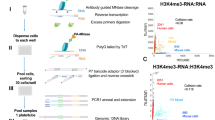
Abstract
The three-dimensional structure of chromatin plays a crucial role in development and disease, both of which are associated with transcriptional changes. However, given the heterogeneity in single-cell chromatin architecture and transcription, the regulatory relationship between the three-dimensional chromatin structure and gene expression is difficult to explain based on bulk cell populations. Here we develop a single-cell, multimodal, omics method allowing the simultaneous detection of chromatin architecture and messenger RNA expression by sequencing (single-cell transcriptome sequencing (scCARE-seq)). Applying scCARE-seq to examine chromatin architecture and transcription from 2i to serum single mouse embryonic stem cells, we observe improved separation of cell clusters compared with single-cell chromatin conformation capture. In addition, after defining the cell-cycle phase of each cell through chromatin architecture extracted by scCARE-seq, we find that periodic changes in chromatin architecture occur in parallel with transcription during the cell cycle. These findings highlight the potential of scCARE-seq to facilitate comprehensive analyses that may boost our understanding of chromatin architecture and transcription in the same single cell.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Sequencing data have been deposited at the NCBI GEO with accession number GSE211395, using mm10 reference genome. Other public datasets used in this study were downloaded from NCBI GEO with accession numbers as follows: ChIP-seq (GSE90895; CTCF and H3K27ac), in situ Hi-C (GSE124342), 2013-Nagano (GSE48262), 2017-Flyamer (GSE80006), 2017-Nagano (GSE94489), 2017-Steven (GSE80280), 2019-Tan (GSE121791), 2021-Tan (GSE162511), sci-CAR (GSE117089), SNARE-seq (GSE126074), CoTECH (GSE158435), Paired-Tag (GSE152020) and Paired-seq (GSE130399). Source data are provided with this paper.
Code availability
Custom scripts used in this study are available from https://github.com/jsun9003/scCARE-seq.
References
Cremer, T. & Cremer, C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat. Rev. Genet. 2, 292–301 (2001).
Dekker, J. & Mirny, L. The 3D genome as moderator of chromosomal communication. Cell 164, 1110–1121 (2016).
Wei, C. et al. CTCF organizes inter-A compartment interactions through RYBP-dependent phase separation. Cell Res. 32, 744–760 (2022).
Bonev, B. et al. Multiscale 3D genome rewiring during mouse neural development. Cell 171, 557–572.e524 (2017).
Du, Z. et al. Allelic reprogramming of 3D chromatin architecture during early mammalian development. Nature 547, 232–235 (2017).
Lupiáñez, D. G. et al. Disruptions of topological chromatin domains cause pathogenic rewiring of gene-enhancer interactions. Cell 161, 1012–1025 (2015).
Lieberman-Aiden, E. et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science 326, 289–293 (2009).
Dixon, J. R. et al. Chromatin architecture reorganization during stem cell differentiation. Nature 518, 331–336 (2015).
Rao, S. S. P. et al. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell 159, 1665–1680 (2014).
Huang, J., Jiang, Y., Zheng, H. & Ji, X. BAT Hi-C maps global chromatin interactions in an efficient and economical way. Methods 170, 38–47 (2020).
Quinodoz, S. A. et al. Higher-order inter-chromosomal hubs shape 3D genome organization in the nucleus. Cell 174, 744–757.e724 (2018).
Kempfer, R. & Pombo, A. Methods for mapping 3D chromosome architecture. Nat. Rev. Genet. 21, 207–226 (2020).
Mortazavi, A., Williams, B. A., McCue, K., Schaeffer, L. & Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 5, 621–628 (2008).
Marioni, J. C., Mason, C. E., Mane, S. M., Stephens, M. & Gilad, Y. RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res. 18, 1509–1517 (2008).
Gertz, J. et al. Transposase mediated construction of RNA-seq libraries. Genome Res. 22, 134–141 (2012).
Lianoglou, S., Garg, V., Yang, J. L., Leslie, C. S. & Mayr, C. Ubiquitously transcribed genes use alternative polyadenylation to achieve tissue-specific expression. Genes Dev. 27, 2380–2396 (2013).
Schwartzman, O. & Tanay, A. Single-cell epigenomics: techniques and emerging applications. Nat. Rev. Genet. 16, 716–726 (2015).
Wen, L. & Tang, F. Single-cell sequencing in stem cell biology. Genome Biol. 17, 71 (2016).
Nagano, T. et al. Single-cell Hi-C reveals cell-to-cell variability in chromosome structure. Nature 502, 59–64 (2013).
Nagano, T. et al. Cell-cycle dynamics of chromosomal organization at single-cell resolution. Nature 547, 61–67 (2017).
Stevens, T. J. et al. 3D structures of individual mammalian genomes studied by single-cell Hi-C. Nature 544, 59–64 (2017).
Flyamer, I. M. et al. Single-nucleus Hi-C reveals unique chromatin reorganization at oocyte-to-zygote transition. Nature 544, 110–114 (2017).
Ramani, V. et al. Massively multiplex single-cell Hi-C. Nat. Methods 14, 263–266 (2017).
Tan, L., Xing, D., Chang, C.-H., Li, H. & Xie, X. S. Three-dimensional genome structures of single diploid human cells. Science 361, 924–928 (2018).
Tang, F. et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382 (2009).
Svensson, V., Vento-Tormo, R. & Teichmann, S. A. Exponential scaling of single-cell RNA-seq in the past decade. Nat. Protoc. 13, 599–604 (2018).
Stark, R., Grzelak, M. & Hadfield, J. RNA sequencing: the teenage years. Nat. Rev. Genet. 20, 631–656 (2019).
Stuart, T. & Satija, R. Integrative single-cell analysis. Nat. Rev. Genet. 20, 257–272 (2019).
Miao, Z., Humphreys, B. D., McMahon, A. P. & Kim, J. Multi-omics integration in the age of million single-cell data. Nat. Rev. Nephrol. 17, 710–724 (2021).
Mateo, L. J. et al. Visualizing DNA folding and RNA in embryos at single-cell resolution. Nature 568, 49–54 (2019).
Su, J.-H., Zheng, P., Kinrot, S. S., Bintu, B. & Zhuang, X. Genome-scale imaging of the 3D organization and transcriptional activity of chromatin. Cell 182, 1641–1659.e1626 (2020).
Cardozo Gizzi, A. M. et al. Direct and simultaneous observation of transcription and chromosome architecture in single cells with Hi-M. Nat. Protoc. 15, 840–876 (2020).
Takei, Y. et al. Integrated spatial genomics reveals global architecture of single nuclei. Nature 590, 344–350 (2021).
Cao, J. et al. Comprehensive single-cell transcriptional profiling of a multicellular organism. Science 357, 661–667 (2017).
Lee, D.-S. et al. Simultaneous profiling of 3D genome structure and DNA methylation in single human cells. Nat. Methods 16, 999–1006 (2019).
Yang, T. et al. HiCRep: assessing the reproducibility of Hi-C data using a stratum-adjusted correlation coefficient. Genome Res. 27, 1939–1949 (2017).
Bakken, T. E. et al. Single-nucleus and single-cell transcriptomes compared in matched cortical cell types. PLoS ONE 13, e0209648 (2018).
Wu, H., Kirita, Y., Donnelly, E. L. & Humphreys, B. D. Advantages of single-nucleus over single-cell RNA sequencing of adult kidney: rare cell types and novel cell states revealed in fibrosis. J. Am. Soc. Nephrol. 30, 23–32 (2019).
Tan, L., Xing, D., Daley, N. & Xie, X. S. Three-dimensional genome structures of single sensory neurons in mouse visual and olfactory systems. Nat. Struct. Mol. Biol. 26, 297–307 (2019).
Tan, L. et al. Changes in genome architecture and transcriptional dynamics progress independently of sensory experience during post-natal brain development. Cell 184, 741–758.e717 (2021).
Li, G. et al. Joint profiling of DNA methylation and chromatin architecture in single cells. Nat. Methods 16, 991–993 (2019).
Zhu, C. et al. An ultra high-throughput method for single-cell joint analysis of open chromatin and transcriptome. Nat. Struct. Mol. Biol. 26, 1063–1070 (2019).
Zhu, C. et al. Joint profiling of histone modifications and transcriptome in single cells from mouse brain. Nat. Methods 18, 283–292 (2021).
Xiong, H., Luo, Y., Wang, Q., Yu, X. & He, A. Single-cell joint detection of chromatin occupancy and transcriptome enables higher-dimensional epigenomic reconstructions. Nat. Methods 18, 652–660 (2021).
Chen, S., Lake, B. B. & Zhang, K. High-throughput sequencing of the transcriptome and chromatin accessibility in the same cell. Nat. Biotechnol. 37, 1452–1457 (2019).
Cao, J. et al. Joint profiling of chromatin accessibility and gene expression in thousands of single cells. Science 361, 1380–1385 (2018).
Mimitou, E. P. et al. Multiplexed detection of proteins, transcriptomes, clonotypes and CRISPR perturbations in single cells. Nat. Methods 16, 409–412 (2019).
Ficz, G. et al. FGF signaling inhibition in ESCs drives rapid genome-wide demethylation to the epigenetic ground state of pluripotency. Cell Stem Cell 13, 351–359 (2013).
Marks, H. et al. The transcriptional and epigenomic foundations of ground state pluripotency. Cell 149, 590–604 (2012).
McLaughlin, K. et al. DNA methylation directs polycomb-dependent 3D genome re-organization in naive pluripotency. Cell Rep. 29, 1974–1985.e1976 (2019).
Naumova, N. et al. Organization of the mitotic chromosome. Science 342, 948–953 (2013).
Gibcus, J. H. et al. A pathway for mitotic chromosome formation. Science 359, eaao6135 (2018).
Stuparević, I. et al. Regulation of the conserved 3′–5′ exoribonuclease EXOSC10/Rrp6 during cell division, development and cancer. Biol. Rev. Camb. Philos. Soc. 96, 1092–1113 (2021).
Zhu, C., Preissl, S. & Ren, B. Single-cell multimodal omics: the power of many. Nat. Methods 17, 11–14 (2020).
Picelli, S. et al. Tn5 transposase and tagmentation procedures for massively scaled sequencing projects. Genome Res. 24, 2033–2040 (2014).
Lai, B. et al. Trac-looping measures genome structure and chromatin accessibility. Nat. Methods 15, 741–747 (2018).
Servant, N. et al. HiC-Pro: an optimized and flexible pipeline for Hi-C data processing. Genome Biol. 16, 259 (2015).
Imakaev, M. et al. Iterative correction of Hi-C data reveals hallmarks of chromosome organization. Nat. Methods 9, 999–1003 (2012).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2009).
Ma, S. et al. Chromatin potential identified by shared single-cell profiling of RNA and chromatin. Cell 183, 1103–1116.e1120 (2020).
Wolff, J. et al. Galaxy HiCExplorer 3: a web server for reproducible Hi-C, capture Hi-C and single-cell Hi-C data analysis, quality control and visualization. Nucleic Acids Res. 48, W177–W184 (2020).
van der Weide, R. H. et al. Hi-C analyses with GENOVA: a case study with cohesin variants. NAR Genom. Bioinform. 3, lqab040 (2021).
Lopez-Delisle, L. et al. pyGenomeTracks: reproducible plots for multivariate genomic datasets. Bioinformatics 37, 422–423 (2020).
Durand, N. C. et al. Juicer provides a one-click system for analyzing loop-resolution Hi-C experiments. Cell Syst. 3, 95–98 (2016).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Shin, H. et al. TopDom: an efficient and deterministic method for identifying topological domains in genomes. Nucleic Acids Res. 44, e70 (2015).
Zhang, R., Zhou, T. & Ma, J. Multiscale and integrative single-cell Hi-C analysis with Higashi. Nat. Biotechnol. 40, 254–261 (2022).
Zhang, S. et al. DeepLoop robustly maps chromatin interactions from sparse allele-resolved or single-cell Hi-C data at kilobase resolution. Nat. Genet. 54, 1013–1025 (2022).
Chronis, C. et al. Cooperative binding of transcription factors orchestrates reprogramming. Cell 168, 442–459.e420 (2017).
Satija, R., Farrell, J. A., Gennert, D., Schier, A. F. & Regev, A. Spatial reconstruction of single-cell gene expression data. Nat. Biotechnol. 33, 495–502 (2015).
Acknowledgements
We are grateful to members of the Ding laboratories for discussions on the paper. This research was funded by grants from the National Natural Science Foundation of China (grant nos 31970811 and 32170798), the Guangdong Basic and Applied Basic Research Foundation (grant no. 2021B1515120063), the Guangdong Regenerative Medicine and Health of Guangdong Laboratory Frontier Exploration Project (grant no. 2018GZR110105007) and the Guangdong Innovative and Entrepreneurial Research Team Program (grant no. 2016ZT06S029) to J.D.; the Natural Science Foundation of Guangdong Province, China (grant nos 2021A1515010938 and 2023A1515010148) to J.S.; the National Natural Science Foundation of China (grant no. 32100497), the Natural Science Foundation of Guangdong Province, China (grant no. 2023A1515010197) and Postdoctoral Program (grant no. 2021M703760) to C.W.; the Fundamental Research Funds for the Central Universities of Jinan University (Natural Science) (grant no. 2162004), China Postdoctoral Science Foundation (grant no. 2021M701441), China Postdoctoral Special Grant Foundation (grant no. 2022T150269), Guangdong Basic and Applied Basic Research Foundation (grant no. 2021A1515111056) and Guangzhou Basic and Applied Basic Research Foundation (grant no. 202201010961) to L.F.; and the National Natural Science Foundation of China (grant no. 32100927) to H.Y.
Author information
Authors and Affiliations
Contributions
J.D. and J.Q. conceived and designed the study. J.Q. designed and performed all experiments. J.Q., J.S., X.L., C.Z. and X. Zhang performed the data analysis. J.Q., J.S. and C.Z. wrote the paper with input from all other authors. S.J. and C.W. discussed results and edited the manuscript. H.Y., X. Zeng and L.F. provided support. J.D. supervised the research.
Corresponding authors
Ethics declarations
Competing interests
J.D., J.Q. and J.S. are listed as inventors of a patent application titled ‘Single-cell simultaneous detection of 3D chromatin structure and gene expression by sequencing’. The other authors declare no competing interests.
Peer review
Peer review information
Nature Structural & Molecular Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available. Primary Handling Editor: Dimitris Typas, in collaboration with the Nature Structural & Molecular Biology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Schematics for pre-amplification and construction of DNA/RNA libraries.
a, Pre-amplification was achieved by primers mix to amplify nucleic acid numbers. DNA/RNA libraries construction was worked by specific DNA or RNA primers. b, Scatter plots showing the mapped DNA reads of Hi-C data (left) and mapped RNA -reads of RNA-seq data (middle) in hg19 and mm10 for each cell. And the fraction of human reads in DNA and RNA libraries for each cell (right). HEK293T refers to human embryonic kidney 293 T, and mESCs refers to mouse embryonic stem cells.
Extended Data Fig. 2 Overview of our method to simultaneously detect 3D chromatin structure and transcription.
a,b, Track view displaying both contact matrices and RNA signals from 60-63 Mb of chromosome 10 (a) and 80-83 Mb of chromosome 15 (b).
Extended Data Fig. 3 Performance of CARE-seq in comparative analyses.
a, Saddle plots: average contact enrichment between pairs of 500 kb regions arranged by their compartment scores and the difference was Hi-C compared to CARE-seq. The upper right quarter represents A-A interactions, the bottom left quarter represents B-B interactions. b, The similarity of different bulk 3D chromatin structure data was evaluated by HiCRep at 500 kb resolution per euchromosome (n = 19). The values represented the mean Stratum-adjusted Correlation Coefficient (SCC). The boxplots were drawn from lower quartile (Q1) to upper quartile (Q3), with the middle line denoting the median, whiskers with maximum 1.5 interquartile range (IQR) and outliers were not indicated. c, Dependence of contact probability on genomic separation for single cells from CARE-seq data (orange) and Hi-C data (black). d, Insulation profiles of CARE-seq and Hi-C over 40 kb bins in chromosomes 2. e-g, Scatter plots show the strong concordance of gene expression signals from two technical replicates in nuclear mRNA (g) and total mRNA (f), and total mRNA versus CARE-seq (g).
Extended Data Fig. 4 Comparison of CARE-seq and typical RNA-seq in gene expression profiles.
Representative regions showing a consistent pattern of gene expression across datasets.
Extended Data Fig. 5 Comparison of 2i and serum mESCs from scCARE-seq.
a, The tables summarized the Hi-C (top) and RNA-seq data (bottom) of Supplementary Table 2, respectively. b,c, Comparison of scCARE-seq data from 192 2i mESCs and 192 serum mESCs. The contacts numbers (left) and cis-to-trans ratio (right) (b); UMIs (left) and expressed gene numbers (right) (c). The boxplots were drawn from lower quartile (Q1) to upper quartile (Q3), with the middle line denoting the median, whiskers with maximum 1.5 interquartile range (IQR) and outliers were not indicated.
Extended Data Fig. 6 scCARE-seq data quality in mESCs.
a, Distribution of cis long-range interactions (>20 kb) in the scCARE-seq data (n = 384, median = 52.04%). b, The similarity between scCARE-seq Hi-C data and Hi-C or CARE-seq Hi-C data was evaluated by HiCRep at 500 kb resolution per euchromosome (n = 19). The values represented the mean Stratum-adjusted Correlation Coefficient (SCC). The boxplots were drawn from lower quartile (Q1) to upper quartile (Q3), with the middle line denoting the median, whiskers with maximum 1.5 interquartile range (IQR) and outliers were not indicated. c, Comparison of contact heatmap of chromosome 3 between scCARE-seq and CARE-seq, at 1 Mb resolution (left); 50–140 Mb/250 kb resolution (right). Matrix similarity is evaluated by HiCRep at the corresponding resolution. SCC, Stratum-adjusted Correlation Coefficient. d, Dependence of contact probability on genomic separation for single cells from scCARE-seq data (n = 192, yellow), combined scCARE-seq data from all cells (orange) and bulk CARE-seq data (black). e, Cumulative coverage percentage of genes detected in single cells compared to the bulk data. f, A representative region showing a consistent pattern of gene expression across datasets generated using scCARE-seq and CARE-seq. The transcriptional profiles are gene expression read counts from bulk (upper) and a total of 83 single cells (bottom).
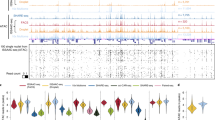
Extended Data Fig. 7 The relationship between chromatin architecture and gene expression in the different cell clusters.
a,b, Pearson’s correlation matrixes from different cell clusters. Contacts numbers in different clusters were sampled to same numbers and plot the balanced matrixes in Juicebox (version 1.11.08). Pearson’s correlation coefficient was calculated under 1-Mb resolution. White frame shows the difference regions. c,d, Showing change in expression of the different clusters (top) in the compartment A to B (c) and compartment B to A (d), where the compartment switch was defined by published Hi-C data (bottom). 2i and serum have two replications, respectively. CS represents log2(compartment scores+1). The boxplots were drawn from lower quartile (Q1) to upper quartile (Q3), with the middle line denoting the median, whiskers with maximum 1.5 interquartile range (IQR) and outliers were not indicated.
Extended Data Fig. 8 The relationship between chromatin architecture and gene expression in the cell cycle.
a, Saddle plots: average contact enrichment between pairs of 100 kb regions arranged by their compartment scores in the different cell cycle phases. b, Uniform manifold approximation and projection (UMAP) embedding showing the clustering of single cells from scCARE-seq 3D chromatin structural profiles. Each dot represents an individual cell and each color represents a cell cluster. c, GO enriched by marker genes of clusters in b. d, Percentage of inter-chromosomal contacts per single mESC in 2i and serum were ordered by cell-cycle phasing and each cell was annotated by cell type colored the same as in a. The black line represents mean trend. Shadow represents the confidence intervals of 0.95. e, Similar to d, Contacts, E-P interactions, UMIs and number of expressed genes per single serum mESCs from left to right. f, Comparison of UMI in single cells of top and bottom group in 2i mESCs. The top and bottom groups were selected based on the top and the bottom each 48 single cells (25%) ranked by number of E-P interactions from highest to lowest. The boxplots were drawn from lower quartile (Q1) to upper quartile (Q3), with the middle line denoting the median, whiskers with maximum 1.5 interquartile range (IQR) and outliers were not indicated. P value, one-sided Wilcoxon signed-rank test. g, Venn diagrams showing the expressed genes in E-P interactions of Early-S and LateS-G2 in e (right). h, Partial gene ontology (GO) terms, enriched by specific genes of Early-S and LateS-G2 in g, respectively.
Supplementary information
Supplementary Tables
Supplementary Table 1. The sequences of primers used in this study. Supplementary Table 2. The data quality of scCARE-seq DNA and RNA in this study. Supplementary Table 3. The phasing of cell cycle in scCARE-seq. Supplementary Table 4. The known cell cycle marker genes list.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qu, J., Sun, J., Zhao, C. et al. Simultaneous profiling of chromatin architecture and transcription in single cells. Nat Struct Mol Biol 30, 1393–1402 (2023). https://doi.org/10.1038/s41594-023-01066-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41594-023-01066-9