Abstract
Interleukin 38 (IL-38) is a novel identified cytokine of IL-1 family in which some members are important in inflammation and angiogenesis. However, the role of IL-38 in regulating angiogenesis is unknown. The aim of the present study is to explore the effect of IL-38 on angiogenesis. Oxygen-induced retinopathy (OIR) of C57BL/6 J mice was induced by exposure of hyperoxia (75% oxygen) from postnatal day 7 (P7) to P12 and then returned to room air. The mice were injected with IL-38. At P17, neovascular region (tufts) and avascular area of the retinas were analyzed. The data showed that administration of IL-38 in vivo inhibited retinal angiogenesis significantly. Furthermore, the addition of IL-38 to the cell cultures attenuated the proliferation, scratch wound healing and tube formation of vascular endothelial cells induced by VEGF significantly. Our findings suggest that IL-38 is an antiangiogenic cytokine in pathophysiological settings and may have therapeutic potential for angiogenesis related diseases.
Similar content being viewed by others
Introduction
Angiogenesis is a process of new blood vessels sprouting from pre-existing vasculature, which has an important role in reproduction, organ regeneration and wound repair1, 2. Pathological angiogenic signaling results in disordered neovascularization and persist for years, such as retinopathy of prematurity (ROP), age-related macular degeneration (AMD), corneal neovascularization (CNV), tumor formation, rheumatoid arthritis and atherosclerosis. Angiogenesis is tightly orchestrated by a range of angiogenic cytokines such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), transforming growth factor β (TGF-β) and interleukin 1β (IL-1β)3 and antiangiogenic cytokines. These cytokines act on the process of proliferation and migration of endothelial cells (ECs) which is regarded as the hallmark of angiogenesis4, 5. The current intervention of modulating the prominent angiogenic cytokines such as anti-VEGF therapy has shown efficacy in ocular neovascularisation6. However, a proportion of patients are refractory to anti-VEGF agents, suggesting other angiogenic or antiangiogenic cytokines that coordinately contribute to angiogenesis remain to be identified7.
Interleukin 38 (IL-38, also named IL-1F10, IL1HY2, FIL-1θ, or IL-1θ) is a novel member of interleukin 1 family. Human IL-38 encodes a 152 amino acid protein with 16.9 kD molecular mass8, 9. It has been detected in a range of tissues including heart, placenta, fetal liver, skin, spleen, thymus and tonsil by multitissue first-strand cDNA PCR analysis8. Human IL-38 gene is located in the IL-1 family cluster on chromosome 2q13-14.1, between the genes encoding IL-36Ra and IL-1Ra10. IL-38 gene polymorphisms are associated with psoriatic arthritis (PsA), ankylosing spondylitis (AS)11,12,13 and cardiovascular disease14. As is typical of the IL-1 family, including IL-36Ra, IL-36α, IL-36β and IL-36γ, IL-38 lacks a signal peptide and caspase 1 consensus cleavage site8, 15. However, the natural N terminus for IL-38 is still unclear15. In addition, similar to the crystal structure of IL-1Ra and IL-18, 16, IL-38 displays a 12-β-stranded trefoil structure and shares. These features of IL-38 further prove that it belongs to the IL-1 family.
IL-1 family molecules play a prominent role in inflammatory and immune responses, acting as the first line of defense against invasive pathogenic microorganisms and physical damage. However, many cytokines of the IL-1 family, such as IL-1α, IL-1β, IL-18, IL-33 and IL-37 contribute importantly to angiogenesis7, 17,18,19. IL-38 shares high sequence homology (41% and 43%) with IL-1Ra and IL-36Ra9, 15 and lower homology (14–30%) with IL-1β and other IL-1 family proteins. IL-38 was recently suggested to bind to IL-36R and exerted antagonistic effects similar to those of IL-36Ra20. In a report by van de Veerdonk, IL-38 bound to IL-36R but did not bind to the immobilized IL-1RI, IL-18R and IL-1R accessory proteins (IL-1RAP, IL-1RAcP)20. However, IL-1RI was once considered a receptor for IL-388, 20, although with lower affinity than IL-1 or IL-1Ra8. The issue about receptor of IL-38 is still in dispute. The studies suggest that IL-38 may act as an IL-1 family antagonist (likely belongs to IL-36 subfamily) exerting its function and is probably involved in angiogenesis-associated pathophysiologies. For example, a recent study showed that IL-38 was upregulated in the serum of patients with systemic lupus erythematosus (SLE), probably exerting antiinflammatory functions21. Moreover, IL-38 is associated with pathogenesis of spondyloarthritis22, 23 and is upregulated in the salivary glands of patients with primary Sjoren’s syndrome24. To date, no reports have described the effect of IL-38 on angiogenesis.
In this study, we found that administration of IL-38 in vivo inhibited retinal angiogenesis significantly. IL-38 attenuated the proliferation, migration, and tube formation of endothelial cells in a dose-dependent manner. The inhibitory effect of IL-38 was eliminated by adding anti-IL-38 antibody. These results suggest that IL-38 is a regulator of pathophysiological angiogenesis.
Results
IL-38 Regulates Developmental Angiogenesis in Neonatal Mice
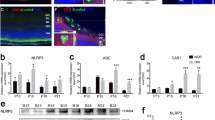
We first investigated whether IL-38 regulates neovascularization under physiological conditions. The retinal vascularization is a widely used model for physiological angiogenesis. Neonatal mice were intraperitoneally administrated with IL-38 (1 ng/g body weight) from postnatal day 1 (P1) to P4 (Fig. 1a). The mice were euthanatized at P5 and the retinas were harvested, fixed and flat-mounted for isolectin B4 (IB4) staining (Fig. 1a). After IL-38 administration, the vascular area and relative vascular area were significantly attenuated compared to the control group (Fig. 1b). The vessel density and tip cell numbers observed under immunofluorescence microscopy were also decreased in IL-38-treated mice (Fig. 1c and d).
IL-38 inhibits developmental angiogenesis. (a) Neonatal mice were intraperitoneally administrated with IL-38 (1 ng per gram bodyweight) from P1 to P4. Vascular area of the retina whole mounts was assessed by fluorescence microscopy. The retina whole mounts and vascular area were respectively delineated by green and red boundary line. Scale bars, 500 μm. (b) Vascular area and relative vascular area from control group and IL-38-treated group were summarized; n = 8. (c) High-magnification images of retinal vasculature from control mice (top) or IL-38-treated mice (bottom). (d) Densities of tip cells (white arrow) and branching points were quantified. Data from 8 animals were summarized in the graphs. Scale bar, 50 μm. Data are presented as mean ± SEM. *P < 0.05 Student t test.
IL-38 Inhibits Pathological Angiogenesis
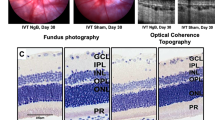
In the mouse model of oxygen-induced retinopathy (OIR), hypoxia-triggered angiogenic response induces the formation of pathological neovessels and neovascularization tufts are considered as the hallmark for characterization of pathological angiogenesis. The retinas of OIR mice at P17 were harvested, fixed and flat-mounted for IB4 staining. As is shown in Fig. 2a, the retinas of the mice developed extensive vascular regression in the central region and pathologic neovascular tufts in the middle and peripheral regions. The avascualar area and neovascular tufts in the groups with IL-38 intraperitoneal admistration (1 or 5 ng/g body weight) were reduced significantly, as compared with that of control group (Fig. 2b–d). Figure 2e is the representative, illustrating neovascular cell nuclei anterior to the internal limiting membrane (ILM). Consistent with IB4 staining analysis, the number of neovascular cell nuclei in the IL-38-treated group was significantly decreased compared with the counterpart control (Fig. 2f).
Systemic administration of IL-38 inhibits pathological angiogenesis in mouse OIR model. (a) Neovascular region, avascular region and the retina whole mounts delineated respectively by red, white and green boundary line. Representative images from the vehicle (OIR mouse model) are shown. (b) Mice were administrated with PBS, 1 or 5 ng/g bodyweight of IL-38 at P12, P14, and P16 during normoxia phase. (c) Disordered neovascular regions and avascular regions were quantified. (d) The relative retinal neovascular and avascular areas were quantified. (e) Neovascular cell nuclei anterior to internal limiting membrane (ILM) represented extent of retinal neovascularization and are indicated with black arrows in retina. (f) Statistical analysis showed that the number of neovascular cell nuclei anterior to the ILM in the IL-38-treated group is significantly decreased compared with the counterpart mice at P17. Scale bars, 500 μm. n = 8 per group. *P < 0.05 Student t test. BW, bodyweight.
In addition, the neovascularization tufts of the OIR mice administrated intravitreally with IL-38 (1 or 5 ng) at P12, was significantly decreased in a dose-dependent manner by IB4-isolectin staining imaging analysis at P17 (Fig. 3a). The sizes of vascular and avascular region were markedly reduced compared with the vehicle treated group (Fig. 3b and c).
Local administration of IL-38 inhibits pathological angiogenesis in mouse OIR model. (a) Mouse retinas were harvested at P17 and subjected to whole mount immunostaining with isolectin B4. Mice were injected inravitreally with 1 μm l IL-38 dissolved in PBS at different concentrations (1 or 5 ng/μl) at P12, during normoxia phase. Disordered neovascular growth (tufts; lower) in retina were highlighted in red and quantified. (b) The retinal neovascular and avascular areas were quantified. (c) The relative retinal neovascular and avascular areas were quantified. Scale bars, 500 μm (a, upper) and 100 μm (a, lower). n = 8 per group. *P < 0.05 Student t test.
IL-38 Attenuates Endothelial Cell Proliferation
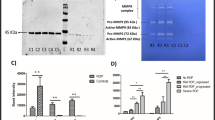
The hallmark of angiogenesis is the proliferation and migration of endothelial cells5. We examined the effects of IL-38 on proliferation of cultured human retinal endothelial cells (HRECs) and human umbilical vein endothelial cells (HUVECs). We found that supplementation of IL-38 in cell culture significantly reduced VEGF-induced HREC proliferation in comparison to the control cells at concentration of 1 or 5 ng/mL (Fig. 4). The inhibitory effect of IL-38 on HRECs was eliminated by adding anti-IL-38 antibodies (Fig. 4). Similar to HRECs, addition of IL-38 to the culture reduced the proliferation of HUVECs (Supplementary Figure 3). The results demonstrate the inhibitory effect of IL-38 on ECs proliferation.
IL-38 attenuates endothelial cell proliferation. HRECs (1 × 104) were cultured with VEGF/IL-38/anti-IL-38/IgG at 37 °C in a CO2 incubator and the proliferation was determined by MTT method. Shown are results from one representative experiment of three performed (in triplicates). *P < 0.05 Student t test.
Effects of IL-38 on Endothelial Cell Migration
Migration of ECs toward the angiogenic stimulus is an important early process in angiogenesis25. We next determined the effect of IL-38 on EC migration. After a scratch wound was created on the monolayer of HRECs, 1 or 5 ng/mL of IL-38 were added to the culture and the cells were monitored 0 and 8 hours (Fig. 5a). The healing area of HRECs in IL-38-treated group was reduced than that of control group. The inhibitory effect of IL-38 was eliminated by adding anti-IL-38 antibodies (Fig. 5). Similar to HRECs, addition of IL-38 to the culture reduced the migration of HUVECs (Supplementary Figure 4).
IL-38 reduces endothelial cell migration. (a) The effect of IL-38 on HRECs migration in scratch wound assay. Cells were treated with indicated concentrations of IL-38. Representative images after 0 and 8 hours after scratch wounding were shown. Scale bars, 100 μm. (b) Quantification of IL-38-treated HRECs migration in monolayer scratch wound assay. *P < 0.05 Student t test.
IL-38 Suppresses Endothelial Cell Tube Formation
We then performed tube formation assay to identify the in vitro anti-angiogenic activity of IL-38. Capillary-like tube formation was induced in VEGF-stimulated HRECs (Fig. 6a). IL-38 effectively inhibited the tube formation in dose-dependent manner as quantified by total tubule length and branching points (Fig. 6b). The inhibitory effect of IL-38 on tuber formation was eliminated by adding anti-IL-38 antibodies. Similar to HRECs, addition of IL-38 to the culture reduced the tube formation of HUVECs (Supplementary Figure 5).
IL-38 reduces endothelial cell tube formation. (a) 40,000 HRECs/well were seeded on Matrigel containing VEGF/IL-38/anti-IL-38/IgG in depleted medium. The cells were cultured for 18 h at 37 °C 5% CO2. Tube formation was quantified by counting the tube-like structures in the gel and data were presented as the number of branches per field. Scale bar, 200 μm. (b) Total length of tubule structure were quantified. *P < 0.05 Student t test.
Discussion
Our results identified IL-38 as an inhibitor in both pathological and physiological settings. IL-38 is the 10th member of IL-1 family and emerging regarded as an important anti-inflammatory cytokine. The most identified IL-1 family members are widely expressed in inflammatory cells and exert important role in inflammatory responses. Recently, the relationship between inflammation and angiogenesis has attracted more attention. Many cytokines of IL-1 family are closely associated with angiogenesis7, 17,18,19. To our knowledge, the role of IL-38 on angiogenesis has not been investigated before. We found that IL-38 was in low expression at P17 in normal mice, but it was up-regulated at P17 in OIR mice (Supplementary Figure 1a,b). IL-38 was highly expressed at P0 but the expression at P7, P12, P15 and P17 was decreased as compared with P0 in normal mice (Supplementary Figure 1c). That is, IL-38 is highly expressed at avascular situation and but decreased after vascular growth. Whether IL-38 is involved in regulating angiogenesis when the retinal vasculature is fully developed need further study.
Retinal neovascularization is a main cause of proliferative diabetic retinopathy (PDR), which can lead to vision loss due to secondary complications such as blood retinal barrier breakdown, vascular leakage, inflammation and edema26,27,28,29. In this study, we further demonstrated that IL-38 significantly suppressed neovascularization in the mouse model of OIR. Based on our results, local and systemic application of IL-38 had an equivalent effect on inhibiting angiogenesis. These results suggest that IL-38 is an antiangiogenic cytokine that inhibits retinal angiogenesis. In addition, we found that IL-38 expression was increased at P12 in OIR mice but decreased after that (Supplementary Figure 1d). The result indicates that IL-38 expression is increased in hyperoxia situation, a well-known anti-angiogenic conditions that stimulates vascular vaso-obliteration. Furthermore, we have demonstrated the antiangiogenic effect of IL-38 in vitro model of the cells. IL-38 attenuates VEGF-induced EC proliferation, migration and tube formation. The inhibitory effect of IL-38 was eliminated by adding anti-IL-38 antibodies. However, the mechanism by which IL-38 inhibits angiogenesis needs further investigation.
In the last decade, the link between inflammation and angiogenesis has become recognized. Extensive angiogenesis was observed in chronic inflammation, for example in rheumatoid arthritis and inflammatory bowel disease30, 31. It was found that IL-38 reduced the production of proinflammatory cytokines20, 32 and raises the question whether IL-38 modulates angiogenic response by modulating proinflammatory reaction. During inflammation, ECs become activated and recruit immune cells from the circulation into the underlying tissue where they play a role in angiogenesis with the up-regulation of growth factors and cytokines. IL-1 and most related family members are primarily inflammatory-associated cytokines and the role of IL-1 molecules in promoting angiogenesis had been studied, such as IL-1α, IL-1β, IL-18, IL-33 and IL-377, 17,18,19. It was found that IL-1β can mobilize endothelial progenitor cells (EPCs) by regulation of VEGF and VEGFR2 expression on ECs in a VEGF-dependent manner33. Supernatant from macrophages of IL-1β knockout mice did not induce inflammatoy or angiogenic responses34. We also found that the expression of pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 were decreased in IL-38-treated mice (Supplementary Figure 2). These results indicate that IL-38 modulates angiogenic response by modulating proinflammatory reaction. In addition, systemic administration with IL-1Ra prevented neovascularization formation in corneas by regulation of VEGF or bFGF35. The binding of IL-1Ra and IL-36Ra to their receptor reduces inflammation by blocking the binding of receptor ligands. IL-38 was reported to bind to the IL-1RI because of its highly amino acid homology to the IL-1Ra and IL36-Ra. IL-38 may via binding of IL-1RI inhibit the function of IL-1β and IL-1α to and thus regulates the inflammation-induced angiogenic responses. However, subsequent study indicated that IL-38 binds to the IL-36R not to the IL-1RI, IL-18R and IL-1RAcP20. The issues need further study.
The link of IL-38 and Th17 cells was attracted attention recently. Study demonstrated that IL-38 modulates the production of characteristic cytokines of the Th17 response, such as IL-17, IL-22, IL-23, IL-6 and IL-8 by blocking the IL-1R, IL-18R and IL-36R pathways20. IL-38 reduced the expression of C. albicans-induced IL-17 and IL-22 from peripheral blood mononuclear cells by reducing the stimulation of proinflammatory cytokines such as IL-8 in the tissues, similar to IL-36Ra20, 32. IL-8 as a known angiogenic factors which promotes angiogenic responses in vivo models, has been confirmed by many researchers36,37,38. This might be another mechanism of IL-38 that exerting antiangiogenic function, which needs further study. The identification of IL-38 as antiangiogenic cytokine and its mechanism have important clinical implications. However, the IL-38-related signaling pathway and many others are poorly understood and require further investigation.
Materials and Methods
Animals
C57BL/6 mice for oxygen-induced retinopathy model were purchased from the Animal Laboratory of Zhongshan Ophthalmic Center (Guangzhou, China). All animal experiments were approved by the Animal Ethical Committee at Zhongshan Ophthalmic Center, Sun Yat-sen University (Permit Number 2013–084) and were performed in accordance with the guidelines of the Association for Research in Vision and Ophthalmology (ARVO) for the use of animals in research. Animals were kept in a specific pathogen-free facility. Animals care and use were in compliance with institutional guidelines.
Cells and Culture
Human retinal endothelial cells (HRECs) and Human umbilical vein endothelial cells (HUVECs) were purchased from ScienceCell (Carlsbad, CA) and maintained in ECM according to company’s instructions. Specifically, ECM (ScienCell) supplemented with 5% FBS and endothelial cell growth supplement (ECGS, ScienCell) according to company’s instructions.
Reagents
Recombinant human IL-38 was purchased from R&D Systems (Minneapolis, MN). Recombinant mouse IL-38 was purchased from Adipogen (AG-40B-0101). Purified Human IgG was purchased from R&D Systems (Minneapolis, MN). For Western blot analysis, antibody against IL-38 was purchased from Abcam (Cambridge, MA). Recombinant human VEGF-A was obtained from R&D Systems (Minneapolis, MN). Endothelial Cell Medium (ECM) were purchased from ScienCell (Carlsbad, CA)
Mouse Retinal Vascularization
Neonatal mice were intraperitoneally administrated with IL-38 at the concentration of 1 ng/gram bodyweight from P1 to P4. Littermate controls were administrated with the same volume of PBS. The whole retina mounts were isolated at P5 and stained with FITC-conjugated Isolectin B4 (IB4). Retinal vasculature was assessed by fluorescent microscopy and vascular area was analyzed with Image J.
Mouse Model of Oxygen-induced Retinopathy
Neonatal mice and their nursing mothers were placed in a 75% oxygen supply chamber from postnatal day 7 (P7) to P12. Mice were returned to normal oxygen supply (21%) at P12. Mice were intravitreal administration with 1 μl of IL-38 dissolved in phosphate buffered saline (PBS) at different concentrations (0, 1, or 5 ng/μl) at P12 or intrapertoneal administration with PBS or IL-38 at 1 ng/gram bodyweight at P12, P14 and P16. At P17, the whole retinal mounts were isolated and stained with Isolectin B4 (Sigma-Aldrich, St. Louis, MO). Neovascular region (tufts) and avascular area were analyzed using Image J. Significant differences were determined using Student’s t test.
Real-time quantitative RT-PCR
For IL-38 expression, total RNAs were extracted from normal and OIR mouse retinas, and real-time PCR was performed. For pro-inflammatory cytokines expression under IL-38 administration in OIR model, total RNAs IL-38-treated retinas and control retinas, and real-time PCR was performed. Each reaction contained 12.5 μl of 2 × SYBR green Master Mix, 300 nM oligonucleotide primers (Table 1) synthesized by Invitrogen Biotechnology Co. Ltd, (Shanghai, China), 10 μl of 1 in 10 dilution of the cDNA and water, to a total of 25 μl. The tested mRNA expression was finally determined after correction with GAPDH expression.
Western Blot
For IL-38 expression, proteins were extracted from normal and OIR mouse retinas at P17. Proteins were detected by antibodies against IL-38 (R&D Systems, Minneapolis, MN). Densitometric quantitation of was performed and analyzed using two-tailed Student’s t test.
Proliferation
HUVECs were cultured in human endothelial-SFM (Invitrogen, Carlsbad, CA). Triplicate 0.2-ml cultures containing 2 × 104 cells were seeded in round-bottom 96-well microtiter plates. The cells were treated with VEGF/IL-38/anti-IL-38/IgG at 37 °C in a CO2 incubator for 48 h. MTT solution (10 μl of 5 mg/mL) was added to each well and the cells were further incubated for 4 h at 37 °C. The cells were then resuspended in 100 μl of 0.04 M HCl/isopropanol solution and the incubation was continued for 2 h to solubilize formazan violet crystals in the cells. The absorbance in each well was determined by spectrophotometry at the dual wavelengths of 570 and 630 nm on a microplate reader (Pharmacia, Swedn).
Migration assays
HUVECs were seeded in a 6-well plate at a density of 1 × 106 cells/well in growth medium until they reached 90% confluence. A scratch was made through each well using a sterile tip. The monolayer was incubated with a migration assay buffer consisting of serum-free medium and VEGF/IL-38/anti-IL-38/IgG. Images were captured at 0 h and 8 h. The area of healing wound was calculated with Image J software39.
Tube formation assay
Tube formation assay was performed as described in detail40,41,42. Briefly, HUVECs were seeded on Matrigel at 40,000 cells/well in 96-well plates with VEGF/IL-38/anti-IL-38/IgG in depleted medium. The cells were cultured 18 h at 37 °C in a 5% CO2 incubator. Photographs were taken on an inverted microscope using a 10 × objective. Tube formation in cell culture was quantified by counting the tube-like structures in the gel and data were presented as the number of branches per field ( × 100). In each experiment, 4 fields were calculated simultaneously and 3 independent experiments were performed.
Statistical Analyses
Data are presented as means ± SEM. Prism software was used for statistical analyses. Significant differences between paired samples were analyzed with two-tailed Student’s t test. A value of P < 0.05 was considered significant for all analyses.
References
Folkman, J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 6, 273–286, doi:10.1038/nrd2115 (2007).
Jang, Y. J., Kim, D. S., Jeon, O. H. & Kim, D. S. Saxatilin suppresses tumor-induced angiogenesis by regulating VEGF expression in NCI-H460 human lung cancer cells. J Biochem Mol Biol 40, 439–443 (2007).
Folkman, J. & D’Amore, P. A. Blood vessel formation: what is its molecular basis? Cell 87, 1153–1155 (1996).
Chung, A. S. & Ferrara, N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol 27, 563–584, doi:10.1146/annurev-cellbio-092910-154002 (2011).
Sholley, M. M., Ferguson, G. P., Seibel, H. R., Montour, J. L. & Wilson, J. D. Mechanisms of neovascularization. Vascular sprouting can occur without proliferation of endothelial cells. Lab Invest 51, 624–634 (1984).
Kamba, T. & McDonald, D. M. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer 96, 1788–1795, doi:10.1038/sj.bjc.6603813 (2007).
Voronov, E. et al. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA 100, 2645–2650, doi:10.1073/pnas.0437939100 (2003).
Lin, H. et al. Cloning and characterization of IL-1HY2, a novel interleukin-1 family member. J Biol Chem 276, 20597–20602, doi:10.1074/jbc.M010095200 (2001).
Bensen, J. T., Dawson, P. A., Mychaleckyj, J. C. & Bowden, D. W. Identification of a novel human cytokine gene in the interleukin gene cluster on chromosome 2q12-14. J Interferon Cytokine Res 21, 899–904, doi:10.1089/107999001753289505 (2001).
Nicklin, M. J. et al. A sequence-based map of the nine genes of the human interleukin-1 cluster. Genomics 79, 718–725, doi:10.1006/geno.2002.6751 (2002).
Rahman, P. et al. Association between the interleukin-1 family gene cluster and psoriatic arthritis. Arthritis Rheum 54, 2321–2325 (2006).
Chou, C. T. et al. Replication of association of IL1 gene complex members with ankylosing spondylitis in Taiwanese Chinese. Ann Rheum Dis 65, 1106–1109, doi:10.1136/ard.2005.046847 (2006).
Guo, Z. S. et al. Association of IL-1 gene complex members with ankylosing spondylitis in Chinese Han population. Int J Immunogenet 37, 33–37, doi:10.1111/j.1744-313X.2009.00889.x (2010).
Dehghan, A. et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation 123, 731–738, doi:10.1161/CIRCULATIONAHA.110.948570 (2011).
Kumar, S. et al. Identification and initial characterization of four novel members of the interleukin-1 family. J Biol Chem 275, 10308–10314 (2000).
Shen, H., Goodall, J. C. & Hill Gaston, J. S. Frequency and phenotype of peripheral blood Th17 cells in ankylosing spondylitis and rheumatoid arthritis. Arthritis Rheum 60, 1647–1656, doi:10.1002/art.24568 (2009).
Matsuo, Y. et al. IL-1alpha secreted by colon cancer cells enhances angiogenesis: the relationship between IL-1alpha release and tumor cells’ potential for liver metastasis. J Surg Oncol 99, 361–367, doi:10.1002/jso.21245 (2009).
Park, C. C. et al. Evidence of IL-18 as a novel angiogenic mediator. J Immunol 167, 1644–1653 (2001).
Choi, Y. S. et al. Interleukin-33 induces angiogenesis and vascular permeability through ST2/TRAF6-mediated endothelial nitric oxide production. Blood 114, 3117–3126, doi:10.1182/blood-2009-02-203372 (2009).
van de Veerdonk, F. L. et al. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc Natl Acad Sci U S A 109, 3001–3005, doi:10.1073/pnas.1121534109 (2012).
Rudloff, I. et al. Brief Report: Interleukin-38 Exerts Antiinflammatory Functions and Is Associated With Disease Activity in Systemic Lupus Erythematosus. Arthritis Rheumatol 67, 3219–3225, doi:10.1002/art.39328 (2015).
Monnet, D. et al. Association between the IL-1 family gene cluster and spondyloarthritis. Ann Rheum Dis 71, 885–890, doi:10.1136/annrheumdis-2011-200439 (2012).
Lea, W. I. & Lee, Y. H. The associations between interleukin-1 polymorphisms and susceptibility to ankylosing spondylitis: a meta-analysis. Joint Bone Spine 79, 370–374, doi:10.1016/j.jbspin.2011.06.010 (2012).
Ciccia, F. et al. Interleukin-36alpha axis is modulated in patients with primary Sjogren’s syndrome. Clin Exp Immunol 181, 230–238, doi:10.1111/cei.12644 (2015).
Carmeliet, P. & Jain, R. K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307, doi:10.1038/nature10144 (2011).
Vinores, S. A. et al. Implication of the hypoxia response element of the Vegf promoter in mouse models of retinal and choroidal neovascularization, but not retinal vascular development. J Cell Physiol 206, 749–758, doi:10.1002/jcp.20525 (2006).
Zhang, S. X. et al. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J 20, 323–325, doi:10.1096/fj.05-4313fje (2006).
Reynolds, L. E. et al. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med 8, 27–34, doi:10.1038/nm0102-27 (2002).
Ishida, S. et al. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med 198, 483–489, doi:10.1084/jem.20022027 (2003).
Folkman, J. Angiogenesis-dependent diseases. Semin Oncol 28, 536–542 (2001).
Folkman, J. Fundamental concepts of the angiogenic process. Curr Mol Med 3, 643–651 (2003).
Vigne, S. et al. IL-36R ligands are potent regulators of dendritic and T cells. Blood 118, 5813–5823, doi:10.1182/blood-2011-05-356873 (2011).
Amano, K. et al. Mechanism for IL-1 beta-mediated neovascularization unmasked by IL-1 beta knock-out mice. J Mol Cell Cardiol 36, 469–480, doi:10.1016/j.yjmcc.2004.01.006 (2004).
Carmi, Y. et al. The role of macrophage-derived IL-1 in induction and maintenance of angiogenesis. J Immunol 183, 4705–4714, doi:10.4049/jimmunol.0901511 (2009).
Coxon, A. et al. Inhibition of interleukin-1 but not tumor necrosis factor suppresses neovascularization in rat models of corneal angiogenesis and adjuvant arthritis. Arthritis Rheum 46, 2604–2612, doi:10.1002/art.10546 (2002).
Waugh, D. J. & Wilson, C. The interleukin-8 pathway in cancer. Clin Cancer Res 14, 6735–6741, doi:10.1158/1078-0432.CCR-07-4843 (2008).
Koch, A. E. et al. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science 258, 1798–1801 (1992).
Strieter, R. M. et al. Interleukin-8. A corneal factor that induces neovascularization. Am J Pathol 141, 1279–1284 (1992).
Liang, C. C., Park, A. Y. & Guan, J. L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2, 329–333, doi:10.1038/nprot.2007.30 (2007).
Lin, C. M. et al. Protective role of wogonin against lipopolysaccharide-induced angiogenesis via VEGFR-2, not VEGFR-1. Int Immunopharmacol 6, 1690–1698, doi:10.1016/j.intimp.2006.07.003 (2006).
Zhou, Y. et al. Interleukin-12 inhibits pathological neovascularization in mouse model of oxygen-induced retinopathy. Sci Rep 6, 28140, doi:10.1038/srep28140 (2016).
Sachdev, U. et al. High mobility group box 1 promotes endothelial cell angiogenic behavior in vitro and improves muscle perfusion in vivo in response to ischemic injury. J Vasc Surg 55, 180–191, doi:10.1016/j.jvs.2011.07.072 (2012).
Acknowledgements
We are grateful to Dr. Ji Ming Wang, National Cancer Institute, National Institutes of Health for his critique of the manuscript. This project was supported in part by the grants from the National Natural Science Foundation of China (31471122) and the grants from the Science and Technology Planning Project of Guangdong Province, China (2014A030313047).
Author information
Authors and Affiliations
Contributions
J.Z., R.Z., J.C., J.J., Y.Y., Y.T., W.L., W.W., H.Z. and S.B.S. were deeply involved in the conception and design of the study. J.Z. and S.B.S. were responsible for the analyses of the data and drafted the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhang, J., Zhao, R., Chen, J. et al. The Effect of Interleukin 38 on Angiogenesis in a Model of Oxygen-induced Retinopathy. Sci Rep 7, 2756 (2017). https://doi.org/10.1038/s41598-017-03079-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-03079-z
This article is cited by
-
An overview of the biological and multifunctional roles of IL-38 in different infectious diseases and COVID-19
Immunologic Research (2022)
-
Retinopathy of prematurity: contribution of inflammatory and genetic factors
Molecular and Cellular Biochemistry (2022)
-
IL-38 has an anti-inflammatory action in psoriasis and its expression correlates with disease severity and therapeutic response to anti-IL-17A treatment
Cell Death & Disease (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.