Abstract
Stress during gestation has harmful effects on pregnancy outcome and can lead to spontaneous abortion. Few studies, however, have addressed the impact of gestational stress, particularly auditory stress, on behavioural performance and pregnancy outcome in mice. This study aimed to examine the effect of two types of gestational stress on uterus receptivity and behavioural performance. Pregnant C57BL/6 mice were randomly assigned to either auditory or physical stress conditions or a control condition from gestational days 12–16. The auditory stress regimen used loud 3000 Hz tone, while the physical stressor consisted of restraint and exposure to an elevated platform. Three behavioural tests were performed in the dams after weaning. Uterine receptivity was investigated by counting the number of sites of implantation and fetal resorption. Also, the offspring survival rates during the early postnatal period were calculated. Auditory stress caused an increase in anxiety-like behaviour, reduced time spent exploring new object/environment, and reduced balance when compared to the physical stress and control groups. Auditory stress also caused higher rates of resorbed embryos and reduction of litter size. Our results suggest that the adverse effect of noise stress is stronger than physical stress for both uterus receptivity and behavioural performance of the dams.
Similar content being viewed by others
Introduction
The nervous system has high resilience to the influence of diverse components of the maternal environment during fetal development. The noxious effects of maternal environmental factors such as alcohol, drugs, smoking, and stress cause significant stress on fetal development in humans and experimental animals1,2,3,4. Specifically, environmental aversive stimuli such as noise exposure play a critical role in shaping brain and behaviour5. In addition to the harmful effect of gestational stress on offspring’s brain structure and function, the adverse effect of stress during pregnancy on the female’s physiology and behaviour can persist even beyond parturition6,7,8. Furthermore, stress during gestation can constantly affect endocrine state, body weight9, 10, and maternal performance in pregnant and lactating laboratory rodents6, 11, 12.
Clinical studies show higher incidences of autoimmune diseases, allergies, infections, and cancer following stressful situations13,14,15. The adverse effect of stress on reproductive function has been reported in many studies16,17,18,19,20. For instance, the effect of stress on implantation and fetal growth during pregnancy can lead to spontaneous abortion16, 20, 21. Stress also alters the immune system in mammals, and altered immune function is likely a principal cause of spontaneous abortions14, 16, 22. Moreover, exposing pregnant mice to a short period of ultrasonic-acoustic stress early in gestation can significantly increase the rate of spontaneous abortion14, 16, 20. Such an increase in abortion rate could be the result of inhibition of protective suppression and promotion of tumor necrosis factor-alpha (TNF-alpha) release in the uterus decidua16, 20.
Infanticide is observed in approximately 35 to 50% of adult male mice and rats and 10% or less in adult females23,24,25. Although numerous studies have demonstrated detrimental effects from crowded housing conditions, daily handling, forced swimming, loud noise, heat and bright light, and physical restraint on pregnancy outcome in rodents20, 26, 27, the effects of gestational stress on the loss of pups due to eating or killing them by mothers in the early postnatal period is unknown. Pregnant mice in previous studies were typically euthanized at a specific gestational age, to study the effect of gestational auditory stress on uterus receptivity by counting the number of implanted and resorbed embryos16, 18, 19. Therefore, the impact of auditory stress during pregnancy on loss of pups in the first postnatal days remains unclear.
Auditory stress is often defined as an unwanted and unpleasant auditory stimulus that leads to physiological, behavioural, and biochemical changes in humans and nonhuman animals. High levels of acoustic stimulation (90–105 dB) severely impair the hypothalamic–pituitary–adrenal (HPA) axis activation in both dams and offspring28. In the present study, we examined the effects of two types of stress, specifically auditory stress (AS) and physical stress (PS), on pregnancy outcome and postpartum behaviours of mice. We acquired several measures of pregnancy outcomes for analysis including weight gain during pregnancy, pregnancy duration, the number of implantation sites, the number of reabsorption signs on the uterus, and the number of live and lost pups. To address the influence of gestational stress on behaviour, we employed three behavioural tests to examine animals cognitive function, motor control and balance performance: novel object recognition (NOR) test29, 30, elevated plus maze (EPM)31,32,33,34,35, and balance beam test (BBT)36. Although there are many behavioural tests designed to score animals behaviour in mice, we used these behavioural tests for two main reasons. First, they require no training, external motivation, or reward enabling us to monitor signs of implantation sites and resorbed embryos within a short time after weaning. Second, their ability to show the effects of stress on rodents’ behaviour has been previously well documented36,37,38,39. The NOR test, which assesses recognition memory, is widely used for investigating a wide range of cognitive, memory, and neuropsychological functions30, 40, 41. The EPM is a popular measure of laboratory rodent anxiety levels, where the difference between time spent in the open and closed arms provides an index of anxiety-like behavior in mice31, 32, 42. Similarly, the BBT is a sensitive test for early detection of balance deficits in rodents43. In view of the high rate of abortion in mice exposed to AS in the past studies16, 18,19,20, we hypothesised that AS would have a more detrimental effect on both pregnancy outcome and behavioural performance compared to PS.
Results
The ages of the female mice were similar across the groups (F2,27 = 0.697, p = 0.507). Since no significant differences were observed between the two control groups in any of the measures used in this study (p > 0.05), the results of the control groups were pooled together.
Behavioural tests
Novel object recognition (NOR) test
Mice exposed to the AS spent significantly less time (sec) with the new object compared to those mice in the control group (Fig. 1A). Mice in both stress groups (AS and PS) spent more time with the old object compared to those animals in the control group but the difference was not statistically significant (F2,27 = 2.526, p = 0.101, η2 = 0.114). The ratio of time spent with old compared to the new object was significantly higher in the AS group than the two other groups (Fig. 1B). This experiment thus suggests that the gestational exposure to auditory stress reduces the dams’ ability to distinguish a new object from one that has been encountered previously (Table 1).
The novel object recognition (NOR) test: (A) the auditory stress group significantly spent shorter time with the new object compared with the control group. (B) The ratio of time spent with old compared with new object was significantly higher in the auditory stress group than the two other groups. N = 10 in all groups. Results are reported as mean ± S.E.M. Asterisks indicate *p < 0.05 or **p < 0.01.
Elevated plus maze (EPM) test
Table 1 shows that the AS group spent significantly less time (sec) in the open arms and had a lower number of entries to open arms, when compared to the other two groups. Figure 2 compares means of variables in all groups. This experiment thus demonstrates the anxioselectivity of the gestational exposure to auditory stress on dams on plus maze anxiety.
Balance beam test (BBT)
The AS dams were slower in crossing the beam (latency to cross, sec), had higher number of foot slips, and higher number of turns compared to the other groups (Table 1). Figure 3 illustrates summary results for measured variables among the three groups.
The balance beam test (BBT): The auditory stress group revealed (A) longer latency to travel across the beam (sec), (B) higher number of foot slips, and (C) higher number of turns compared with the two other groups. N = 10 in all groups. Results are reported as mean ± S.E.M. Asterisks indicate *p < 0.05 or **p < 0.01.
Mouse uterus receptivity and pregnancy outcomes
Pregnancy duration was the same across all three groups (control group mean: 21.80 ± 0.91 day, PS group mean: 21.30 ± 1.33 day, and AS group mean: 21.5 ± 0.97 day; F2,27 = 0.531, p = 0.594, η2 = 0.038). Table 2 provides a summary of the statistical analyses of uterus receptivity and pregnancy outcome among the three groups. The dams in the AS group had significantly lower weight gain during pregnancy than either the PS or control dams (Fig. 4A). The number of live pups on the first PD (Fig. 4B), as well as the number of live pups on the second PD (Fig. 4C) was significantly lower in the AS group than the other groups. The difference between the number of pups on the first and the second PDs was not significant (F2,27 = 2.103, p = 0.159, η2 = 0.087). Moreover, the number of pups did not change after the second day of birth. Although the number of observed uterus implantation sites were similar in the three groups (control group mean: 5.78 ± 2.11; PS group mean: 6.1 ± 1.44, and AS group mean: 5.0 ± 2.22, F2,27 = 0.715, p = 0.498, η2 = 0.053), the number of resorbed signs, and the number of lost pups was significantly higher in the AS group compared to the other two groups (Fig. 4D–F). The difference between the number of surviving pups and the number of implantation sites was significant in the AS group (control group: F1,9 = 1.011, p = 0.343, η2 = 0.100; PS group: F1,9 = 2.250, p = 0.168, η2 = 0.200; AS group: F1,9 = 11.676, p = 0.008, η2 = 0.565). Figure 4Fi-iii, shows representative examples comparing the implantation sites and the reabsorption signs of uteri from each of the groups.
Pregnancy outcome: The auditory stress group obtained (A) lower weight gain during pregnancy (g), (B) decreased number of pups on the first postnatal day (PD), (C) fewer pups on the second PD, (D) higher number of resorbed signs, and (E) higher number of lost pups. N = 10 in all groups. Results are reported as mean ± S.E.M. Asterisks indicate *p < 0.05. Uterus receptivity: (F) A sample of marked uterus from each study group (fi = control, fii = physical stress, fiii = auditory stress). Black lines show the implantation sites and red lines show the resorbed signs.
Overall, the PS group showed a trend toward the same pattern of results as the AS group (Figs 1–4), but the differences with the control group were not significant (p’s > 0.05).
Discussion
Pregnant mice were assessed on three behavioural tests to investigate the effects of gestational acoustic and physical stress on exploratory, cognitive, and balance abilities on the dams. To evaluate recognition memory30 in our control and stressed groups, we used the novel object recognition (NOR) test. In the NOR test, the dams in the AS group spent significantly less time with the new object than the old one when compared to the control and PS groups. This reduced tendency for exploring the new object in the AS group suggests that the gestational exposure to auditory stress may cause a cognitive memory impairment. The EPM measures anxiety and was designed based on the competing natural tendencies of rodents to explore novel places and their innate behaviour to avoid unprotected, bright, and elevated places. The AS dams spent significantly less time in the open arms and showed a lower number of entries into open arms44. The reduced number of entries into open arms and time spent in the open arms in the AS mice indicates higher anxiety levels than the PS and control groups31, 32, 35, 44, 45.
Noise exposure can markedly produce behavioural and biochemical changes in both animals and humans. In general, subjecting animals to stress causes the dysregulation of HPA axis and can lead to various types of mood disorders, such as depression and anxiety15, 31, 46, 47. Specifically, high levels of environmental noise correlates with the psychological symptoms and the occurrence of psychiatric disorders48. Furthermore, similar to other types of stress, the AS can increase levels of stress hormones such as corticosterone and norepinephrine49,50,51. In recent decades, there is growing evidence showing detrimental effects of the long-term outcomes of the gestational stressful experiences on the psychological functioning of offspring8. Moreover, various epidemiological studies in humans demonstrate that stress during pregnancy increases the risk for anxiety- and depression-related disorders in children15, 52,53,54,55,56,57. Hence, the results from the NOR and EPM in the present study are consistent with previous literature, and demonstrate the long lasting effects of gestational stress on the mental wellbeing of mothers.
Balance behaviour is a complex ability that requires vestibular, skeletal muscle, and cerebellar-circuits for motor control. Abnormal physiological functions of these key systems are influenced by aging, structural damage, and genetic factors and can lead to balance-related dysfunction (e.g., deficits in posture, foot placement, and targeting)58. However, there is little information regarding how balance ability is affected by exposing mice to gestational stressors. In the present study, we investigated the crossing time, the number of foot slips and number of turns to assess balance and motor coordination. Mice in the AS group showed negative impacts in all measures compared to the PS and control groups. These results are consistent with a previous finding36 indicating that chronic exposure to low frequency noise at moderate levels (100 Hz with 70 dB SPL) impairs balance coordination in mice. The authors proposed that the effect was largely due to vestibular abnormalities. In view of the few studies regarding the effect of noise stress during gestation on the motor and balance coordination in the dams and offspring, we suggest investigations into the influence of noise characteristics (i.e., intensity, frequency spectrum, and duration) on balance performance as well as neuroendocrine system in mice.
The negative effect of gestational stress on reproductive function is a topic of interest that has been previously well-documented14, 16, 18, 19. Loud noise and physical restraint may represent stressful conditions that can influence pregnancy outcomes in rodents16, 20. Stress, for instance, has detrimental effects on fertility, mating behaviour, ovulation, implantation, fetal growth, and lactation in animals21, 59,60,61. Also, low birth weight, reduced litter sizes, and lower survival rates are adverse pregnancy outcomes induced by gestational stress62,63,64,65,66. In the present study, average weight gain was lower in the stressed groups and in particular, it was significantly lower in the AS group compared to the other groups. This lower weight gain could be the result of both abortion (resorbed embryos) and implantation rate in the AS group. In addition to significantly higher resorption rate in the AS group, the average number of implantations was lower in the AS group than the PS and the control groups, although the difference was not significant. In this regard, Zamora et al.67 reported significantly decreased blastocyst production in mice exposed to increased housing rack noise. The mean noise was 80.4 dBC in the ventilated rack and 69.2 dBC in the static rack, and the authors showed a reduced blastocyst count in bred C57BL/6 donor mice housed in ventilated cages.
Furthermore, the number of the live pups observed on the first PD in the current study was significantly lower in the AS group than the other groups, and it was slightly decreased on the second PD. It was not possible in this study to determine the pups’s health status at that age point. The number of live pups, however, did not change in the next post-delivery days. In addition, investigating uterus receptivity by counting the number of resorbed signs and implantation sites14, 16 indicated a remarkably higher number of resorbed signs in the AS group than the control group. The number of survived pups in the AS group was also significantly lower than the number of implantation sites compared with the other groups. However, no significant difference was observed among the three groups in terms of the number of implantations. In accordance with the behavioural findings, the reduced uterus receptivity and the low rate of survived pups in the AS group indicated a stronger effect of the AS than the PS exposure in the dams. An altered immune response due to gestational stress is the most likely reason for the high rate of spontaneous abortion in mammals14, 16, 22. Subjecting pregnant mice to a brief period of ultrasonic- acoustic stress during gestation increases the abortion rate owing to increased levels of TNFα and transforming growth factor β2 (TGFβ2). Both factors decrease the immune system activity and negatively affect the uterus receptivity14, 16, 20.
While the adverse effect of stress on reproduction by influencing the implantation of embryos and increasing the rate of abortion has been previously addressed16, 20, 21, there is no report of eating or killing pups as a result of gestational stress. Our results indicated that AS reduced the number of pups on the first early PD. It is difficult to determine how much of this loss is specifically the result of killing pups, because the health condition of eaten pups was unknown. Although it was not investigated in the current study, changes in nest building can provide a general view of a mouse’s anxiety state, and is an indication of psychological distress68 in gestationally stressed dams. Thus, assessing nest structure and quality in a systematic manner69, 70 in future studies would be a useful way to test anxiety-like behaviour in stressed mice during peripartum and the early postpartum days.
Gestational stress can produce long-lasting dysfunction of the HPA and hypothalamo–gonadal (HPG) axes71, 72, and can elevate corticosterone levels in dams and fetuses73. Brummelte and Galea74 demonstrated that injecting corticosterone into pregnant dams negatively affected postpartum maternal care and decreased the time spent in nursing pups. The 11þ-hydroxysteroid dehydrogenase-2 (11þ-HSD-2) is an enzyme that is present in the placenta and central nervous system, and converts extra levels of corticosterone to relatively inactive products in normal pregnancies. In mid-gestation the expression of this enzyme is greatly decreased in the rodents75,76,77, and it seems that stress during pregnancy can decrease it further. While the loss of pups in our study could be associated with maternal stress and excess levels of corticosterone in the dams, female testosterone is another possible mechanism for this behaviour. Infanticide by adult males is modulated by the presence or absence of testosterone, and castration can reduce this behaviour78. Furthermore, depending upon the dose and duration of testosterone treatment, 35% to 100% of the rodent dams can be induced to kill their pups79. Therefore, investigating how hormones and other biochemical, epigenetic, or neurogenic factors change in the female mice under gestational AS needs to be addressed in future studies.
The PS paradigm used in our study failed to induce anxiety-like behaviour. Previous studies used a period of restraint ranging from 30 or 45 min three times a day for a week, either as a single stressor or combined with other stressors80,81,82,83,84,85,86. A recent study used a 30 min restraint stress paradigm for three times a day between GDs 5 and 19 in mice, affected the dams’ depressive-like, but not their anxiety-like behaviour86. Due to procedural and organizational demands, we were unable to apply a longer period of restraint stress in the present study. Hence, our results could be affected by the shorter period of stress exposure than previous studies. Thus, it seems that replication of our methodology in the same mouse strain with longer PS exposure could provide further information on the effects of stress on pregnant mice. Contrary to the effects of PS, AS induced greater anxiety-like behaviour along with significant alterations in pregnancy outcome.
Loud noises are usually a source of information, such as warnings for risks, strains, or dangers in many species, and they readily induce HPA, autonomic, and behavioural responses87. Also, long term exposure to high levels of corticosterone downregulates the glucocorticoid receptors and makes a vicious endocrine cycle of increased corticosterone levels and enhanced responsiveness to stress. On the other hand, the glucocorticoid receptors of the organ of Corti that respond to both systemic stress and acoustic stimulation play an important role in the mechanism of down regulation of glucocorticoid receptors88. Sleep deprivation and disruption is also a factor that might influence our findings in the AS group, since it can activate the stress-responsive regulatory systems89 and significantly affect corticosterone and adrenocorticotropic hormone secretion90. In addition, noise exposure leads to increased aggression91, interpersonal animosity92, 93, and curtailment of prosocial behaviour in humans94, 95. These can be taken as indications of hostility and generalized aggression96. These findings from human studies along with our findings here, highlight the importance of further studies on noxious effects of gestational AS on brain and behaviour in human and laboratory animals in the future.
There are some procedural limitations that should be considered in this study. First, although we used well-known protocols for exposing animals to AS and PS, these types of experimental stressors are different to some extent than those typical auditory and physical stress observed in real life. Thus, experimental conditions that provide more analogous models of stress to natural stressors are recommended for future studies. The second limitation of our finding is that we did not measure corticosterone levels, which might have allowed us to compare the relative severity of the two different types of stressors. Nevertheless, even in the absence of hormonal indicator of the stress response, we believe that the alterations found in the present study demonstrates a difference in the effects of the two stressors. In addition, investigating the health condition of the AS pups who did not survive after birth, as well as exploring maternal behaviour of the dams during the late peripartum and the early postpartum periods68, 70 can help to make clear the influence of pups health as well as maternal anxiety on loss of pups in the early PD. Finally, pre-exposure to an elevated platform as a stressor for the PS group could influence the later behaviour of the PS dams in both EPM and BBT. It might cause an attenuating or boosting effect on the results that can be examined in the future by adding another PS group free of elevated platform stress.
Methods
Animals
All experiments were carried out in accordance with the Canadian Council of Animal Care and approved by the University of Lethbridge Animal Care Committee. All animals were given access to food and water ad libitum and were maintained on a 12:12-h light:dark cycle in a temperature-controlled breeding room (21 °C) with less than 66 ± 2 dBC room noise level. Thirty female C57BL/6 mice were individually mated with thirty male C57BL/6 mice in standard shoe-box cages. Mice were singly housed once the pregnancy was confirmed. The rate of weight gain during pregnancy (grams), duration of pregnancy (days), number of the live pups on the first PD, and number of the survived pups during the early postnatal period (P0-P4) were calculated for each mouse.
Recordings of gestational length
Female mice between 8 to 11 weeks of age were housed with a male at 4:00 pm. The female mice were assessed three hours later at 7:00 pm and the next morning for breeding signs such as sperm plug and red/swollen vaginal opening28. The female mice were considered possibly pregnant only if the breeding signs were present on both observations. Female mice with a negative sign of the breeding were not paired with male mice for the period of 11 days until the lack of pregnancy was confirmed. The weight gain of the female mice was followed every day to confirmed pregnancy. On the gestational day (GD) 11, a weight gain of at least 3.5 g usually signifies conception has occurred. This method allows a determination of the length of gestation with a 0.5-day precision.
Experimental design
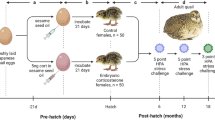
Pregnant mice were randomly assigned into either auditory or physical stress groups or a control group. We administered gestational stress during days 12–16 of gestation because the significant section of the corticogenesis process occurs between embryonic days 12–162, 97.
Gestational AS group
On gestational days (GDs) 12, 14, and 16 a pregnant mouse was transferred into a standard cage and moved to a sound chamber. Dams (n = 10) were presented with an intermittent 1 sec tone (3 KHz; 90 dB)14, 16, 19 with an inter-stimulus interval of 15 seconds for 24 hrs period starting at 8:00 am.
Gestational PS group
Two stressors, restraint and elevated platform (EP), were applied daily from GDs 12 through 16. For restraint, mice (n = 10) were maintained in a transparent Plexiglas container (5 cm inner diameter), 20 minutes per day at 10:00 am. The container maintained the mice in a standing position without compression of the body98, 99. For the EP stressor, each mouse was placed on an elevated platform (1 m height, 21 × 21 cm), 30 minutes twice a day at 9:00 am and 3:00 pm4, 38.
Control group
There were two sets of control animals: one served as a control for AS dams and another was a control for PS dams. In AS control group, pregnant mice (n = 5) on GDs 12, 14, and 16 were individually transferred into a standard cage and moved to the sound chamber. The dams were left undisturbed for 24hrs starting at 8:00am. In PS control group, pregnant mice during GDs 12–16 (n = 5) were transferred daily from their home cage to the same testing room used for the PS group. The dams left undisturbed for 20 or 30 minutes (depending on the type of stressor) and later were returned to their home cages.
Behavioural assessments
We used three behavioural tests after weaning (21–23 days after birth) to measure the effect of gestational stress on the dams’ cognitive and balance performance. The behavioural tests began one day after weaning and were completed 4 days later. NOR, EPM, and BBT tests were conducted respectively on separate days every morning between 9–10 am.
Novel object recognition (NOR) test
Each mouse was placed in an open-field arena (47 cm width × 50 cm length × 30 cm height) made of white Plexiglas. In the first trial, the mouse was placed in the arena with two identical objects and explored the field for 5 min. The animal was removed and placed in a transport box for 3 min, and one of the objects was randomly replaced with a new object. The mouse was then returned to the arena, and the animal’s exploration was filmed (30 frames/second) for 3 minutes. Time spent with each object was only calculated during the second session. If the nose of the mouse was within 1 cm of the object, it was considered to be in contact with an object38, 39. The ratio of time spent with the old compared to the new object was calculated by subtracting times spent with ‘old’ from the new object divided by the total time spent for exploration100.
Elevated plus maze (EPM) test
The EPM apparatus consisted of 3 main components: a base (48 cm), two open arms (5 cm × 27 cm), and two closed arms (5 cm × 27 cm × 21 cm). The apparatus, made of black Plexiglas®, was housed in a well-lit empty testing room. The camera for filming was placed at the end of an open arm slightly above the maze. Mice were placed with their front paws in the centre area and facing a closed arm. Each mouse was filmed (30 frames/sec) for 5 min and scored for time spent in open arms and number of entries to open arms32, 42. Animals were considered in an arm when the front half of the body was within the arm34, 101. The center zone connecting all arms was excluded33.
Balance beam test (BBT)
Mice were required to traverse an elevated, narrow aluminium beam (1 cm diameter, 100 cm long and 50 cm above a foam pad to cushion falling mice) to reach an enclosed escape box. Mice were first trained (4–5 trials) and were tested (3 trials) on the next day. We calculated the mean latency (sec), distance travelled, number of foot slips, number of turns, and number of falls across the 3 testing trials102. Only the BBT needed two test days, one day for the training trials and the following day for the testing trials.
Mouse uterus receptivity
Upon completing the behavioural tests, all dams were euthanized, 28–40 days after parturition and their uteri were removed. Uterus receptivity was investigated by counting the number of implantation sites and reabsorption signs17. The placenta is a flattened circular organ in the uterus of pregnant mammals, nourishing and maintaining the fetus through the umbilical cord103. In Figure 4F, each dark spot marked by a black line reflects an implantation site or the place of an embryo connected to the placenta; and each pale sign marked by a red line, indicates a resorbed sign or the place of an embryo aborted or disconnected from the placenta17. To measure the effect of gestational stress on the number of surviving pups, we periodically observed each pregnant mouse inside her standard shoe-box cage and looked for the new pups or any changes in the number of pups within 3 days before the estimated birth time, in order to determine if there were any preterm deliveries, as well as 5 days after the parturition. The number of lost pups during the early postnatal period (P0–P4) was calculated by subtracting the number of surviving pups from the number of identified implantation sites in the uteri.
Statistical analysis
All statistical analyses were done using SPSS Statistics 24.0 using an alpha level of 0.05. We used the Kolmogorov–Smirnov test for normally distributed data. To test for differences between the three studied groups for age, different parameters of the three behavioural tests, as well as the pregnancy outcome and the uterus receptivity including weight gain due to pregnancy (gram), pregnancy duration (day), number of the live pups on the first PD, number of surviving pups, number of the resorbed signs, and number of the implantation sites we used Multivariate analysis of variance (MANOVA). To compare the number of live pups on the first and the second PDs as well as the number of surviving pups and the number of implantation sites a repeated measures ANOVA was used. For multiple comparisons of group means in each measurement, the Tukey post-hoc test was performed.
References
Huizink, A. C. & Mulder, E. J. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev 30, 24–41 (2006).
Kolb, B., Mychasiuk, R., Muhammad, A. & Gibb, R. Experience and the developing prefrontal cortex. Proc Natl Acad Sci USA 109, 17186–17193 (2012).
Mychasiuk, R., Muhammad, A., Carroll, C. & Kolb, B. Does prenatal nicotine exposure alter the brain’s response to nicotine in adolescence? A neuroanatomical analysis. Eur J Neurosci 38, 2491–2503 (2013).
Mychasiuk, R., Gibb, R. & Kolb, B. E. Prenatal stress alters dendritic morphology and synaptic connectivity in the prefrontal cortex and hippocampus of developing offspring. Synapse 66, 308–314 (2012).
Jafari, Z., Kolb, B. E. & Mohajerani, M. H. Effect of acute stress on auditory processing: a systematic review of human studies. Rev Neurosci 28, 1–13 (2017).
Klaus, T., Schopper, H. & Huber, S. Effects of chronic stress during pregnancy on maternal performance in the guinea pig (Cavia aperea f. porcellus). Behav Processes 94, 83–88 (2013).
Yao, Y. et al. Ancestral exposure to stress epigenetically programs preterm birth risk and adverse maternal and newborn outcomes. BMC Med 12, 121 (2014).
Babenko, O., Kovalchuk, I. & Metz, G. A. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci Biobehav Rev 48, 70–91 (2015).
Baker, S. et al. Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain Res 1213, 98–110 (2008).
Heiming, R. S. et al. Living in a dangerous world decreases maternal care: a study in serotonin transporter knockout mice. Horm Behav 60, 397–407 (2011).
Meek, L. R., Dittel, P. L., Sheehan, M. C., Chan, J. Y. & Kjolhaug, S. R. Effects of stress during pregnancy on maternal behavior in mice. Physiol Behav 72, 473–479 (2001).
Smith, J. W., Seckl, J. R., Evans, A. T., Costall, B. & Smythe, J. W. Gestational stress induces post-partum depression-like behaviour and alters maternal care in rats. Psychoneuroendocrinology 29, 227–244 (2004).
McGregor, A. M. Immunoendocrine interactions and autoimmunity. N Engl J Med 322, 1739–1741 (1990).
Arck, P. C. et al. Stress-induced murine abortion associated with substance P-dependent alteration in cytokines in maternal uterine decidua. Biol Reprod 53, 814–819 (1995).
Fine, R., Zhang, J. & Stevens, H. E. Prenatal stress and inhibitory neuron systems: implications for neuropsychiatric disorders. Mol Psychiatry 19, 641–651 (2014).
Arck, P. C., Merali, F. S., Manuel, J., Chaouat, G. & Clark, D. A. Stress-triggered abortion: inhibition of protective suppression and promotion of tumor necrosis factor-alpha (TNF-alpha) release as a mechanism triggering resorptions in mice. Am J Reprod Immunol 33, 74–80 (1995).
Joachim, R. et al. The progesterone derivative dydrogesterone abrogates murine stress-triggered abortion by inducing a Th2 biased local immune response. Steroids 68, 931–940 (2003).
Haque, S. F. et al. Anesthesia and acoustic stress-induced intra-uterine growth retardation in mice. J Reprod Dev 50, 185–90 (2004).
Kondoh, E. et al. Stress affects uterine receptivity through an ovarian-independent pathway. Hum Reprod 24, 945–953 (2009).
Clark, D. A., Banwatt, D. & Chaouat, G. Stress-triggered abortion in mice prevented by alloimmunization. Am J Reprod Immunol 29, 141–147 (1993).
Wiebold, J. L., Stanfield, P. H., Becker, W. C. & Hillers, J. K. The effect of restraint stress in early pregnancy in mice. J Reprod Fertil 78, 185–192 (1986).
Dorian, B. & Garfinkel, P. E. Stress, immunity and illness: a review. Psychol Med 17, 393–407 (1987).
Rosenberg, K. M., Denenberg, V. H., Zarrow, M. X. & Frank, B. L. Effects of neonatal castration and testosterone on the rat’s pup-killing behavior and activity. Physiol Behav 7, 363–368 (1971).
Gandelman, R. Induction of pup killing in female mice by androgenization. Physiol Behav 9, 101–102 (1972).
Svare, B. Steroidal influences on pup-killing behavior in mice. Horm Behav 13, 153–164 (1979).
Mirkes, P. E. & Cornel, L. A comparison of sodium arsenite- and hyperthermia-induced stress responses and abnormal development in cultured postimplantation rat embryos. Teratology 46, 251–259 (1992).
Glockner, R., Schwarz, S. & Jahne, F. Enhanced effect of chronic stress on pregnancy outcome in Uje:WIST rats by prenatal treatment with lithium. Exp Toxicol Pathol 45, 35–37 (1993).
Barzegar, M., Sajjadi, F. S., Talaei, S. A., Hamidi, G. & Salami, M. Prenatal exposure to noise stress: anxiety, impaired spatial memory, and deteriorated hippocampal plasticity in postnatal life. Hippocampus 25, 187–196 (2015).
Burgado, J. et al. Two weeks of predatory stress induces anxiety-like behavior with co-morbid depressive-like behavior in adult male mice. Behav Brain Res 275, 120–125 (2014).
Grayson, B. et al. Assessment of disease-related cognitive impairments using the novel object recognition (NOR) task in rodents. Behav Brain Res 285, 176–193 (2015).
Amico, J. A., Mantella, R. C., Vollmer, R. R. & Li, X. Anxiety and stress responses in female oxytocin deficient mice. J Neuroendocrinol 16, 319–324 (2004).
Calabrese, E. J. An assessment of anxiolytic drug screening tests: hormetic dose responses predominate. Crit Rev Toxicol 38, 489–542 (2008).
McBrayer, Z. L. et al. Forebrain-Specific Loss of BMPRII in Mice Reduces Anxiety and Increases Object Exploration. PLoS One 10, e0139860 (2015).
Raza, S., Harker, A., Richards, S., Kolb, B. & Gibb, R. Tactile stimulation improves neuroanatomical pathology but not behavior in rats prenatally exposed to valproic acid. Behav Brain Res 282, 25–36 (2015).
Solati, J., Kleehaupt, E., Kratz, O., Moll, G. H. & Golub, Y. Inverse effects of lipopolysaccharides on anxiety in pregnant mice and their offspring. Physiol Behav 139, 369–374 (2015).
Tamura, H. et al. Chronic exposure to low frequency noise at moderate levels causes impaired balance in mice. PLoS One 7, e39807 (2012).
Pellow, S. & File, S. E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol. Biochem Behav 24, 525–529 (1986).
Muhammad, A., Carroll, C. & Kolb, B. E. Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience 216, 103–109 (2012).
Richards, S., Mychasiuk, R., Kolb, B. & Gibb, R. Tactile stimulation during development alters behaviour and neuroanatomical organization of normal rats. Behav Brain Res 231, 86–91 (2012).
Dere, E., Huston, J. P. & De Souza Silva, M. A. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev 31, 673–704 (2007).
Ennaceur, A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res 215, 244–254 (2010).
Campos, A. C., Fogaça, M. V., Aguiar, D. C. & Guimarães, F. S. Animal models of anxiety disorders and stress. Rev Bras Psiquiatr 35(Suppl 2), S101–111 (2013).
Brooks, S. P. & Dunnett, S. B. Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci 10, 519–529 (2009).
Muhammad, A. & Kolb, B. E. Prenatal tactile stimulation attenuates drug-induced behavioral sensitization, modifies behavior, and alters brain architecture. Brain Res 1400, 53–65 (2011).
File, S. E. Behavioural detection of anxiolytic action. In: Experimental approaches to anxiety and depression. Elliott, J. M., Heal, D. J. & Marsden, C. A. Editors Wiley: New York, 25–44 (1992).
Cryan, J. F. & Holmes, A. The ascent of mouse: advances in modelling human depression and anxiety. Na. Rev Drug Discov 4, 775–790 (2005).
Kim, M. H. & Leem, Y. H. Chronic exercise improves repeated restraint stress-induced anxiety and depression through 5HT1A receptor and cAMP signaling in hippocampus. J Exerc Nutrition Biochem 18, 97–104 (2014).
Stansfeld, S. A. & Matheson, M. P. Noise pollution: non-auditory effects on health. Br Med Bull 68, 243–257 (2003).
Brandenberger, G., Follenius, M., Wittersheim, G. & Salame, P. Plasma catecholamines and pituitary adrenal hormones related to mental task demand under quiet and noise conditions. Biol Psychol 10, 239–252 (1980).
Agnes, F., Sartorelli, P., Abdi, B. H. & Locatelli, A. Effect of transport loading or noise on blood biochemical variables in calves. Am J Vet Res 51, 1679–1681 (1990).
Chandralekha, G., Jeganathan, R., Viswanathan & Charan, J. C. Serum leptin and corticosterone levels after exposure to noise stress in rats. Malays J Med Sci 12, 51–56 (2005).
Torrey, E. F., Rawlings, R. R., Ennis, J. M., Merrill, D. D. & Flores, D. S. Birth seasonality in bipolar disorder, schizophrenia, schizoaffective disorder and stillbirths. Schizophr Res 21, 141–149 (1996).
Watson, J. B., Mednick, S. A., Huttunen, M. & Wang, X. Prenatal teratogens and the development of adult mental illness. Dev Psychopathol 11, 457–466 (1999).
Brown, A. S., van Os, J., Driessens, C., Hoek, H. W. & Susser, E. S. Further evidence of relation between prenatal famine and major affective disorder. Am J Psychiatry 157, 190–195 (2000).
O’Connor, T. G., Heron, J., Golding, J., Beveridge, M. & Glover, V. Maternal antenatal anxiety and children’s behavioural/emotional problems at 4 years. Report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry 180, 502–508 (2002).
O’Connor, T. G., Heron, J., Golding, J., Glover, V. & ALSPAC Study Team. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry 44, 1025–1036 (2003).
Van den Bergh, B. R., Van Calster, B., Smits, T., Van Huffel, S. & Lagae, L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology 33, 536–545 (2008).
Matsumura, B. A. & Ambrose, A. F. Balance in the elderly. Clin Geriatr Med 22, 395–412 (2006).
Weir, M. W. & De Fries, J. C. Blocking of pregnancy in mice as a function of stress. Psychol Rep 13, 365–366 (1963).
Pratt, N. C. & Lisk, R. D. Effects of social stress during early pregnancy on litter size and sex ratio in the golden hamster (Mesocricetus auratus). J Reprod Fertil 87, 763–769 (1989).
Tennessen, J. B., Parks, S. E. & Langkilde, T. Traffic noise causes physiological stress and impairs breeding migration behaviour in frogs. Conserv Physiol 2, cou032 (2014).
de Catanzaro, D. Effect of predator exposure upon early pregnancy in mice. Physiol Behav 43, 691–696 (1988).
Pratt, N. C. & Lisk, R. D. Role of progesterone in mediating stress-related litter deficits in the golden hamster (Mesocricetus auratus). J Reprod Fertil 92, 139–146 (1991).
Brunton, P. J. & Russell, J. A. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J Neuroendocrinol 22, 258–271 (2010).
Paris, J. J., Brunton, P. J., Russell, J. A. & Frye, C. A. Immune stress in late pregnant rats decreases length of gestation and fecundity, and alters later cognitive and affective behaviour of surviving pre-adolescent offspring. Stress 14, 652–664 (2011).
Brunton, P. J. Effects of maternal exposure to social stress during pregnancy: consequences for mother and offspring. Reproduction 146, R175–189 (2013).
Zamora, B. M. et al. Decreased blastocyst production in mice exposed to increased rack noise. J Am Assoc Lab Anim Sci 48, 486–491 (2009).
Jirkof, P. Burrowing and nest building behavior as indicators of well-being in mice. J Neurosci Methods 234, 139–146 (2014).
Deacon, R. M. Assessing nest building in mice. Nature protocols 1, 1117–1119 (2006).
Okayama, T., Goto, T. & Toyoda, A. Assessing nest-building behavior of mice using a 3D depth camera. J Neurosci Methods 251, 151–157 (2015).
Pedersen, C. A., Caldwell, J. D., McGuire, M. & Evans, D. L. Corticotropin-releasing hormone inhibits maternal behavior and induces pup-killing. Life Sci 48, 1537–1546 (1991).
Del Cerro, M. C., Ortega, E., Gómez, F., Segovia, S. & Pérez-Laso, C. Environmental prenatal stress eliminates brain and maternal behavioral sex differences and alters hormone levels in female rats. Horm Behav 73, 142–147 (2015).
Ohkawa, T. et al. Effect of an acute maternal stress on the fetal hypothalamo-pituitary-adrenal system in late gestational life of the rat. Exp Clin Endocrinol 98, 123–129 (1991).
Brummelte, S. & Galea, L. A. Chronic corticosterone during pregnancy and postpartum affects maternal care, cell proliferation and depressive-like behavior in the dam. Horm Behav 58, 769–779 (2010).
Burton, P. J., Smith, R. E., Krozowski, Z. S. & Waddell, B. J. Zonal distribution of 11 beta-hydroxysteroid dehydrogenase types 1 and 2 messenger ribonucleic acid expression in the rat placenta and decidua during late pregnancy. Biol Reprod 55, 1023–1028 (1996).
Diaz, R., Brown, R. W. & Seckl, J. R. Distinct ontogeny of glucocorticoid and mineralocorticoid receptor and 11beta-hydroxysteroid dehydrogenase types I and II mRNAs in the fetal rat brain suggest a complex control of glucocorticoid actions. J Neurosci 18, 2570–2580 (1998).
Cottrell, E. C. & Seckl, J. R. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci 3, 19 (2009).
Gandelman, R. & Vom Saal, F. S. Pup-killing in mice: the effects of gonadectomy and testosterone administration. Physiol Behav 15, 647–651 (1975).
Rosenberg, K. M. Effects of pre- and postpubertal castration and testosterone on pup-killing behavior in the male rat. Physiol Behav 13, 159–161 (1974).
Fleming, D. E., Anderson, R. H., Rhees, R. W., Kinghorn, E. & Bakaitis, J. Effects of prenatal stress on sexually dimorphic asymmetries in the cerebral cortex of the male rat. Brain Res Bull 16, 395–398 (1986).
Szuran, T., Zimmermann, E. & Welzl, H. Water maze performance and hippocampal weight of prenatally stressed rats. Behav Brain Res 65, 153–155 (1994).
Jones, H. E. et al. Prenatal stress alters the size of the rostral anterior commissure in rats. Brain Res Bull 42, 341–346 (1997).
Weinstock, M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev 32, 1073–1086 (2008).
Charil, A., Laplante, D. P., Vaillancourt, C. & King, S. Prenatal stress and brain development. Brain Res Rev 65, 56–79 (2010).
Salari, A. A., Fatehi-Gharehlar, L., Motayagheni, N. & Homberg, J. R. Fluoxetine normalizes the effects of prenatal maternal stress on depression- and anxiety-like behaviors in mouse dams and male offspring. Behav Brain Res 311, 354–367 (2016).
Weinstock, M. Prenatal stressors in rodents: Effects on behavior. Neurobiol Stress 6, 3–13 (2016).
Radley, J., Morilak, D., Viau, V. & Campeau, S. Chronic stress and brain plasticity: Mechanisms underlying adaptive and maladaptive changes and implications for stress-related CNS disorders. Neurosci Biobehav 58, 79–91 (2015).
Hebert, S., Paiement, P. & Lupien, S. J. A physiological correlate for the intolerance to both internal and external sounds. Hear Res 190, 1–9 (2004).
Meerlo, P., Sgoifo, A. & Suchecki, D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep Med Rev 12, 197–210 (2008).
Born, J. et al. Night-time plasma cortisol secretion is associated with specific sleep stages. Biol Psychiat 21, 1415–1424 (1986).
Dzhambov, A. & Dimitrova, D. Neighborhood noise pollution as a determinant of displaced aggression: a pilot study. Noise Health 16, 95–101 (2014).
Bull, A. J. et al. Effects of noise and intolerance of ambiguity upon attraction for similar and dissimilar others. J Soc Psychol 88, 151–152 (1972).
Ward, L. M. & Suedfeld, P. Human responses to highway noise. Environ Res 6, 306–326 (1973).
Sherrod, D. R. & Downs, R. Environmental determinants of altruism: The effects of stimulus overload and perceived control on helping. J Exp Soc Psychol 10, 468–479 (1974).
Mathews, K. E. & Canon, L. K. Environmental noise level as a determinant of helping behavior. J Pers Soc Psychol 32, 571–577 (1975).
Geen R.G. Effects of attack and uncontrollable noise on aggression. J Res Pers 12 (1978).
Kolb, B., Mychasiuk, R., Muhammad, A. & Gibb, R. Brain plasticity in the developing brain. Prog Brain Res 207, 35–64 (2013).
Ward, I. D. et al. Transgenerational programming of maternal behaviour by prenatal stress. BMC Pregnancy Childbirth 13, S9 (2013).
Erickson, Z. T., Falkenberg, E. A. & Metz, G. A. Lifespan psychomotor behaviour profiles of multigenerational prenatal stress and artificial food dye effects in rats. PLoS One 9, e92132 (2014).
Hannesson, D. K., Howland, J. G. & Phillips, A. G. Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats. J Neurosci 24, 4596–4604 (2004).
Muhammad, A. et al. Training on motor and visual spatial learning tasks in early adulthood produces large changes in dendritic organization of prefrontal cortex and nucleus accumbens in rats given nicotine prenatally. Neuroscience 252, 178–189 (2013).
Stover, K. R., Campbell, M. A., Van Winssen, C. M. & Brown, R. E. Analysis of motor function in 6-month-old male and female 3xTg-AD mice. Behav Brain Res 281, 16–23 (2015).
Chamley, L. W. et al. Review: where is the maternofetal interface? Placenta 35(Suppl), S74–80 (2014).
Acknowledgements
We would like to acknowledge Navvab Afrashteh, Erin Falkenberg, and Nasrin Soltanpour for their assistance with the experiment. We also would like to thank Dr. Michael Kyweriga for critical reading of the manuscript. This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant #40352 (MHM), Campus Alberta for Innovation Program Chair, Alberta Alzheimer Research Program (MHM), a Canadian Institute for Advanced Research grant (BK), and Canadian Institutes of Health Research Grant #102652 (GM). This study was part of a postdoctoral fellowship to ZJ in Canadian Center for Behavioural Neuroscience (CCBN) at the University of Lethbridge. ZJ would like to thank the Iran University of Medical Sciences (IUMS) sabbatical leave committee for their approval of her study leave.
Author information
Authors and Affiliations
Contributions
Z.J., B.E.K. and M.H.M. designed the study, Z.J., B.M.A. and J.F. performed the experiments. Z.J. analyzed the data, Z.J., M.H.M. and B.E.K. wrote the manuscript, which all authors commented on and edited.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jafari, Z., Faraji, J., Mirza Agha, B. et al. The Adverse Effects of Auditory Stress on Mouse Uterus Receptivity and Behaviour. Sci Rep 7, 4720 (2017). https://doi.org/10.1038/s41598-017-04943-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-04943-8
This article is cited by
-
Noise and mental health: evidence, mechanisms, and consequences
Journal of Exposure Science & Environmental Epidemiology (2024)
-
Local immune recognition of trophoblast in early human pregnancy: controversies and questions
Nature Reviews Immunology (2023)
-
Developmental Programming in Animal Models: Critical Evidence of Current Environmental Negative Changes
Reproductive Sciences (2023)
-
Prenatal noise stress impairs HPA axis and cognitive performance in mice
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.