Abstract
Many large molecular machines are too elaborate to assemble spontaneously and are built through ordered pathways orchestrated by dedicated chaperones. During assembly of the core particle (CP) of the proteasome, where protein degradation occurs, its six active sites are simultaneously activated via cleavage of N-terminal propeptides. Such activation is autocatalytic and coupled to fusion of two half-CP intermediates, which protects cells by preventing activation until enclosure of the active sites within the CP interior. Here we uncover key mechanistic aspects of autocatalytic activation, which proceeds through alignment of the β5 and β2 catalytic triad residues, respectively, with these triads being misaligned before fusion. This mechanism contrasts with most other zymogens, in which catalytic centers are preformed. Our data also clarify the mechanism by which individual subunits can be added in a precise, temporally ordered manner. This work informs two decades-old mysteries in the proteasome field, with broader implications for protease biology and multisubunit complex assembly.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jäger, S., Groll, M., Huber, R., Wolf, D. H. & Heinemeyer, W. Proteasome beta-type subunits: unequal roles of propeptides in core particle maturation and a hierarchy of active site function. J. Mol. Biol. 291, 997–1013 (1999).
Schnell, H. M., Walsh, R. M., Rawson, S. & Hanna, J. Chaperone-mediated assembly of the proteasome core particle – recent developments and structural insights. J. Cell Sci. 135, jcs259622 (2022).
Watanabe, A., Yashiroda, H., Ishihara, S., Lo, M. & Murata, S. The molecular mechanisms governing the assembly of the immuno- and thymoproteasomes in the presence of constitutive proteasomes. Cells 11, 1580 (2022).
Chen, P. & Hochstrasser, M. Autocatalytic subunit processing couples active site formation in the 20S proteasome to completion of assembly. Cell 86, 961–972 (1996).
Hirano, Y. et al. Dissecting beta-ring assembly pathway of the mammalian 20S proteasome. EMBO J. 27, 2204–2213 (2008).
Schnell, H. M. et al. Structures of chaperone-associated assembly intermediates reveal coordinated mechanisms of proteasome biogenesis. Nat. Struct. Mol. Biol. 28, 418–425 (2021).
Seemüller, E., Lupas, A. & Baumeister, W. Autocatalytic processing of the 20S proteasome. Nature 382, 468–470 (1996).
Huber, E. M. et al. A unified mechanism for proteolysis and autocatalytic activation in the 20S proteasome. Nat. Commun. 7, 10900 (2016).
Groll, M. et al. Structure of 20S proteasome from yeast at 2.4 Å resolution. Nature 386, 463–471 (1997).
Ramos, P. C., Marques, A. J., London, M. K. & Dohmen, R. J. Role of C-terminal extensions of subunits beta2 and beta7 in assembly and activity of eukaryotic proteasomes. J. Biol. Chem. 279, 14323–14330 (2004).
Walsh, R. M. et al. Structure of the preholoproteasome reveals late steps in proteasome core particle biogenesis. Nat. Struct. Mol. Biol. 30, 1516–1524 (2023).
Gerlinger, U. M., Gückel, R., Hoffmann, M., Wolf, D. H. & Hilt, W. Yeast cycloheximide-resistant crl mutants are proteasome mutants defective in protein degradation. Mol. Biol. Cell 8, 2487–2499 (1997).
Kock, M. et al. Proteasome assembly from 15S precursors involves major conformational changes and recycling of the Pba1–Pba2 chaperone. Nat. Commun. 6, 6123 (2015).
Li, X., Li, Y., Arendt, C. S. & Hochstrasser, M. Distinct elements in the proteasomal β5 subunit propeptide required for autocatalytic processing and proteasome assembly. J. Biol. Chem. 291, 1991–2003 (2016).
Xie, Y. & Varshavsky, A. RPN4 is a ligand, substrate, and transcriptional regulator of the 26S proteasome: a negative feedback circuit. Proc. Natl Acad. Sci. USA 98, 3056–3061 (2001).
Guerra-Moreno, A. & Hanna, J. Induction of proteotoxic stress by the mycotoxin patulin. Toxicol. Lett. 276, 85–91 (2017).
Ramos, P. C., Höckendorff, J., Johnson, E. S., Varshavsky, A. & Dohmen, R. J. Ump1p is required for proper maturation of the 20S proteasome and becomes its substrate upon completion of the assembly. Cell 92, 489–499 (1998).
Marques, A. J., Glanemann, C., Ramos, P. C. & Dohmen, R. J. The C-terminal extension of the beta7 subunit and activator complexes stabilize nascent 20S proteasomes and promote their maturation. J. Biol. Chem. 282, 34869–34876 (2007).
Arendt, C. S. & Hochstrasser, M. Eukaryotic 20S proteasome catalytic subunit propeptides prevent active site inactivation by N-terminal acetylation and promote particle assembly. EMBO J. 18, 3575–3585 (1999).
Matias, A. C., Matos, J., Dohmen, R. J. & Ramos, P. C. Hsp70 and Hsp110 chaperones promote early steps of proteasome assembly. Biomolecules 13, 11 (2022).
Khan, A. R. & James, M. N. Molecular mechanisms for the conversion of zymogens to active proteolytic enzymes. Protein Sci. 7, 815–836 (1998).
Richter, C., Tanaka, T. & Yada, R. Y. Mechanism of activation of the gastric aspartic proteinases: pepsinogen, progastricsin and prochymosin. Biochem. J. 335, 481–490 (1998).
Arolas, J. L., Goulas, T., Cuppari, A. & Gomis-Rüth, F. X. Multiple architectures and mechanisms of latency in metallopeptidase zymogens. Chem. Rev. 118, 5581–5597 (2018).
Poli, M. C. et al. Heterozygous truncating variants in POMP escape nonsense-mediated decay and cause a unique immune dysregulatory syndrome. Am. J. Hum. Genet. 102, 1126–1142 (2018).
de Jesus, A. A. et al. Novel proteasome assembly chaperone mutations in PSMG2/PAC2 cause the autoinflammatory interferonopathy CANDLE/PRAAS4. J. Allergy Clin. Immunol. 143, 1939–1943.e8 (2019).
Dahlqvist, J. et al. A single-nucleotide deletion in the POMP 5′ UTR causes a transcriptional switch and altered epidermal proteasome distribution in KLICK genodermatosis. Am. J. Hum. Genet. 86, 596–603 (2010).
Ansar, M. et al. Biallelic variants in PSMB1 encoding the proteasome subunit β6 cause impairment of proteasome function, microcephaly, intellectual disability, developmental delay and short stature. Hum. Mol. Genet. 29, 1132–1143 (2020).
Hwang, G.-W., Ishida, Y. & Naganuma, A. Identification of F-box proteins that are involved in resistance to methylmercury in Saccharomyces cerevisiae. FEBS Lett. 580, 6813–6818 (2006).
Wani, P. S., Rowland, M. A., Ondracek, A., Deeds, E. J. & Roelofs, J. Maturation of the proteasome core particle induces an affinity switch that controls regulatory particle association. Nat. Commun. 6, 6384 (2015).
Weisshaar, N., Welsch, H., Guerra-Moreno, A. & Hanna, J. Phospholipase Lpl1 links lipid droplet function with quality control protein degradation. Mol. Biol. Cell 28, 716–725 (2017).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Wagner, T. et al. SPHIRE-crYOLO is a fast and accurate fully automated particle picker for cryo-EM. Commun. Biol. 2, 218 (2019).
Scheres, S. H. W. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. CryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Zhong, E. D., Bepler, T., Berger, B. & Davis, J. H. CryoDRGN: reconstruction of heterogeneous cryo-EM structures using neural networks. Nat. Methods 18, 176–185 (2021).
Morin, A. et al. Collaboration gets the most out of software. eLife 2, e01456 (2013).
Jamali, K. et al. Automated model building and protein identification in cryo-EM maps. Nature https://doi.org/10.1038/s41586-024-07215-4 (2024).
Pettersen, E. F. et al. UCSF Chimera – a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Croll, T. I. ISOLDE: a physically realistic environment for model building into low-resolution electron-density maps. Acta Crystallogr. D Struct. Biol. 74, 519–530 (2018).
Liebschner, D. et al. Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 75, 861–877 (2019).
Kao, A. et al. Development of a novel cross-linking strategy for fast and accurate identification of cross-linked peptides of protein complexes. Mol. Cell Proteomics 10, M110.002212 (2011).
Gutierrez, C. B. et al. Developing an acidic residue reactive and sulfoxide-containing MS-cleavable homobifunctional cross-linker for probing protein–protein interactions. Anal. Chem. 88, 8315–8322 (2016).
Wiśniewski, J. R., Zougman, A., Nagaraj, N. & Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 6, 359–362 (2009).
Jiao, F. et al. Two-dimensional fractionation method for proteome-wide cross-linking mass spectrometry analysis. Anal. Chem. 94, 4236–4242 (2022).
Jiao, F. et al. Exploring an alternative cysteine-reactive chemistry to enable proteome-wide PPI analysis by cross-linking mass spectrometry. Anal. Chem. 95, 2532–2539 (2023).
Finley, D., Ozkaynak, E. & Varshavsky, A. The yeast polyubiquitin gene is essential for resistance to high temperatures, starvation, and other stresses. Cell 48, 1035–1046 (1987).
Gietz, R. D. & Sugino, A. New yeast–Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74, 527–534 (1988).
Acknowledgements
We thank J. Roelofs (University of Kansas Medical Center) for the Pba1/2 antibody; H. Schnell for early contributions to the project; and B. Schulman, F. Adolf, J. Du, E. Goodall, J. W. Harper and D. Finley for helpful discussions. This work was supported by National Institutes of Health grants R01-GM144367 and R01-GM135337 (to J.H.), and R35-GM145249 (to L.H.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
B.V., J.H., L.A., E.F.P., M.B., A.R., T.R. and D.F. performed the biochemical aspects of the work. R.M.W. and S.R. performed cryo-EM sample preparation, data collection, data processing, model building and refinement. R.M.W., S.R. and A.R. performed the data analysis. F.J. and L.H. performed cross-linking mass spectrometry. A.R. and J.H. prepared the figures. J.H. wrote the paper with assistance from B.V., R.M.W. and S.R., and input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks Youdong Mao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Dimitris Typas was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Conservation of midline stabilizing aspartates and arginines.
a, Comparison of the C-termini for all 7 yeast β-subunits. Only β3 and β6 end in aspartate. b, Evolutionary conservation of the β3/β5 midline salt bridge residues, β3-D205 and β5-R94. β5′s nearby catalytic triad residue, D92, is shown for reference. c, Evolutionary conservation of the β2/β6 midline salt bridge residues, β6-D241 and β2-R94. β2′s nearby catalytic triad residue, D46, is shown for reference.
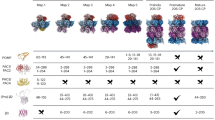
Extended Data Fig. 2 Cryo-EM classification of CP species.
Shown is the processing scheme for classification and refinement of proteasome species. ‘Junk” classes are colored grey while identifiable species are colored by species. The asterisk denotes cryoDRGN classification - displayed classes show representative maps generated via k-means clustering and are not necessarily correlated to the particle selection carried out by clustering of the latent embeddings. All other 3D classification steps were carried out in cryoSPARC.
Extended Data Fig. 3 Cryo-EM data analysis for CP species.
a, Representative micrograph of proteasome particles embedded in vitreous ice. Scale bar, 500 Å. A total of 375,915 individual particles were analyzed. b, Selected 2D class averages of 20S, preholoproteasome, and Blm10-CP particles. c, Proteasome reconstructions were filtered and colored by local resolution (left panels), gold-standard Fourier shell correlation (FSC) curves from cryoSPARC (center panels), and viewing direction distribution plots (right panels). Resolution was determined at FSC = 0.143.
Extended Data Fig. 4 Comparison of Ump1 before and after CP fusion.
a, Molecular model of Ump1 from pre-15S (PDB: 7LS6) and the β3-D205Δ preholoproteasome. The first and last resolved residues are shown. b, Overlay of the two models, showing no significant conformational changes.
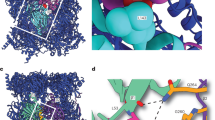
Extended Data Fig. 5 Detailed analysis of the β5-propeptide.
a, Comparison of β5 between mature wild-type 20S (PDB: 5CZ4) and the β3-D205Δ preholoproteasome. The mature portions of β5 largely overlap, although there is a slight rotation of β5 towards the CP midline in the mature 20S, which may reflect final tightening of the CP upon completion of assembly. The first and last resolved residues are indicated. b, Overlay of the two structures from panel A, highlighting the differences between them. Arrows designate portions of β5 that are unresolved in preholoproteasome. c, Overlay of the molecular model of the β5-propeptide onto its primary map density, confirming the validity of the model. Boxed panels show close-up views of selected regions and arrows indicate their position in the overall propeptide.
Extended Data Fig. 6 Validation of the β5 Propeptide Structure by Crosslinking Mass Spectrometry.
Crosslinking mass spectrometry was performed on β3-D205Δ proteasomes, which identified two crosslinks involving the β5-propeptide: β5-D61/D62 with β5-D193, and β5-E27 with α5-E131. A third crosslink, β5-K16 with α6-K115, was detected in a prior study13. All three crosslinks are within the crosslinkable distance for the respective Lys-Lys and Asp/Glu-Asp/Glu crosslinkers, and strongly support the modeled structure of the β5-propeptide. The orange dashed lines indicate the crosslinked residues. The other colored dashed lines indicate unresolved residues.
Extended Data Fig. 7 Graphical Representation of the Crosslinking Mass Spectrometry Data for the Propeptides, Pba1/2, and Ump1.
Intersubunit (green lines) and intrasubunit (blue lines) crosslinks are shown for the β-subunit propeptides (panel A), Pba1/2 (panel B), and Ump1 (panel C). Shaded areas indicate the propeptides.
Extended Data Fig. 8 Analysis of the β1 catalytic triad.
Arg38 in β1 shows a similar orientation to the corresponding arginine residues in β5 and β2, and is hydrogen bonded (dashed lines) to the triad aspartate and lysine residues, suggesting that it may play an important role in β1 activation. However, unlike the situation in β5 and β2, β1′s Arg38 does not form a salt bridge or even contact its midline partner, β7, suggesting that additional mechanisms may account for β1 activation.
Supplementary information
Source data
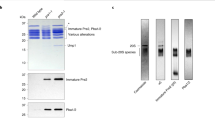
Source Data Fig. 3g
Unprocessed gel and blots.
Source Data Fig. 4b,e
Unprocessed gel and blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Velez, B., Walsh, R.M., Rawson, S. et al. Mechanism of autocatalytic activation during proteasome assembly. Nat Struct Mol Biol (2024). https://doi.org/10.1038/s41594-024-01262-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41594-024-01262-1