Abstract
In a relatively short period of time, transition metal-mediated radiofluorination reactions have changed the PET radiochemistry landscape. These reactions have enabled the radiofluorination of a wide range of substrates, facilitating access to radiopharmaceuticals that were challenging to synthesize using traditional fluorine-18 radiochemistry. However, the process of adapting these new reactions for automated radiopharmaceutical production has revealed limitations in fitting them into the confines of traditional radiochemistry systems. In particular, the presence of bases (e.g. K2CO3) and/or phase transfer catalysts (PTC) (e.g. kryptofix 2.2.2) associated with fluorine-18 preparation has been found to be detrimental to reaction yields. We hypothesized that these limitations could be addressed through the development of alternate techniques for preparing [18F]fluoride. This approach also opens the possibility that an eluent can be individually tailored to meet the specific needs of a metal-catalyzed reaction of interest. In this communication, we demonstrate that various solutions of copper salts, bases, and ancillary ligands can be utilized to elute [18F]fluoride from ion exchange cartridges. The new procedures are effective for fluorine-18 radiochemistry and, as proof of concept, have been used to optimize an otherwise base-sensitive copper-mediated radiofluorination reaction.
Similar content being viewed by others
Introduction
Positron emission tomography (PET) imaging is a functional nuclear medicine imaging technique in which radiotracers (bioactive molecules labeled with a positron-emitting radionuclide) are administered to a patient or animal1, 2. When a positron is emitted from the radiotracer, it annihilates with an electron and generates two 511 keV gamma photons that are detected by the PET scanner. Mapping these gamma rays over the entire duration of the PET scan leads to an image with high spatial resolution that can provide functional information about biochemical and metabolic processes in the body. Fluorine-18 is the most common PET radionuclide because of its excellent imaging properties (a clean decay process involving 97% β+ emission and limited positron migration, leading to highly resolved images), favorable half-life (109.77 min), and the ready accessibility of Curie amounts of no-carrier-added [18F]fluoride from small medical cyclotrons.
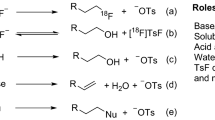
[18F]Fluoride is produced in a cyclotron by bombarding a target loaded with [18O]H2O, via the 18O(p,n)18F nuclear reaction, and then delivered to an automated radiochemistry synthesis module as a solution in [18O]H2O. [18F]Fluoride is generally considered to be strongly hydrated in polar protic solvents and thereby deactivated for nucleophilic reactions (although several recent examples suggest that this might not always be the case)3,4,5,6. As such, a well-established three-step process is typically used to increase the reactivity of nucleophilic [18F]fluoride (Fig. 1)7, 8. This involves: (i) trapping [18F]fluoride on an ion exchange cartridge to recover [18O]H2O and remove impurities; (ii) eluting of X+18F− (X = K+, Cs+, or R4N+) into a reactor using aqueous base (e.g. K2CO3, Cs2CO3 or R4NHCO3) followed by the addition of acetonitrile (and a PTC/metal-chelating crytpand such as kryptofix 2.2.2 (K2.2.2) if needed), and (iii) azeotropic drying of the resulting [18F]fluoride salt. After drying, the “activated” [18F]fluoride is employed in nucleophilic radiofluorination reactions. This classical approach has worked satisfactorily for numerous nucleophilic radiofluorination reactions9 since its introduction in 198610,11,12,13.
Recently there has been renewed interest in developing novel approaches for “late-stage fluorination”14,15,16,17,18,19. Spectacular advances in transition-metal catalyzed fluorination reactions, as well as the development of many new precursors amenable to late-stage fluorination, have greatly expanded the scope of radiopharmaceuticals that can be accessed from [18F]fluoride14, 17. However, the complicated reaction pathways involved in these new reactions have exposed limitations in the traditional method for handling cyclotron-produced [18F]fluoride. For example, the Cu-mediated fluorination of organoboron compounds, originally developed by the Sanford group20 and subsequently adapted for radiolabeling by Sanford and Scott21, Gouverneur22, 23, and Neumaier24, 25, has been used to prepare a range of radiofluorinated arenes in small-scale manual reactions. However, automation and scale-up to the Curie levels of [18F]fluoride used in typical production of radiopharmaceuticals for clinical use have proven challenging21, 24. The inorganic bases (e.g. K2CO3) and cryptands (e.g. K2.2.2) used for standard [18F]fluoride processing result in suppressed radiochemical yields in this Cu-mediated [18F]fluorination reaction, possibly due to the formation of unproductive copper adducts24. The presence of K2CO3 and/or K2.2.2 can also be detrimental to subsequent reactions and/or protecting group manipulations, as we have discovered in recent PET radiotracer development efforts26.
To address these limitations, we considered the use of alternative eluents to process [18F]fluoride. Several recent reports have focused on the development of eluent systems including transition-metal complexes27,28,29, as well as bases, salts or neutral onium salts intended as milder replacements for K2CO3 and/or to reduce (or even eliminate) the need for K2.2.2 30,31,32,33,34,35,36,37,38,39. For example, Lemaire and colleagues used strong bases to generate hydroxide and elute [18F]fluoride from ion exchange cartridges34, while Wessmann and co-workers showed that azeotropic drying could be eliminated if [K+K2.2.2]OH- in acetonitrile was used to elute fluoride39.
Building on these concepts, we sought to replace deleterious bases and/or chelating agents in [18F]fluoride processing with metal salts and/or ancillary ligands necessary for the metal-catalyzed reaction. We initially explored aqueous solutions of pyridinium p-toluenesulfonate (PPTS) or KOTf for the Cu-mediated fluorination of organoborons21. Based on these initial promising results, we considered whether other aqueous solutions could be used to elute [18F]fluoride. This would open the intriguing possibility of tailoring the [18F]fluoride eluent to the reaction at hand, rather than defaulting to the K2CO3/K2.2.2 system. Herein we demonstrate proof-of-concept by matching new [18F]fluoride processing conditions to the copper-mediated [18F]fluorination (and subsequent reactions) of aryl boron derivatives.
Results and Discussion
Alternative Eluent Studies
Our studies commenced with an examination of the use of copper salts from our recently reported Cu-mediated fluorination reactions to prepare [18F]fluoride. We initially investigated elution of [18F]fluoride from tetramethylammonium anion exchange cartridges (QMA cartridges) using Cu(OTf)2, and found that 0.025 M aqueous solutions of Cu(OTf)2 resulted in >85% [18F]fluoride recovery from the QMA (see Supporting Information (SI) for full details of Cu studies). On the basis of these initial findings, we used Cu(OTf)2 as the [18F]fluoride eluent in the automated synthesis of [18F]fluoroacetophenone ([18F]FAP, 2) from 4-acetylphenylboronic acid (1-B(OH) 2 ), using our previously reported [18F]fluorodeboronation chemistry21. However, in automated fluorination reactions, the radiochemical conversion (RCC) to [18F]FAP was dramatically lower when eluting with Cu(OTf)2 (4–5%) than with our previously reported KOTf/K2CO3 elution (yields of [18F]FAP were 61 ± 8% using optimized manual conditions but lower yields of 8–12% were obtained in the automated [18F] fluorination). Thus we next turned our attention to eluting [18F]fluoride using ancillary ligands that are commonly employed in transition-metal mediated reactions.
As ligands are often present in excess in metal-mediated reactions, they are generally less constrained to specific concentration ranges than the metal salt and are typically more redox stable than transition metal cations. This lessens the importance of variable final concentrations resulting from, for example, inconsistent retention on the QMA cartridge during elution. In aqueous solution weak bases undergo equilibrium protonation in water to form BH+-OH− ion pairs. As such, we hypothesized that basic ligands or other non-ionic weak bases with a high enough conjugate pKa in water would generate sufficient OH− to elute [18F]fluoride from QMA cartridges via anion exchange. We first tested a range of concentrations of aqueous KOH to determine the concentration of OH− required to elute [18F]fluoride from QMA cartridges preconditioned with either 0.5 M NaHCO3 or KOTf. [18F]Fluoride recovery from the QMA cartridges was predicted to be related to KOH concentration (Table 1). This relationship was found to be sigmoidal in nature, consistent with previously reported ionic eluent systems30.
We noted that [18F]fluoride recovery was consistently greater from cartridges preconditioned with NaHCO3 when compared to KOTf, suggesting that preconditioning can also impact [18F]fluoride recovery. To further explore this effect, we evaluated a series of preconditioning agents, and observed a clear trend. [18F]Fluoride adsorption (loading) onto the QMA cartridge was not affected by choice of preconditioning agent, while [18F]fluoride recovery was found to be proportional to the valency and ionic character of the preconditioning salt (Table 1). These data suggest that the ability for weak bases to elute [18F]fluoride is dependent on both OH− concentration (which can be predicted from the conjugate acid (BH+) pKa value), and the cartridge preconditioning agent, which can be changed to affect [18F]fluoride recovery and ostensibly the reaction conditions.
In light of these results, aqueous solutions of weak nitrogenous bases encompassing a range of pKa values were next tested as [18F]fluoride eluents using QMA cartridges pre-conditioned with either NaHCO3, KOTf, or Na2SO4 (Table 2). As anticipated, a positive sigmoidal relationship between [18F]fluoride recovery and pKa of conjugate acid (BH+) was observed (Fig. 2). However, the weak base solutions eluted a greater proportion of [18F]fluoride from the QMA cartridge than would be predicted on the basis of OH− concentration alone. For example, a 0.1 M aqueous solution of Et3N (conjugate pKa = 11.1) has a predicted OH− concentration of 0.011 M, which should correspond to a [18F]fluoride recovery of 29–31% based on the 0.01–0.02 M KOH elution data (Table 2, KOTf preconditioning). Instead, a [18F]fluoride recovery of 69% was observed (KOTf preconditioning). This trend is observed for all of the bases and QMA preconditioning agents investigated, and may arise from preferential hydrogen bonding interactions between BH+ and [18F]fluoride, assisting in the desorption of [18F]fluoride from the QMA.
To develop a predictive model for [18F]fluoride recovery vs. pKa of conjugate acids, regression analysis was conducted on the data collected in this experiment using GraphPad software (90% confidence level). Regression equations were obtained with high correlation (R2 > 0.9) for each preconditioning agent and serve as a crude predictor of [18F]fluoride recovery when similar non-ionic eluents are used (see SI for full details). For example, the equations predict that when eluting with 500 μl of a 0.1 M solution of aqueous base, then bases with a conjugate acid pKa of ≥10.4 are optimal for recovering >50% [18F]fluoride from ion exchange cartridges preconditioned with KOTf, whereas pKa values ≥8.9 and ≥7.7 are required when preconditioning with NaHCO3 and Na2SO4, respectively.
For all weak base eluents tested, [18F]fluoride recovery was found to be related to preconditioning agent in the following order: KOTf < NaHCO3 < Na2SO4. This suggests that a combination of appropriate preconditioning and increased eluent concentration can improve [18F]fluoride recovery to the levels needed for radiochemical synthesis when eluting with weak bases (pKa < 7). To test this hypothesis, we chose pyridine-based eluents (pyridine and 4-methoxypyridine), as they afforded poor results with KOTf and NaHCO3 preconditioning, but are useful ligands for metal-mediated reactions21. Several divalent preconditioning agents (Table 3, entries 1–6) were re-evaluated with 0.1 M solutions of these bases, and Na2SO4 preconditioning resulted in the greatest [18F]fluoride recovery. Both pyridines were then examined at higher concentrations with Na2SO4 QMA preconditioning. [18F]Fluoride recoveries increased steadily to a maximum of 46% and 66% for 500 μL of 1 M pyridine and 4-methoxypyridine, respectively. Notably, this is equivalent to adding 500 µmol of pyridine to a reaction, which is approximately the optimal amount needed for the Cu-mediated fluorination of organoborons21. We also tested the impact of preconditioning agent when eluting [18F]fluoride with Cu(OTf)2, to see if this effect was unique for basic eluents, but results were less conclusive (see SI).
Novel Synthesis of 18F-fluoroarenes Using a Novel Dimethylaminopyridine (DMAP) Elution Method
Finally, we tested whether these new elution approaches could enhance the synthesis of 18F-fluoroarenes that have proven difficult to access by other labeling strategies. For example, we have a long standing interest in accessing [18F]4-fluorophenacylbromide ([18F]FPB, 3) via [18F]FAP (2), due to its potential as a PET radiotracer for glycogen synthase kinase-3 (GSK-3)40. Typical yields of [18F]FAP synthesized via traditional SNAr chemistry (e.g. from the nitro-precursor) are 60–70%41. However, existing methods for conversion to [18F]FPB have proven problematic in our hands as well as when attempted by others41, because of the need to brominate [18F]FAP42,43,44. Most previously reported syntheses of [18F]FPB have utilized Br2 as a brominating reagent. However, we sought to avoid this reagent due to its volatility, toxicity, and the incompatibility of our synthesis module components with this strong oxidant. We likewise wished to avoid “inconvenient” reagents such as perbromide resins or other solid-phase reagents44, because of their incompatibility with modern automated synthesis modules. Moreover, the reported bromination methods suffer from well documented reproducibility issues41. We hypothesize that these reproducibility problems stem from complication of the acid-catalyzed bromination by the presence of residual K2CO3 and/or K2.2.2 from [18F]fluoride processing. As such, this is an ideal system for proof-of-concept demonstration that elution conditions can be customized to enhance a given radiochemical synthesis.
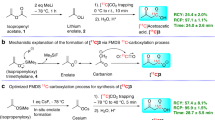
We previously demonstrated a Cu-mediated synthesis of [18F]FAP (2) in an automated module starting from either 4-acetylphenylboronic acid pinacol ester (4-BPin-acetophenone, 1-BPin) or the corresponding arylboronic acid (1-B(OH) 2 ), using KOTf (5 mg spiked with 50 μg of K2CO3, corresponding to a 73/1 molar ratio) to process the [18F]fluoride21. Yields of [18F]FAP were comparable to those obtained using traditional SNAr: 61 ± 8% using optimized manual conditions and 8–12% when the process was automated21, 41. We used N-bromosuccinimide (NBS) in the presence of methanesulfonic acid for the bromination, as it is a relatively mild method for the α-bromination of ketones that is amenable to automation45, 46. However, attempts to brominate the [18F]FAP (2) produced in this fashion proceeded in ≤5% RCC (Fig. 3a), and provided insufficient quantities [18F]FPB (3) for pre-clinical imaging studies. We hypothesize that K2CO3 from [18F]fluoride processing, as well as pyridine left over from the [18F]fluorination, interfere with the acid-catalyzed bromination, in a similar manner to K2CO3/K2.2.2 described above.
Comparison of existing method for the [18F]fluorination of arylboronates (A)21, and new modified method (B). Changing the base from pyridine to DMAP is necessary for successful one-pot acid-catalyzed bromination of [18F]FAP (2) to [18F]FPB (3).
In our studies of the radiofluorination of arylboron reagents, we discovered that DMAP can be used in place of pyridine for the [18F]fluorination of certain organoboron derivatives. While DMAP is not compatible with the fluorination of boronic acid precursors, likely due to the presence of acidic protons, we obtained modest RCC values (20–40%) when fluorinating arylBpin derivatives using a 1: 2: 2.5 ratio of arylBpin: Cu(OTf)2: DMAP21. As aqueous solutions of DMAP can be used to elute [18F]fluoride at similarly low concentrations (Table 2), this serves as an ideal system to test this new elution strategy.
We first optimized the radiolabeling reagent loading to accommodate the amount of DMAP (500 µL of a 0.1 M solution, or 50 µmol) required for satisfactory [18F]fluoride recovery. In preliminary studies, the highest RCC was observed with NaHCO3 preconditioning (5%). Given the potential improvements offered by QMA preconditioning with Na2SO4, we also tested this possibility. However there was no product formation, likely due to deactivation of the catalyst via the coordination of SO4 2− to Cu2+. This result was not entirely unexpected, as it is in line with our previous findings that CuSO4 is an inadequate catalyst for this chemistry21 and prior reports have shown that QMA preconditioning agents end up in reaction mixtures32. This finding demonstrates the need to carefully consider each aspect of fluoride processing when designing and/or optimizing late-stage fluorination approaches.
Through a further series of reaction screens (see SI), we found that 50 µmol of 4-BPin-acetophenone (1-BPin), 8 µmol of Cu(OTf)2, and 50 µmol of DMAP in 1 mL of DMF led to an optimal 58% RCC to [18F]FAP (2) when the reaction mixture was heated to 110 °C for 20 minutes (Fig. 3b). Importantly, the 50 µmol DMAP used in this process is an order of magnitude less than the 500 µmol of pyridine required in our original method21, and was therefore expected to be far less of a complicating factor in the subsequent bromination step.
We next revisited the automated synthesis of [18F]FAP (2) and subsequent conversion to [18F]FPB (3). The optimized method, using NaHCO3 as the preconditioning agent, was transferred to a TRACERLab FXFN synthesis module. The order of reagent addition was then evaluated to examine if it impacts the reaction (see SI for full details). The best yields were obtained by pre-mixing a solution of [18F]fluoride and arylBpin 1-BPin at 100 °C for 5 min, followed by addition of Cu(OTf)2 and DMAP. Conducting the reaction at 110 °C for 20 min resulted in 45% RCC to 2. This result suggests that dissolution of [18F]fluoride is a critical step in this synthesis, and must occur prior to the addition of [18F]fluoride or substrate to Cu(OTf)2. Mechanistic studies into these effects are currently underway.
Finally, investigation of the bromination of [18F]FAP (2) to generate [18F]FPB (3) revealed that subjecting [18F]FAP, prepared from 1-BPin using the new DMAP elution method, to identical bromination conditions as those described above (NBS/MsOH) resulted in 69% RCC to [18F]FPB (3) (the dibrominated product was also obtained in 24% RCC). Putting this all together and running a fully automated synthesis of [18F]FPB provided 13 mCi of isolated and formulated product (1.5% non-corrected radiochemical yield (RCY), 99% radiochemical purity and 8,097 Ci/mmol specific activity), enough to conduct GSK-3 preclinical PET imaging in rodents and primates and these studies are ongoing.
Substrate Scope of DMAP Elution Method
While these reaction conditions were specifically designed to enable an automated one-pot two step synthesis of [18F]FPB, we sought to establish whether they were generally applicable to the fluorination of arylBPin esters. In addition to 1-BPin, we subjected a small series of substrates (4-BPin – 8-BPin) to the optimized conditions, and found that the method was suitable for fluorinating a range of different arylBPin esters (Fig. 4). In general, yields were comparable to those obtained using our previously reported radiofluorination of arylboron reagents21. Both methods are tolerant of a variety of functional groups and unprotected heteroatoms and, like our recently developed radiofluorination of arylstannanes47, can be conducted under an inert atmosphere making then straightforward to automate using radiochemistry synthesis modules. The results suggest that for many substrates these methods are interchangeable. However, for complex precursors, or cases where further chemistry is required downstream on labeled intermediates, one or the other can be selected (and/or further optimized) using the strategies introduced herein.
Conclusion
In conclusion, this paper introduces the concept of tailoring [18F]fluoride processing conditions to enhance late-stage radiofluorination reactions. The appropriate choice of [18F]fluoride processing conditions can lead to milder, simpler, and higher yielding radiofluorinations as well as improve downstream reactions with labeled intermediates. For example, we have demonstrated that aqueous solutions of non-ionic bases and/or ligands used in copper-mediated fluorination reactions, in combination with an appropriate QMA preconditioning agent, can be utilized as eluents for fluorine-18 processing. The resultant conjugate acid [18F]fluoride salts are fully soluble in most organic solvents, negating the need for additional phase transfer catalysts. As a proof-of-concept, we show that an elution method using DMAP enables the copper-mediated synthesis of [18F]FAP and subsequent conversion to [18F]FPB, which has proven problematic using other synthetic approaches. Ultimately, we anticipate that the concept of tailoring [18F]fluoride processing conditions to a given reaction will prove broadly applicable to diverse radiofluorinations in the future.
Methods
Full details of experimental procedures as well as associated analytical data can be found in the Supporting Information.
General [18F]fluoride elution studies method
Waters QMA-light Sep-Paks were washed sequentially with ethanol (10 mL), 0.5 M preconditioning agent in water (10 mL), and deionized water (10 mL). Aqueous [18F]fluoride (0.5 mL) was passed through a QMA cartridge followed by 2 mL air, and the activity of the QMA cartridge was determined with a Capintec dose calibrator. [18F]Fluoride was then eluted from the QMA cartridge into a 4 mL vial with 0.5 mL eluent solution, followed by 2 mL of air. Activity of the 4 mL vial (eluate) and QMA cartridge (residual [18F]fluoride) were determined with a Capintec dose calibrator. Activity data was used to calculate % fluoride recovery.
General radiofluorination details
Fluorine-18 was produced via the 18O(p,n)18F nuclear reaction using a GE PETTrace cyclotron (40 μA beam for 2 min generated ca. 150 mCi of fluorine-18 as measured by synthesis module detector). The [18F]fluoride was then processed and either employed in manual reactions, or automated syntheses using a TRACERLab FXFN radiochemistry synthesis module, according to methods described in the SI. Total recovered activity at end-of-synthesis was measured with a Capintec dose calibrator.
Quality control analysis
Reactions were analyzed by radio-TLC using a Bioscan AR-2000 TLC scanner, and/or HPLC using a Shimadzu LC-2010A HT system equipped with a Bioscan B-FC-1000 radiation detector, according to the methods described in the SI.
References
Van de Bittner, G. C., Ricq, E. L. & Hooker, J. M. A Philosophy for CNS Radiotracer Design. Acc. Chem. Res. 47, 3127–3134 (2014).
Liang, S. H. & Vasdev, N. Total Radiosynthesis: Thinking Outside ‘the Box’. Aust. J. Chem. 68, 1319–1328 (2015).
Stewart, M. N., Hockley, B. G. & Scott, P. J. H. Green Approaches to Late-stage Fluorination: Radiosyntheses of 18F-Labelled Radiopharmaceuticals in Ethanol and Water. Chem. Commun. 51, 14805–14808 (2015).
Kim, D. W. et al. Facile Nucleophilic Fluorination Reactions using tert-Alcohols as a Reaction Medium: Significantly Enhanced Reactivity of Alkali Metal Fluorides and Improved Selectivity. J. Org. Chem. 73, 957–962 (2008).
Sergeev, M. E., Morgia, F., Lazari, M., Wang, C. & van Dam, R. M. Titania-catalyzed Radiofluorination of Tosylated Precursors in Highly Aqueous Medium. J. Am. Chem. Soc. 137, 5686–5694 (2015).
Chun, J.-H., Telu, S., Lu, S. & Pike, V. W. Radiofluorination of Diaryliodonium Tosylates under Aqueous-organic and Cryptand-free Conditions. Org. Biomol. Chem. 11, 5094–5099 (2013).
Sachinidis, J. I., Poniger, S. & Tochon-Danguy, H. J. Automation for Optimised Production of Fluorine-18-labelled Radiopharmaceuticals. Curr. Radiopharm. 3, 248–253 (2010).
Hjelstuen, O. K., Svadberg, A., Olberg, D. E. & Rosser, M. Standardization of Fluorine-18 Manufacturing Processes: New Scientific Challenges for PET. Eur. J. Pharm. Biopharm. 78, 307–313 (2011).
Cai, L., Lu, S. & Pike, V. W. Chemistry with [18F]Fluoride Ion. Eur. J. Org. Chem. 2853–2873 (2008).
Coenen, H. H., Schüller, M., Stöcklin, G., Klatte, B. & Knöchel, A. Preparation of N.C.A. [17-18F]-Fluoroheptadecanoic Acid in High Yields via Aminopolyether Supported, Nucleophilic Fluorination. J. Labeled Cmpd. Radiopharm. 23, 455–466 (1986).
Block, D., Klatte, B., Knöchel, A., Beckmannm, R. & Holm, U. N. C. A. [18F]-Labelling of Aliphatic Compounds in High Yields via Aminopolyether-supported Nucleophilic Substitution. J. Labeled Cmpd. Radiopharm. 23, 467–477 (1986).
Hamacher, K., Coenen, H. H. & Stöcklin, G. Efficient Stereospecific Synthesis of No-Carrier-Added 2-[18F]-Fluoro-2-Deoxy-D-Glucose Using Aminopolyether Supported Nucleophilic Substitution. J. Nucl. Med. 27, 235–238 (1986).
Hamacher, K., Coenen, H. H. & Stöcklin, G. NCA Radiofluorination of Spiperone and N-methylspiperone via Aminopolyether Supported Direct Nucleophilic Substitution. J. Labeled Cmpd. Radiopharm. 23, 1047 (1986).
Brooks, A. F., Topczewski, J. J., Ichiishi, N., Sanford, M. S. & Scott, P. J. H. Late-stage [18F]Fluorination: New Solutions to Old Problems. Chem. Sci. 5, 4545–4553 (2014).
Jacobson, O., Kiesewetter, D. O. & Chen, X. Fluorine-18 Radiochemistry, Labeling Strategies and Synthetic Routes. Bioconjugate Chem 26, 1–18 (2015).
Sanford, M. S. & Scott, P. J. H. Moving Metal-Mediated 18F-Fluorination from Concept to Clinic. ACS Cent. Sci. 2, 128–130 (2016).
Preshlock, S., Tredwell, M. & Gouverneur, V. 18F-Labeling of Arenes and Heteroarenes for Applications in Positron Emission Tomography. Chem. Rev. 116, 719–766 (2016).
Campbell, M. G. & Ritter, T. Late-Stage Fluorination: from Fundamentals to Application. Org. Process Res. Dev. 18, 474–480 (2014).
Neumann, C. N. & Ritter, T. Late-Stage Fluorination: Fancy Novelty or Useful Tool? Angew. Chem. Int. Ed. 54, 3216–3221 (2015).
Ye, Y., Schimler, S. D., Hanley, P. S. & Sanford, M. S. Cu(OTf)2-mediated Fluorination of Aryltrifluoroborates with Potassium Fluoride. J. Am. Chem. Soc. 135, 16292–16295 (2013).
Mossine, A. V. et al. Synthesis of [18F]Arenes via the Copper-mediated [18F]Fluorination of Boronic Acids. Org. Lett. 17, 5780–5783 (2015).
Tredwell, M. et al. A General Copper-Mediated Nucleophilic 18F Fluorination of Arenes. Angew. Chem., Int. Ed. 53, 7751–7755 (2014).
Preshlock, S. et al. Enhanced Copper-Mediated 18F-Fluorination of Aryl Boronic Esters provides Eight Radiotracers for PET Applications. Chem. Commun. 52, 8361–8364 (2016).
Zlatopolskiy, B. D. et al. Copper-Mediated Aromatic Radiofluorination Revisited: Efficient Production of PET Tracers on a Preparative Scale. Chem. - Eur. J 21, 5972–5979 (2015).
Zischler, J., Krapf, P., Richarz, R., Zlatopolskiy, C. D. & Neumaier, B. Automated Synthesis of 4-[18F]fluoroanisole, [18F]DAA106, and 4-[18F]FPhe using Cu-mediated Radiofluorination under “Minimalist” Conditions. Appl. Radiat. Isot. 115, 133–137 (2016).
Mossine, A. V., Brooks, A. F. & Scott Peter, J. H. Synthesis and Evaluation of 4-[18F]Fluorophenacylbromide (4FPB), a Selective and Irreversible Inhibitor of Glycogen Synthase Kinase 3β (GSK-3β). J. Nucl. Med. 57 (Suppl. 2), 1099 (2016).
Graham, T. J. A., Lambert, R. F., Ploessl, K., Kung, H. F. & Doyle, A. G. Enantioselective Radiosynthesis of Positron Emission Tomography (PET) Tracers Containing [18F]Fluorohydrins. J. Am. Chem. Soc. 136, 5291–5294 (2014).
Huang, X., Liu, W., Hooker, J. M. & Groves, J. T. Targeted Fluorination with the Fluoride Ion by Manganese-Catalyzed Decarboxylation. Angew. Chem., Int. Ed. 54, 5241–5245 (2015).
Huang, X. et al. Late Stage Benzylic C-H Fluorination with [18F]Fluoride for PET Imaging. J. Am. Chem. Soc. 136, 6842–6845 (2014).
Katsifis, A., Hamacher, K., Schnitter, J. & Stoecklin, G. Optimization Studies Concerning the Direct Nucleophilic Fluorination of Butyrophenone Neuroleptics. Appl. Radiat. Isot. 44, 1015–1020 (1993).
Lee, S. J., Oh, S. J., Chi, D. Y., Moon, D. H. & Ryu, J. S. High-yielding [18F]Fluorination Method by Fine Control of the Base. Bull. Korean Chem. Soc. 33, 2177–2180 (2012).
Seo, J. W., Lee, B. S., Lee, S. J., Oh, S. J. & Chi, D. Y. Fast and Easy Drying Method for the Preparation of Activated [18F]Fluoride using Polymer Cartridge. Bull. Korean Chem. Soc. 32, 71–76 (2011).
Brichard, L. & Aigbirhio, F. I. An Efficient Method for Enhancing the Reactivity and Flexibility of [18F]Fluoride Towards Nucleophilic Substitution Using Tetraethylammonium Bicarbonate. Eur. J. Org. Chem. 2014, 6145–6149 (2014).
Lemaire, C. F. et al. Fast Production of Highly Reactive No-Carrier-Added [18F]Fluoride for the Labeling of Radiopharmaceuticals. Angew. Chem., Int. Ed. 49, 3161–3164 (2010).
Richarz, R. et al. Neither Azeotropic Drying, nor Base nor Other Additives: a Minimalist Approach to 18F-Labeling. Org. Biomol. Chem. 12, 8094–8099 (2014).
Neumann, C. N., Hooker, J. M. & Ritter, T. Concerted nucleophilic aromatic substitution with 19F− and 18F−. Nature 534, 369–373 (2016).
Katsifis, A., Hamacher, K., Schnitter, J. & Stöcklin, G. Optimization Studies Concerning the Direct Nucleophilic Fluorination of Butyrophene Neuroleptics. Appl. Radiat. Isot. 44, 1015–1020 (1993).
Kostikov, A. P. et al. Oxalic Acid Supported Si-18F-Radiofluorinaton: One-step Radiosytnehsis of N-Succinimidyl 3-(Di-tert-butyl[18F]fluorosilyl)benzoate ([18F]SiFB) for Protein Labeling. Bioconjugate Chem. 23, 106–114 (2012).
Wessman, S. H., Henriksen, G. & Wester, H.-J. Cryptate Mediated Nucleophilic 18F-fluorination Without Azeotropic Drying. Nuklearmedizin 51, 1–8 (2012).
Perez, D. I. et al. Switching Reversibility to Irreversibility in Glycogen Synthase Kinase 3 Inhibitors: Clues for Specific Design of New Compounds. J. Med. Chem. 54, 4042–4056 (2011).
Mühlhausen, U., Ermert, J., Herth, M. M. & Coenen, H. H. Synthesis, Radiofluorination and First Evaluation of (±)-[18F]MDL 100907 as Serotonin 5-HT2A Receptor Antagonist for PET. J. Label. Compd. Radiopharm. 52, 6–12 (2008).
Hwang, D. R., Dence, C. S., Gong, J. & Welch, M. J. A New Procedure for Labeling Alkylbenzenes with Fluorine-18 [18F]Fluoride. Appl. Radiat. Isot. 42, 1043–1047 (1991).
de Vries, E. F. J., Vroegh, J., Elsinga, P. H. & Vaalburg, W. Evaluation of Fluorine-18-labeled Alkylating Agents as Potential Synthons for the Labeling of Oligonucleotides. Appl. Radiat. Isot. 58, 469–476 (2003).
Dence, C. S., McCarthy, T. J. & Welch, M. J. Improved Synthesis of p-[18F]fluorophenacyl Bromide: the Use of Polymer Supported Perbromide. Appl. Radiat. Isot. 44, 981–983 (1993).
Saikia, I., Borah, A. J. & Phukan, P. Use of Bromine and Bromo-organic Compounds in Organic Synthesis. Chem. Rev. 116, 6837–7042 (2016).
Vekariya, R. H. & Patel, H. D. Synthesis of α-Bromocarbonyl Compounds: Recent Advances. Tetrahedron 70, 3949–3961 (2014).
Makaravage, K., Brooks, A. F., Mossine, A. V., Sanford, M. S. & Scott, P. J. H. Copper-Mediated Radiofluorination of Arylstannanes with [18F]KF. Org. Lett. 18, 5440–5443 (2016).
Acknowledgements
We acknowledge the NIH (R01EB021155 to M.S.S. and P.J.H.S.), US DOE/NIBIB (DE-SC0012484 to P.J.H.S.), and Merck (M.S.S. and P.J.H.S.) for financial support.
Author information
Authors and Affiliations
Contributions
P.J.H.S. and M.S.S. conceived and designed experiments. A.V.M., A.F.B., K.M. and N.I. performed the experiments. All authors analyzed data and wrote the paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Mossine, A.V., Brooks, A.F., Ichiishi, N. et al. Development of Customized [18F]Fluoride Elution Techniques for the Enhancement of Copper-Mediated Late-Stage Radiofluorination. Sci Rep 7, 233 (2017). https://doi.org/10.1038/s41598-017-00110-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-00110-1
This article is cited by
-
Radiosynthesis and biological evaluation of [18F]AG-120 for PET imaging of the mutant isocitrate dehydrogenase 1 in glioma
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Multi-patient dose synthesis of [18F]Flumazenil via a copper-mediated 18F-fluorination
EJNMMI Radiopharmacy and Chemistry (2022)
-
Closing the gap between 19F and 18F chemistry
EJNMMI Radiopharmacy and Chemistry (2021)
-
Automated synthesis of 18F radiolabelled indole containing Oncrasin-like molecules; a comparison of iodonium salts and boronic ester chemistry
EJNMMI Radiopharmacy and Chemistry (2020)
-
Synthesis of high-molar-activity [18F]6-fluoro-l-DOPA suitable for human use via Cu-mediated fluorination of a BPin precursor
Nature Protocols (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.