Abstract
Although scleral buckling is a well-established surgical treatment for rhegmatogenous retinal detachment (RRD), the procedure can reportedly cause problems in the ocular circulation. Segmental scleral bucking without a concomitant encircling procedure was performed on 46 eyes with successfully reattached macula-on RRD. Choroidal blood flow was assessed using laser speckle flowgraphy. Spectral-domain optical coherence tomography was used to image macular regions, to measure the subfoveal choroidal thickness (SFCT), and to calculate the luminal and the stromal areas by the binarization method preoperatively and 1, 4, 8 and 12 weeks postoperatively. Choroidal mean blur rate at the macula did not significantly change, while that at the buckle and unbuckle side significantly reduced at 8 weeks postoperatively in the operated eye (P = 0.007 and P = 0.017, respectively). The SFCT and the luminal area increased temporarily 1 week following surgery in the operated eye (P < 0.001). The trend of SFCT with time coincided with that of the luminal area (P < 0.001). Venous drainage obstruction induced by compression force of scleral buckling leads to SFCT thickening in the acute postoperative phase. The macular choroidal blood flow might be less susceptible because the blood flow at the macula, in contrast to the other areas, does not change following segmental scleral buckling.
Similar content being viewed by others
Introduction
Rhegmatogenous retinal detachment (RRD), which is the detachment of the sensory retina from the retinal pigment epithelium (RPE) caused by breaks in the retina, is a sight-threatening pathology1. RRD is usually treated with either scleral buckling alone or pars plana vitrectomy (PPV) with or without concomitant scleral buckling.
Although scleral buckling is a well-established surgical treatment for RRD and the anatomical success rate is high2, the procedure can reportedly cause problems in the ocular circulation3,4,5,6. Experimentally, it was found that encircling buckling significantly decreased retinal and choroidal circulation using the microsphere technique in rabbit eyes3. Several authors reported that scleral buckling procedures reduced retinal4, 5, 7 and choroidal blood flow8,9,10 due to compressional mechanisms3, 11. However, very few reports evaluated choroidal blood flow following segmental scleral buckling without the encircling procedure for eyes with RRD.
A variety of techniques have been developed for measuring retinal blood flow, including fluorescein angiography, radioactive microspheres, hydrogen clearance, the laser Doppler technique4, 6, colour Doppler ultrasonography12, and the pulsatile technique8. Time intensiveness or problems with reproducibility have, however, hampered their widespread use. Accordingly, the number of eyes is low in most of studies using these methods.
Laser speckle flowgraphy (LSFG) (Softcare Co., Ltd., Fukutsu, Japan) is a non-invasive, real-time method used to measure relative blood flow for 4 s without the administration of contrast agents13, 14. LSFG can detect the speckle contrast pattern produced by the interference of illuminating laser light scattered by the movement of erythrocytes in the blood vessels, and it enables the measurement of relative blood flow in the vessels, expressed as the mean blur rate (MBR)13. The measurements have excellent reproducibility: the coefficient of variation (COV) was 4.7 on choroidal blood flow15. Therefore, LSFG was considered suitable for measuring ocular –including choroidal–blood flow.
Kimura et al. reported a temporary thickening of subfoveal choroidal thickness (SFCT) using spectral domain optical coherence tomography (SD-OCT) after segmental scleral buckling surgery16. They speculate that haemostasis and inflammation caused the temporal increase in SFCT.
The choroid is composed mainly of vessels and stroma (extravascular tissue) lacking a well-organised structure. However, it is difficult to differentiate the luminal area from the stromal area in the choroid in vivo. Recently, Sonoda et al. reported the use of a binarization method involving SD-OCT images, which can differentiate the choroidal luminal area from the stromal area and quantify the areas using Image-J software with high repeatability17. Therefore, it is important to understand what variation in the choroidal structure affects SFCT and to compare choroidal with luminal area blood flow.
Thus, this study aimed to evaluate the change in choroidal blood flow using LSFG following segmental scleral buckling procedures and the relationship between choroidal blood flow change and morphological change using SD-OCT.
Results
Patient demographics
Between August 2013 and March 2015, 114 eyes of 114 patients with RRD underwent segmental scleral buckling at our department for the repair of RRD. Of those, 68 eyes were excluded for macula-off (n = 42), vitreous haemorrhage (n = 1), glaucoma (n = 3), diabetic retinopathy (n = 1), or an incapacity to attend regular follow-up visits (n = 21). Included in final analysis were 46 eyes with macula-on RRD (mean age, 44.6 ± 17.4 years). Patient demographics and surgical procedures are shown in Table 1. The number of eyes undergoing examinations in each observation period was 46 at 1 week, 36 at 4 weeks, 32 at 8 weeks and 31 at 12 weeks following surgery. There were no cases of scleral buckling straddling extraocular vortex veins. The extent of quadrant-wise buckle was 91.5 ± 21.6 degrees.
Changes in choroidal MBR
In eyes affected by RRD, the mean macular choroidal MBR was 9.4 ± 3.2 arbitrary units (AU) before surgery, 9.9 ± 3.7 AU at 1 week, 9.4 ± 3.7 AU at 4 weeks, 8.9 ± 3.1 AU at 8 weeks and 9.3 ± 2.6 AU at 12 weeks following surgery (Fig. 1). There was no significant change in macular choroidal MBR over time in the affected or unaffected opposite eyes (Table 2). There was no significant difference in macular choroidal MBR between the affected eye group and the unaffected opposite eye group in the entire observation period.
Representative composite colour maps using the mean blur rate (MBR) as measured by laser speckle flowgraphy (LSFG) in eyes with rhegmatogenous retinal detachment (RRD) preoperatively and 1, 4 and 12 weeks postoperatively at the macula (A) and the buckle side (B). The red colour indicates a high MBR, and the blue colour indicates a low MBR. To measure choroidal MBR, the centre of a square was set at the fovea (250 × 250 pixels, degree: 6.31° × 6.31°) and the buckle and unbuckle side (60 × 60 pixels, degree: 1.5° × 1.5°). The mean change in MBR in the operated eye and the fellow eye. There was no significant change in macular choroidal blood flow during follow-up in the operated eye group or the fellow eye group (C). There was a significant reduction in choroidal MBR at the buckle and unbuckle side at 8 weeks after surgery compared with before surgery in the affected eyes (P = 0.007, P = 0.017, respectively).
In the eyes affected by RRD, the mean choroidal MBR at the buckle side was 7.4 ± 4.0 AU before surgery, 7.3 ± 3.3 AU at 1 week, 6.9 ± 3.8 AU at 4 weeks, 5.8 ± 2.7 AU at 8 weeks and 6.1 ± 2.6 AU at 12 weeks following surgery, and that at the unbuckle side was 7.8 ± 2.4 AU before surgery, 7.8 ± 2.6 AU at 1 week, 7.6 ± 2.4 AU at 4 weeks, 6.9 ± 2.4 AU at 8 weeks and 7.3 ± 2.5 AU at 12 weeks following surgery. There was a significant reduction in choroidal MBR at the buckle and unbuckle sides at 8 weeks following surgery compared with that before surgery in the affected eyes (P = 0.007 and P = 0.017, respectively).
The change in the choroidal MBR was not significantly correlated with the preoperative parameters of the refractive error, axial length, extent of quadrant-wise buckle (degrees), and extent of retinal detachment.
Morphologic changes in choroid
The mean SFCT in the operated eyes was 237.3 ± 71.1 µm before surgery, 263.5 ± 83.5 µm at 1 week, 240.1 ± 70.3 µm at 4 weeks, 232.6 ± 55.9 µm at 8 weeks and 232.1 ± 65.9 µm at 12 weeks following surgery (Fig. 2A). The mean SFCT at 1 week after surgery was significantly thicker than that before surgery (P < 0.001), while there was no significant difference between the SFCT preoperatively and after postoperative week 4. There was no significant change in SFCT over time in the fellow eyes.
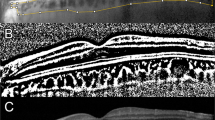
Horizontal SD-OCT images of an eye with macula-on RRD preoperatively and 1, 4 and 12 weeks postoperatively (A). The area of interest of the choroid is demarcated (B). The EDI-OCT image is converted to a binary image using ImageJ software (C). The rectangle surrounded by the red line was excised, and the dark areas were traced by the modified Niblack method. In the merged binarized images and the margins of traced areas, the light pixels were defined as the stromal, and the dark pixels as the luminal areas. SFCT at Week 1 was thicker than that preoperatively, and at weeks 4 and 12 postoperatively. The trend of SFCT with time coincided with that of the luminal area.
The mean choroidal, luminal and stromal areas were 0.360 ± 0.101 mm2, 0.233 ± 0.068 mm2 and 0.123 ± 0.041 mm2, respectively (Fig. 2). These significantly increased to 0.387 ± 0.111 mm2, 0.256 ± 0.077 mm2, 0.131 ± 0.039 mm2, respectively, at one week following surgery (P < 0.001, P < 0.001, P = 0.025, respectively) (Figs 2 and 3), while there was no significant difference in those areas in the fellow eyes throughout the follow-up period.
The mean subfoveal choroidal thickness (SFCT) at 1 week postoperatively was significantly thicker than that preoperatively in the operated eye (P < 0.001), while there was no significant change in the fellow eye throughout the follow-up period (A). The choroidal area (B), the luminal area (C) and the stromal area (D) at 1 week postoperatively were significantly increased compared with those preoperatively in the operated eye (P < 0.001, P < 0.001, P = 0.025, respectively), while there was no significant change in the fellow eye throughout the follow-up period.
SFCT significantly correlated with the choroidal, luminal, and stromal areas in the entire observation period (Table 3). The ratio of postoperative SFCT to preoperative SFCT correlated with that of the luminal area throughout the postoperative period but did not correlate with that of the stromal area throughout this period (Table 3).
Correlation between SFCT and other parameters
Regarding the correlation of variation of SFCT with that of other factors, the trend of SFCT coincided with that of the choroidal and luminal areas, IOP and macular choroidal MBR (P < 0.001, P < 0.001, P = 0.037, P = 0.039, respectively).
Multiple regression analysis in the ratio of SFCT and variable parameters at 1 week postoperatively to that preoperatively shows that only the ratio of SFCT was positively correlated with that of the luminal area (P = 0.018).
Discussion
Our results showed that macular choroidal blood flow determined by LSFG did not change, while the choroidal blood flow at the buckle and unbuckle sides was reduced at 8 weeks following segmental scleral buckling with cryopexy procedures in the operated eye. The SFCT and the luminal area determined by the binarization method increased temporarily 1 week postoperatively. The ratio of postoperative SFCT to preoperative SFCT correlated with the luminal area but did not correlate with the stromal area throughout the postoperative period. In addition, the trend of the SFCT coincided with that of the luminal area postoperatively, while there was no morphologic change over time in the fellow eye. Multiple regression analysis demonstrates the SFCT at 1 week postoperatively:preoperatively was positively correlated with that of the luminal area.
Many reports have described ocular blood flow reduction following scleral bucking, generally including the encircling procedure–the element generally agreed to affect ocular blood flow. While some reports showed a progressive reduction and no return to the baseline value in choroidal blood flow after the buckling procedure18, others found a return in choroidal blood flow 3 to 6 months postoperatively8, 10. All these reports–some controversial–suggested that compression on the peripheral vasculature by indentation caused by the encircling buckle caused the reduction in ocular blood flow. The discrepancy among reports is partly due to the dependence of blood flow rate reduction upon the degree of constriction of the encircling band. However, in the present study, there was no significant reduction in choroidal MBR at the macula postoperatively.
We contrastingly performed segmental (not encircling) scleral buckling with an average of 92 degrees in all cases. Our objectives, follow-up period and measuring method were different from those of other reports, precluding direct comparison. However, our results indicated that partial compression force induced by segmental scleral buckling does not change choroidal blood flow at the macula. Ito et al. reported that scleral buckling procedures can cause subclinical disturbance of the choroidal circulation, even if encircling procedures are avoided. However, their exoplant silicone sponge was much wider (Mira No. 507, 509 in addition to No. 506), and the extent of quadrant-wise buckle, not mentioned. Our result indicates that relatively less invasive procedure of segmental (without encircling) scleral buckling may not affect macular blood flow.
There have been few reports describing the change in SFCT following scleral buckling. Kimura et al. reported a mean 13% temporary thickening in SFCT following segmental scleral buckling, and no significant difference after postoperative month 316. Our result, with a temporary 10% thickening in SFCT, only at 1 week after segmental scleral buckling, demonstrates that our procedure causes less SFCT thickening than Kimura’s16, possibly due to the shorter extent of our quadrant-wise buckle (degrees) compared to Kimura’s average of 107 degrees. On the other hand, encircling reportedly causes greater thickening of SFCT, even in late postoperative phases19.
It has been reported that the percentage increase in SFCT is correlated with the percentage change in IOP after trabeculectomy20. This would indicate that changes in IOP can affect SFCT. However, in the present study, IOP marginally decreased by 2.3% at 1 week after surgery, although SFCT transiently increased by 10%. These results suggest that the decrease in IOP probably does not play the main role in the temporary thickening in SFCT at 1 week after surgery.
The exact mechanism by which scleral buckling affects SFCT remains unclear, but, the postoperative trend of SFCT determined by binarization coincided with that of the luminal area in our study, and multiple regression analysis shows that SFCT at 1 week postoperatively: preoperatively ratio was positively correlated only with that of the luminal area. Temporary alteration of the choroidal vessel area affects mainly the thickening of SFCT. Venous drainage obstruction induced by compression force of the scleral buckle apparently leads to the temporary thickening of SFCT in the acute postoperative phase, which would be related to compensation of the hemostasis in ocular circulation.
The stromal area was also temporally increased 1 week after segmental buckling in our binarization method, but changes did not coincide with those for SFCT. Histologically, the choroid comprises blood vessels and stromal tissues–the latter including pigment cells, smooth muscles, neurons, vascular walls, inflammatory cells and connective tissue–in which differentiation by binarization can increase inflammatory retinochoroidal disease21. Therefore, the temporary increase in stromal area indicates that ocular inflammation might contribute to the thickening of SFCT. One report attributes the temporary increase in SFCT to periocular inflammation caused by cryotherapy16, 19.
We know of no reports describing the relation between macular choroidal blood flow and morphological change following RRD surgery. Our results show that macular choroidal blood flow does not change and is not correlated with choroidal morphological change following segmental scleral buckling, perhaps because neither the increment of SFCT nor the luminal area is extensive, and venous drainage obstruction at the macula is exiguous.
In contrast to the macula, the choroidal blood flow in the buckle side and unbuckle side was significantly reduced at the late postoperative period of 8 weeks following scleral buckling surgery. Using the pulsatile ocular blood flow, Yokota et al. reported that the choroidal blood flow decreases after scleral buckling; this can reflect the whole choroidal blood flow8. It is not possible to accurately measure blood flow in areas with retinal detachment using LSFG. Accordingly, we measured blood flow at the buckle side in area without retinal detachment, which results that the area is not close to the area with the buckling element in the most of the present cases.
The compression force caused by segmental buckling on the peripheral vasculature probably does not increase with time postoperatively. In addition, as a treatment for RRD, significant changes in choroidal blood flow have also been reported following vitrectomy22. Accordingly, alternative mechanisms to compression forces would cause the late appearance of the reduction of the choroidal blood flow except for the macula. Trans-scleral cryopexy leads a devoid of choriocapillaris and choroidal large vessel and loss of the photoreceptor, which has the potential to cause a reduction in choroidal blood flow. The late appearance of the reduction in the choroidal blood flow at the buckle and unbuckle sides would be caused by cryopexy rather than the mechanical compression force from segmental buckling. The choroidal blood flow close to the buckling element should decrease because the choroid undergoing cryopexy gets atrophied with time. These results suggest that the entire choroidal blood flow would be reduced after buckling surgery because of the reduction of blood flow to some areas, except for the macula.
Macular choroidal blood flow is enriched to supply nutrition to the dense macular photoreceptor area for survival. In addition, choroidal thickness in the macular region is higher than in other areas. Taken together, the partial compression force induced by a relatively less invasive procedure of segmental (not encircling) scleral buckling may not affect macular blood flow, differing from other choroidal areas.
Limitations of this study included small sample size and short-term follow-up. Diurnal variations in choroidal thickness23, 24 and blood flow25 reportedly occur; similar diurnal patterns were reported on different days in SFCT24. We performed all examinations at approximately 12 PM to avoid diurnal variations, and SFCT in the fellow eyes showed no significant differences preoperatively and postoperatively, suggesting that diurnal fluctuations had little influence in our study. In addition, the first postoperative examination was at 1 week in our study, and SFCT may have been thicker at an earlier postoperative period than postoperative 1 week. Further studies with a larger number of patients and a wide range of postoperative periods are needed.
Our study indicates that venous drainage obstruction induced by partial compression force of scleral buckling leads to the thickening of SFCT in the acute postoperative phase. Although these results require careful interpretation since previous investigations used different parameters and measurement methods. We conclude that macular choroidal blood flow might be less susceptible to partial compression force because the blood flow at the macula, in contrast to the other areas, does not change following segmental scleral buckling.
Methods
Ethics statement
In this retrospective, cross-sectional single-centre study, the procedures used were approved by the Ethics Committee of the Nagoya University Hospital (Nagoya, Japan). The study conformed to the tenets of the Declaration of Helsinki.
Subjects
We reviewed the medical records of all patients who had undergone segmental scleral buckling procedures at the Nagoya University Hospital from July 2013 to March 2015. All patients signed an informed consent form before surgery.
All patients underwent a comprehensive ophthalmic examination including the measurement of intraocular pressure (IOP) and axial length, slit-lamp examination, fundus examination and OCT preoperatively and 1,4, 8 and 12 weeks postoperatively.
All patients were asked to abstain from alcoholic and caffeinated beverages on the morning of the day of the examination. The pupil was dilated 30 minutes before the examinations. The subjects rested for 10 to 15 min in a quiet dark room before the examination. All examinations were performed in the sitting position at approximately 12 PM to avoid diurnal variations16, 23, 25. The axial lengths were measured with partial optical coherence interferometry (IOLMaster; Carl Zeiss Meditec, La Jolla, CA). The IOP was measured with a handheld tonometer (Icare; TiolatOy, Helsinki, Finland). The systolic blood pressure (SBP) and the diastolic blood pressure (DBP) were measured with an automatic sphygmomanometer (CH-483C; Citizen, Tokyo, Japan). The mean arterial blood pressure (MAP) and mean ocular perfusion pressure (MOPP) were calculated as follows: MAP = DBP + 1/3(SBP-DBP), MOPP = 2/3MAP − IOP.
LSFG
The LSFG-NAVI was used to determine choroidal blood flow. The principles of LSFG have been described in detail26. To evaluate macular choroidal blood flow, the centre of a rectangle (250 × 250 pixels, degree: 6.31° × 6.31°) was placed at the fovea (Fig. 1A). To evaluate choroidal blood flow at the buckle and unbuckle sides, a rectangle (60 × 60 pixels, degree: 1.5° × 1.5°) was placed at the area where visible retinal vessels were not observed (Fig. 1B). The LSFG was measured three times at each time-point in all eyes, and the averages of the variables were calculated. The area same as the operated eye in the fellow eye was used as the control.
Measurement of choroidal thickness and differentiation of luminal and stromal areas
Choroidal images were obtained by SD-OCT (Spectralis OCT, Heidelberg Engineering, Heidelberg, Germany). The choroidal thickness was measured with SD-OCT using the enhanced depth-imaging (EDI) technique. The SFCT was measured as the distance from the hyper-reflective RPE line to the choroid–sclera border with the calliper tool on SD-OCT.
Binarization of the choroidal area in the EDI-OCT image was performed by the modified Niblack’s method17. The EDI-OCT image was analysed using ImageJ software (ImageJ version 1.47, NIH, Bethesda, MD). The examined area was 1,500 μm wide in the subfoveal choroid, extending vertically from the RPE to the chorioscleral border (Fig. 3). The light pixels were defined as the interstitial areas, and the dark pixels were defined as the luminal areas. After adding the data of the distance of each pixel, the luminal and interstitial areas were automatically calculated. Two clinicians –masked to the other findings–measured the area.
Surgical technique
In all patients, retinal breaks were identified and treated by transscleral cryotherapy. Mattress sutures were placed 7.0 to 7.5 mm apart with 4–0 supramid (Kono, Chiba, Japan) for the circumferential segmental buckle and a silicone sponge (Mira No. 506; Mira, Inc, Waltham, MA) was sutured as an explant in all cases. Scleral dissection, extraocular muscle disinsertion and concomitant encircling were not required for any patients. Subretinal fluid drainage was performed if necessary. Intraocular tamponade was not used for all cases. Dexamethasone (MSD K.K., Tokyo, Japan) was injected subconjunctivally at the end of surgery.
No intraoperative complication was encountered. Reattachment of the retina was achieved in all patients in the initial surgery.
Exclusion Criteria
The exclusion criteria included presence of any optic disc abnormalities such as glaucoma or optic disc atrophy, history of intraocular surgery, presence of vitreous haemorrhage, severe cataract and medical conditions that could influence the haemodynamics of the eye, such as diabetes, hypertension, arrhythmia and vascular diseases.
Statistical analyses
We evaluated the changes in choroidal MBR, SFCT, choroidal area, luminal area, stromal area and IOP, MOPP and flare and the correlation of variation of SFCT with that of other factors from five fixed time points (before and 1, 4, 8 and 12-week(s) after surgery). The strength of the mixed model for analysis of dynamic, longitudinal data is that it can describe each individual’s pattern of change even in the face of missing data points27. Therefore, we used a mixed model to incorporate the appropriate covariates between repeated measured values over time.
Specifically, we assumed the following model:
i(subject)=1, …, 46, j(time) = 0, 1, 4, 8, 12 where yij is the variables at time j on subject i; \({a}_{i}\) is a subject-specific random effect; Gi = 1 indicates affected eye and 0 indicates unaffected eye. The function f(tj:b) represents a fixed effect of time on the variables. For the residual term eij of the values, we assumed a heterogeneous compound symmetry structure within patients.
All statistical analyses were performed with SAS9.4 (SAS Inc., Cary). A P value < 0.05 was considered statistically significant.
References
D’Amico, D. J. Clinical practice. Primary retinal detachment. N Engl J Med. 359, 2346–2354 (2008).
Campo, R. V. et al. Pars plana vitrectomy without scleral buckle for pseudophakic retinal detachments. Ophthalmology. 106, 1811–1815; discussion 1816 (1999)
Diddie, K. R. & Ernest, J. T. Uveal blood flow after 360 degrees constriction in the rabbit. Arch Ophthalmol 98, 729–730 (1980).
Ogasawara, H. et al. Retinal blood flow alterations associated with scleral buckling and encircling procedures. Br J Ophthalmol. 76, 275–279 (1992).
Yoshida, A. et al. Retinal circulatory changes after scleral buckling procedures. Am J Ophthalmol. 95, 182–188 (1983).
Yoshida, A., Hirokawa, H., Ishiko, S. & Ogasawara, H. Ocular circulatory changes following scleral buckling procedures. Br J Ophthalmol. 76, 529–531 (1992).
Eshita, T. et al. Retinal blood flow in the macular area before and after scleral buckling procedures for rhegmatogenous retinal detachment without macular involvement. Jpn J Ophthalmol. 48, 358–363 (2004).
Yokota, H., Mori, F., Nagaoka, T., Sugawara, R. & Yoshida, A. Pulsatile ocular blood flow: changes associated with scleral buckling procedures. Jpn J Ophthalmol. 49, 162–165 (2005).
Nagahara, M., Tamaki, Y., Araie, M. & Eguchi, S. Effects of scleral buckling and encircling procedures on human optic nerve head and retinochoroidal circulation. Br J Ophthalmol. 84, 31–36 (2000).
Sugawara, R. et al. Choroidal blood flow in the foveal region in eyes with rhegmatogenous retinal detachment and scleral buckling procedures. Br J Ophthalmol. 90, 1363–1365 (2006).
Dobbie, J. G. Circulatory changes in the eye associated with retinal detachment and its repair. Trans Am Ophthalmol Soc. 78, 503–566 (1980).
Roldan-Pallares, M., Musa, A. S., Hernandez-Montero, J. & Bravo-Llatas, C. Preoperative duration of retinal detachment and preoperative central retinal artery hemodynamics: repercussion on visual acuity. Graefes Arch Clin Exp Ophthalmol. 247, 625–631 (2009).
Sugiyama, T., Araie, M., Riva, C. E., Schmetterer, L. & Orgul, S. Use of laser speckle flowgraphy in ocular blood flow research. Acta Ophthalmol. 88, 723–729 (2010).
Nagahara, M., Tamaki, Y., Tomidokoro, A. & Araie, M. In vivo measurement of blood velocity in human major retinal vessels using the laser speckle method. Invest Ophthalmol Vis Sci. 52, 87–92 (2011).
Aizawa, N. et al. Reproducibility of retinal circulation measurements obtained using laser speckle flowgraphy-NAVI in patients with glaucoma. Clin Ophthalmol. 5, 1171–1176 (2011).
Kimura, M. et al. Subfoveal choroidal thickness change following segmental scleral buckling for rhegmatogenous retinal detachment. Am J Ophthalmol. 154, 893–900 (2012).
Sonoda, S. et al. Choroidal structure in normal eyes and after photodynamic therapy determined by binarization of optical coherence tomographic images. Invest Ophthalmol Vis Sci. 55, 3893–3899 (2014).
Ito, Y. et al. Effects of scleral buckling without encircling procedures on retrobulbar hemodynamics as measured by color Doppler imaging. Arch Ophthalmol. 123, 950–953 (2005).
Odrobina, D., Laudanska-Olszewska, I., Gozdek, P., Maroszynski, M. & Amon, M. Influence of scleral buckling surgery with encircling band on subfoveal choroidal thickness in long-term observations. Biomed Res Int. 2013, 586894 (2013).
Yoshikawa, M. et al. Longitudinal change in choroidal thickness after trabeculectomy in primary open-angle glaucoma patients. Jpn J Ophthalmol. (2016)
Kawano, H. et al. Relative changes in luminal and stromal areas of choroid determined by binarization of EDI-OCT images in eyes with Vogt-Koyanagi-Harada disease after treatment. Graefes Arch Clin Exp Ophthalmol. 254, 421–426 (2016).
Vetrugno, M., Gigante, G. & Cardia, L. The choroidal circulation after retinal detachment surgery. Clin Hemorheol Microcirc. 21, 349–352 (1999).
Usui, S. et al. Circadian changes in subfoveal choroidal thickness and the relationship with circulatory factors in healthy subjects. Invest Ophthalmol Vis Sci. 53, 2300–2307 (2012).
Chakraborty, R., Read, S. A. & Collins, M. J. Diurnal variations in axial length, choroidal thickness, intraocular pressure, and ocular biometrics. Invest Ophthalmol Vis Sci. 52, 5121–5129 (2011).
Iwase, T. et al. Diurnal variations in blood flow at optic nerve head and choroid in healthy eyes: diurnal variations in blood flow. Medicine (Baltimore) 94, e519 (2015).
Sugiyama, T., Utsumi, T., Azuma, I. & Fujii, H. Measurement of optic nerve head circulation: comparison of laser speckle and hydrogen clearance methods. Jpn J Ophthalmol. 40, 339–343 (1996).
Edwards, L. J. Modern statistical techniques for the analysis of longitudinal data in biomedical research. Pediatr Pulmonol. 30, 330–344 (2000).
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
The design and conduct of the study (T.I.); collection of data (T.I., M.K., K.Y., K.Y.); management, analysis, and interpretation of data (T.I., M.K., K.Y., E.R., H.T.); and preparation, review, and approval of the manuscript (T.I., M.K., K.Y., K.Y., E.R., H.T.).
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iwase, T., Kobayashi, M., Yamamoto, K. et al. Change in choroidal blood flow and choroidal morphology due to segmental scleral buckling in eyes with rhegmatogenous retinal detachment. Sci Rep 7, 5997 (2017). https://doi.org/10.1038/s41598-017-05126-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-05126-1
This article is cited by
-
Short-term postoperative changes in the choroidal vascularity index in patients with a unilateral epiretinal membrane
BMC Ophthalmology (2023)
-
A pilot study of scleral thickness in central serous chorioretinopathy using anterior segment optical coherence tomography
Scientific Reports (2021)
-
Choroidal vascular changes after encircling scleral buckling for rhegmatogenous retinal detachment
Eye (2021)
-
Primary rhegmatogenous retinal detachment: risk factors for macular involvement
Graefe's Archive for Clinical and Experimental Ophthalmology (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.