin, F. J. Oxides Which Show A Metal-To-Insulator Transition At The Neel Temperature. Phys Rev Lett 3, 34–36 (1959).
Anderson, G. Studies on VO2. Acta Chemic Scandiavica 10 (1956).
Marezio, M., McWhan, D. B., Remeika, J. P. & Dernier, P. D. Structural Aspects of the Metal-Insulator Transitions in Cr-Doped VO2. Phys Rev B 5, 2541–2551 (1972).
Goodenough, J. B. The two components of the crystallographic transition in VO2. J Solid State Chem 3, 490–500 (1971).
Bianconi, A. Multiplet Splitting Of Final-State Configurations In X-Ray-Absorption Spectrum Of Metal VO2: Effect Of Core-Hole-Screening, Electron Correlation, And Metal-Insulator-Transition. Phys Rev B 26, 2741–2747 (1982).
Christmann, T., Felde, B., Niessner, W., Schalch, D. & Scharmann, A. Thermochromic VO2 thin films studied by photoelectron spectroscopy. Thin Solid Films 287, 134–138 (1996).
Shin, S. et al. Vacuum-ultraviolet reflectance and photoemission study of the metal-insulator phase transitions in VO2, V6O13, and V2O3. Phys Rev B 41, 4993–5009 (1990).
Kang, L., Gao, Y. & Luo, H. A novel solution process for the synthesis of VO2 thin films with excellent thermochromic properties. Acs Appl Mater Interfaces 1, 2211–2218 (2009).
Richardson, M. A. & Coath, J. A. Infrared optical modulators for missile testing. Optics & Laser Technology 30, 137–140 (1998).
Chen, B., Yang, D., Charpentier, P. A. & Zeman, M. Al3+-doped vanadium dioxide thin films deposited by PLD. Sol Energ Mater Sol C 93, 1550–1554 (2009).
Marini, C. et al. Optical properties of V1-xCrxO2 compounds under high pressure. Phys Rev B 77 (2008).
Piccirillo, C., Binions, R. & Parkin, I. P. Synthesis and characterisation of W-doped VO2 by Aerosol Assisted Chemical Vapour Deposition. Thin Solid Films 516, 1992–1997 (2008).
Binions, R., Piccirillo, C. & Parkin, I. P. Tungsten doped vanadium dioxide thin films prepared by atmospheric pressure chemical vapour deposition from vanadyl acetylacetonate and tungsten hexachloride. Surface and Coatings Technology 201, 9369–9372 (2007).
Romanyuk, A., Steiner, R., Marot, L. & Oelhafen, P. Temperature-induced metal–semiconductor transition in W-doped VO2 films studied by photoelectron spectroscopy. Sol Energ Mater Sol C 91, 1831–1835 (2007).
Wang, N., Liu, S., Zeng, X. T., Magdassi, S. & Long, Y. Mg/W-codoped vanadium dioxide thin films with enhanced visible transmittance and low phase transition temperature. Journal of Materials Chemistry C 3, 6771–6777 (2015).
Tang, C. et al. Local atomic and electronic arrangements in WxV1-xO2. Phys Rev B 31, 1000–1011 (1985).
Cao, X., Wang, N., Magdassi, S., Mandler, D. & Long, Y. Europium Doped Vanadium Dioxide Material: Reduced Phase Transition Temperature, Enhanced Luminous Transmittance and Solar Modulation. Science of Advanced Materials 6, 558–561 (2014).
Aliev, R. A. et al. Effect of vacuum heat treatment on the metal-semiconductor phase transition in thin vanadium dioxide films. Tech Phys 50, 754–757 (2005).
Berezina, O. et al. Vanadium oxide thin films and fibers obtained by acetylacetonate sol–gel method. Thin Solid Films 574, 15–19 (2015).
Zhang, C., Cao, W., Adedeji, A. V. & Elsayed-Ali, H. E. Preparation and properties of VO2 thin films by a novel sol–gel process. Journal of Sol-Gel Science and Technology 69, 320–324 (2014).
Wu, J. et al. Effect of annealing temperature on thermochromic properties of vanadium dioxide thin films deposited by organic sol–gel method. Applied Surface Science 268, 556–560 (2013).
Zhang, Y. et al. Direct preparation and formation mechanism of belt-like doped VO2(M) with rectangular cross sections by one-step hydrothermal route and their phase transition and optical switching properties. Journal of Alloys and Compounds 570, 104–113 (2013).
Binions, R., Hyett, G., Piccirillo, C. & Parkin, I. P. Doped and un-doped vanadium dioxide thin films prepared by atmospheric pressure chemical vapour deposition from vanadyl acetylacetonate and tungsten hexachloride: the effects of thickness and crystallographic orientation on thermochromic properties. Journal of Materials Chemistry 17, 4652–4660 (2007).
Yan, J., Huang, W., Zhang, Y., Liu, X. & Tu, M. Characterization of preferred orientated vanadium dioxide film on muscovite (001) substrate. Physica Status Solidi (A) 205, 2409–2412 (2008).
Zhao, Y. et al. Structural, electrical, and terahertz transmission properties of VO2 thin films grown on c-, r-, and m-plane sapphire substrates. J Appl Phys 111, 053533 (2012).
Zhang, J. et al. Self-Assembling VO2 Nanonet with High Switching Performance at Wafer-Scale. Chem Mater 27, 7419–7424 (2015).
Schilbe, P. Raman scattering in VO2. Physica B-Condensed Matter 316, 600–602 (2002).
Arcangeletti, E. et al. Evidence of a pressure-induced metallization process in monoclinic VO2. Phys Rev Lett 98 (2007).
Radue, E. et al. Effect of a substrate-induced microstructure on the optical properties of the insulator-metal transition temperature in VO2 thin films. J Appl Phys 113, 233104 (2013).
Chen, F. H. et al. Control of the metal-insulator transition in VO2 epitaxial film by modifying carrier density. Acs Appl Mater Interfaces 7, 6875–6881 (2015).
Manning, T. D. et al. Intelligent window coatings: atmospheric pressure chemical vapour deposition of vanadium oxides. Journal of Materials Chemistry 12, 2936–2939 (2002).
Strelcov, E. et al. Local coexistence of VO2 phases revealed by deep data analysis. Sci Rep 6, 29216 (2016).
Silversmit, G., Depla, D., Poelman, H., Marin, G. B. & De Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J Electron Spectrosc 135, 167–175 (2004).
Li, W., Ji, S., Qian, K. & Jin, P. Preparation and characterization of VO2(M)–SnO2 thermochromic films for application as energy-saving smart coatings. J Colloid Interface Sci 456, 166–173 (2015).
Ji, S., Zhang, F. & Jin, P. Preparation of high performance pure single phase VO2 nanopowder by hydrothermally reducing the V2O5 gel. Sol Energ Mater Sol C 95, 3520–3526 (2011).
Warwick, M. E. A., Ridley, I. & Binions, R. Thermochromic vanadium dioxide thin films from electric field assisted aerosol assisted chemical vapour deposition. Surface and Coatings Technology 230, 163–167 (2013).
Crane, J., Warwick, M., Smith, R., Furlan, N. & Binions, R. The Application of Electric Fields to Aerosol Assisted Chemical Vapor Deposition Reactions. J Electrochem Soc 158, D62–D67 (2011).
Zhang, Y. et al. Preparation of W- and Mo-doped VO2(M) by ethanol reduction of peroxovanadium complexes and their phase transition and optical switching properties. Journal of Alloys and Compounds 544, 30–36 (2012).
Tan, X. et al. Unraveling Metal-insulator Transition Mechanism of VO2 Triggered by Tungsten Doping. Sci Rep 2, 466 (2012).
Wu, Y. et al. Depressed transition temperature of WxV1-xO2: mechanistic insights from the X-ray absorption fine structure (XAFS) spectroscopy. Physical Chemistry Chemical Physics: PCCP 16, 17705–17714 (2014).
Jeong, J. et al. Suppression of metal-insulator transition in VO2 by electric field-induced oxygen vacancy formation. Science (New York, N.Y.) 339, 1402–1405 (2013).
Appavoo, K. et al. Role of defects in the phase transition of VO2 nanoparticles probed by plasmon resonance spectroscopy. Nano Lett 12, 780–786 (2012).
Li, M. et al. Defect-mediated phase transition temperature of VO2 (M) nanoparticles with excellent thermochromic performance and low threshold voltage. Journal of Materials Chemistry A 2, 4520–4523 (2014).
Li, Y., Liu, Y., Liu, J. & Ren, L. The effects of niobium on the structure and properties of VO2 films. Journal of Materials Science: Materials in Electronics 27, 4981–4987 (2016).
Soltani, M., Chaker, M., Haddad, E., Kruzelecky, R. V. & Margot, J. Effects of Ti–W codoping on the optical and electrical switching of vanadium dioxide thin films grown by a reactive pulsed laser deposition. Applied Physics Letters 85, 1958–1960 (2004).
Chae, B. G. & Kim, H. T. Effects of W doping on the metal–insulator transition in vanadium dioxide film. Physica B: Condensed Matter 405, 663–667 (2010).
Griffiths, C. H. & Eastwood, H. K. Influence of stoichiometry on the metal‐semiconductor transition in vanadium dioxide. J Appl Phys 45, 2201–2206 (1974).
Lopez, R., Haynes, T. E., Boatner, L. A., Feldman, L. C. & Haglund, R. F. Size effects in the structural phase transition of VO2 nanoparticles. Phys Rev B 65, 224113 (2002).
Manning, T. D., Parkin, I. P., Pemble, M. E., Sheel, D. & Vernardou, D. Intelligent window coatings: Atmospheric pressure chemical vapor deposition of tungsten-doped vanadium dioxide. Chem Mater 16, 744–749 (2004).
Acknowledgements
We thank the National Natural Science Foundation of China (Grand No. 11474019) for financial support.
Author information
Authors and Affiliations
Contributions
X.L. supervised and coordinated all aspects of the project. G.P. synthesized and characterized the materials. K.J., H.J. and J.Y. carried out the measurement of optical properties and Raman characterizations. X.C. carried out the XRD characterizations and crystal structure analysis. J.L. carried out the AFM characterization and image analysis. All authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pan, G., Yin, J., Ji, K. et al. Synthesis and thermochromic property studies on W doped VO2 films fabricated by sol-gel method. Sci Rep 7, 6132 (2017). https://doi.org/10.1038/s41598-017-05229-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05229-9
This article is cited by
-
Towards Room Temperature Thermochromic Coatings with controllable NIR-IR modulation for solar heat management & smart windows applications
Scientific Reports (2024)
-
Hydrothermal synthesis and thermochromism effects in Eu-doped VO2 polycrystalline materials
Journal of Materials Science: Materials in Electronics (2023)
-
Composition evolution and electrical properties of VO2 thin films induced by annealing temperature
Journal of Sol-Gel Science and Technology (2022)
-
Iron-Alloyed Vanadium Dioxide Analysis Using X-Ray Photoelectron Spectroscopy Technique
Russian Physics Journal (2020)
-
Phase transition and changing properties of nanostructured V2O5 thin films deposited by spray pyrolysis technique, as a function of tungsten dopant
Applied Physics A (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.