Abstract
To make adaptive decisions, we build an internal model of the associative relationships in an environment and use it to make predictions and inferences about specific available outcomes. Detailed, identity-specific cue–reward memories are a core feature of such cognitive maps. Here we used fiber photometry, cell-type and pathway-specific optogenetic manipulation, Pavlovian cue–reward conditioning and decision-making tests in male and female rats, to reveal that ventral tegmental area dopamine (VTADA) projections to the basolateral amygdala (BLA) drive the encoding of identity-specific cue–reward memories. Dopamine is released in the BLA during cue–reward pairing; VTADA→BLA activity is necessary and sufficient to link the identifying features of a reward to a predictive cue but does not assign general incentive properties to the cue or mediate reinforcement. These data reveal a dopaminergic pathway for the learning that supports adaptive decision-making and help explain how VTADA neurons achieve their emerging multifaceted role in learning.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data that support the findings of this study are available as Supplementary Information. Source data are provided with this paper.
Code availability
Custom-written MATLAB code is available from the corresponding author upon request. The basic code is available via Dryad (https://doi.org/10.5068/D1109S).
References
Steinberg, E. E. et al. A causal link between prediction errors, dopamine neurons and learning. Nat. Neurosci. 16, 966–973 (2013).
Schultz, W., Dayan, P. & Montague, P. R. A neural substrate of prediction and reward. Science 275, 1593–1599 (1997).
Eshel, N., Tian, J., Bukwich, M. & Uchida, N. Dopamine neurons share common response function for reward prediction error. Nat. Neurosci. 19, 479–486 (2016).
Waelti, P., Dickinson, A. & Schultz, W. Dopamine responses comply with basic assumptions of formal learning theory. Nature 412, 43–48 (2001).
Schultz, W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 80, 1–27 (1998).
Montague, P. R., Dayan, P. & Sejnowski, T. J. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J. Neurosci. 16, 1936–1947 (1996).
Delamater, A. R. On the nature of CS and US representations in Pavlovian learning. Learn Behav. 40, 1–23 (2012).
Fanselow, M. S. & Wassum, K. M. The origins and organization of vertebrate Pavlovian conditioning. Cold Spring Harb. Perspect. Biol. 8, a021717 (2015).
Tolman, E. C. Cognitive maps in rats and men. Psychol. Rev. 55, 189–208 (1948).
Sharpe, M. J. et al. Dopamine transients are sufficient and necessary for acquisition of model-based associations. Nat. Neurosci. 20, 735–742 (2017).
Sharp, M. E., Foerde, K., Daw, N. D. & Shohamy, D. Dopamine selectively remediates ‘model-based’reward learning: a computational approach. Brain 139, 355–364 (2015).
Stalnaker, T. A. et al. Dopamine neuron ensembles signal the content of sensory prediction errors. Elife 8, e49315 (2019).
Howard, J. D. & Kahnt, T. Identity prediction errors in the human midbrain update reward-identity expectations in the orbitofrontal cortex. Nat. Commun. 9, 1611 (2018).
Keiflin, R., Pribut, H. J., Shah, N. B. & Janak, P. H. Ventral tegmental dopamine neurons participate in reward identity predictions. Curr. Biol. 29, 93–103 (2019).
Chang, C. Y., Gardner, M., Di Tillio, M. G. & Schoenbaum, G. Optogenetic blockade of dopamine transients prevents learning induced by changes in reward features. Curr. Biol. 27, 3480–3486 (2017).
Wunderlich, K., Smittenaar, P. & Dolan, R. J. Dopamine enhances model-based over model-free choice behavior. Neuron 75, 418–424 (2012).
Seitz, B. M., Hoang, I. B., DiFazio, L. E., Blaisdell, A. P. & Sharpe, M. J. Dopamine errors drive excitatory and inhibitory components of backward conditioning in an outcome-specific manner. Curr. Biol. 32, 3210–3218 (2022).
Langdon, A. J., Sharpe, M. J., Schoenbaum, G. & Niv, Y. Model-based predictions for dopamine. Curr. Opin. Neurobiol. 49, 1–7 (2018).
Gardner, M. P. H., Schoenbaum, G. & Gershman, S. J. Rethinking dopamine as generalized prediction error. Proc. Biol. Sci. 285, 20181645 (2018).
Nasser, H. M., Calu, D. J., Schoenbaum, G. & Sharpe, M. J. The dopamine prediction error: contributions to associative models of reward learning. Front. Psychol. 8, 244 (2017).
Keiflin, R. & Janak, P. H. Error-driven learning: dopamine signals more than value-based errors. Curr. Biol. 27, R1321–R1324 (2017).
Brinley-Reed, M. & McDonald, A. J. Evidence that dopaminergic axons provide a dense innervation of specific neuronal subpopulations in the rat basolateral amygdala. Brain Res. 850, 127–135 (1999).
Beier, K. T. et al. Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 162, 622–634 (2015).
Sias, A. et al. A bidirectional corticoamygdala circuit for the encoding and retrieval of detailed reward memories. ELife 10, e68617 (2021).
Colwill, R. M. & Motzkin, D. K. Encoding of the unconditioned stimulus in Pavlovian conditioning. Anim. Learn. Behav. 22, 384–394 (1994).
Rescorla, R. A. Preservation of Pavlovian associations through extinction. Q. J. Exp. Psychol. B 49, 245–258 (1996).
Costa, K. M., et al. The role of the orbitofrontal cortex in creating cognitive maps. Nature Neurosci. https://doi.org/10.1038/s41593-022-01216-0 (2022).
Lutas, A. et al. State-specific gating of salient cues by midbrain dopaminergic input to basal amygdala. Nat. Neurosci. 22, 1820–1833 (2019).
Corbit, L. H. & Balleine, B. W. Learning and motivational processes contributing to Pavlovian-instrumental transfer and their neural bases: dopamine and beyond. Curr. Top. Behav. Neurosci. 27, 259–289 (2016).
Holland, P. C. & Straub, J. J. Differential effects of two ways of devaluing the unconditioned stimulus after Pavlovian appetitive conditioning. J. Exp. Psychol. Anim. Behav. Process 5, 65–78 (1979).
Delamater, A. R. & Oakeshott, S. Learning about multiple attributes of reward in Pavlovian conditioning. Ann. N. Y. Acad. Sci. 1104, 1–20 (2007).
Kamin, L. J. Predictability, surprise, attention, and conditioning. in Punishment Aversive Behavior (eds Church, R. M. & Campbell, B. A.) 279–296 (Appleton-Century-Crofts, 1969).
Rescorla, R. A. Learning about qualitatively different outcomes during a blocking procedure. Anim. Learn. Behav. 27, 140–151 (1999).
Coddington, L. T. & Dudman, J. T. The timing of action determines reward prediction signals in identified midbrain dopamine neurons. Nat. Neurosci. 21, 1563–1573 (2018).
Niv, Y. & Schoenbaum, G. Dialogues on prediction errors. Trends Cogn. Sci. 12, 265–272 (2008).
Schultz, W. Dopamine reward prediction-error signalling: a two-component response. Nat. Rev. Neurosci. 17, 183–195 (2016).
Kutlu, M. G. et al. Dopamine release in the nucleus accumbens core signals perceived saliency. Curr. Biol. 31, 4748–4761 (2021).
Esber, G. R. et al. Attention-related Pearce-Kaye-Hall signals in basolateral amygdala require the midbrain dopaminergic system. Biol. Psychiatry 72, 1012–1019 (2012).
Seitz, B. M., Blaisdell, A. P. & Sharpe, M. J. Higher-order conditioning and dopamine: charting a path forward. Front. Behav. Neurosci. 15, 745388 (2021).
Berke, J. D. What does dopamine mean? Nat. Neurosci. 21, 787–793 (2018).
Takahashi, Y. K. et al. Dopamine neurons respond to errors in the prediction of sensory features of expected rewards. Neuron 95, 1395–1405 (2017).
Stalnaker, T. et al. Dopamine neuron ensembles signal the content of sensory prediction errors. eLife 8, e49315 (2019).
Corbit, L. H. & Balleine, B. W. Double dissociation of basolateral and central amygdala lesions on the general and outcome-specific forms of Pavlovian-instrumental transfer. J. Neurosci. 25, 962–970 (2005).
Kröner, S., Rosenkranz, J. A., Grace, A. A. & Barrionuevo, G. Dopamine modulates excitability of basolateral amygdala neurons in vitro. J. Neurophysiol. 93, 1598–1610 (2005).
Lorétan, K., Bissière, S. & Lüthi, A. Dopaminergic modulation of spontaneous inhibitory network activity in the lateral amygdala. Neuropharmacology 47, 631–639 (2004).
Bissière, S., Humeau, Y. & Lüthi, A. Dopamine gates LTP induction in lateral amygdala by suppressing feedforward inhibition. Nat. Neurosci. 6, 587–592 (2003).
Vander Weele, C. M. et al. Dopamine enhances signal-to-noise ratio in cortical-brainstem encoding of aversive stimuli. Nature 563, 397–401 (2018).
Lutas, A., Fernando, K., Zhang, S. X., Sambangi, A. & Andermann, M. L. History-dependent dopamine release increases cAMP levels in most basal amygdala glutamatergic neurons to control learning. Cell Rep. 38, 110297 (2022).
Rosenkranz, J. A. & Grace, A. A. Dopamine-mediated modulation of odour-evoked amygdala potentials during Pavlovian conditioning. Nature 417, 282–287 (2002).
Li, C. & Rainnie, D. G. Bidirectional regulation of synaptic plasticity in the basolateral amygdala induced by the D1-like family of dopamine receptors and group II metabotropic glutamate receptors. J. Physiol. 592, 4329–4351 (2014).
Speranza, L., di Porzio, U., Viggiano, D., de Donato, A. & Volpicelli, F. Dopamine: the neuromodulator of long-term synaptic plasticity, reward and movement control. Cells 10, 735 (2021).
Liu, J. et al. Neural coding of appetitive food experiences in the amygdala. Neurobiol. Learn Mem. 155, 261–275 (2018).
Courtin, J. et al. A neuronal mechanism for motivational control of behavior. Science 375, eabg7277 (2022).
Malvaez, M., Shieh, C., Murphy, M. D., Greenfield, V. Y. & Wassum, K. M. Distinct cortical–amygdala projections drive reward value encoding and retrieval. Nat. Neurosci. 22, 762–769 (2019).
Saunders, B. T., Richard, J. M., Margolis, E. B. & Janak, P. H. Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat. Neurosci. 21, 1072–1083 (2018).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates (Academic Press, 1998).
Collins, A. L. et al. Nucleus accumbens cholinergic interneurons oppose cue-motivated behavior. Biol. Psychiatry 86, 388–396 (2019).
Lichtenberg, N. T. et al. The medial orbitofrontal cortex–basolateral amygdala circuit regulates the influence of reward cues on adaptive behavior and choice. J. Neurosci. 1, 7267–7277 (2021).
Malvaez, M. et al. Basolateral amygdala rapid glutamate release encodes an outcome-specific representation vital for reward-predictive cues to selectively invigorate reward-seeking actions. Sci. Rep. 5, 12511 (2015).
Lopes, G. et al. Bonsai: an event-based framework for processing and controlling data streams. Front. Neuroinform. 9, 7 (2015).
Morel, C. et al. Midbrain projection to the basolateral amygdala encodes anxiety-like but not depression-like behaviors. Nat. Commun. 13, 1532 (2022).
Schmider, E., Ziegler, M., Danay, E., Beyer, L. & Bühner, M. Is it really robust? Reinvestigating the robustness of ANOVA against violations of the normal distribution assumption. Methodol. Eur. J. Res. Methods Behav. Soc. Sci. 6, 147–151 (2010).
Knief, U. & Forstmeier, W. Violating the normality assumption may be the lesser of two evils. Behav. Res. Methods 53, 2576–2590 (2021).
Levin, J. R., Serlin, R. C. & Seaman, M. A. A controlled powerful multiple-comparison strategy for several situations. Psychol. Bull. 115, 153–159 (1994).
Lichtenberg, N. T. & Wassum, K. M. Amygdala mu-opioid receptors mediate the motivating influence of cue-triggered reward expectations. Eur. J. Neurosci. 45, 381–387 (2016).
Lichtenberg, N. T. et al. Basolateral amygdala to orbitofrontal cortex projections enable cue-triggered reward expectations. J. Neurosci. 37, 8374–8384 (2017).
Chen, T. W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Schoenbaum, G., Chiba, A. A. & Gallagher, M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat. Neurosci. 1, 155–159 (1998).
Sugase-Miyamoto, Y. & Richmond, B. J. Neuronal signals in the monkey basolateral amygdala during reward schedules. J. Neurosci. 25, 11071–11083 (2005).
Fontanini, A., Grossman, S. E., Figueroa, J. A. & Katz, D. B. Distinct subtypes of basolateral amygdala taste neurons reflect palatability and reward. J. Neurosci. 29, 2486–2495 (2009).
Roesch, M. R., Calu, D. J., Esber, G. R. & Schoenbaum, G. Neural correlates of variations in event processing during learning in basolateral amygdala. J. Neurosci. 30, 2464–2471 (2010).
Acknowledgements
This research was supported by NIH grant DA035443 and MH126285 (to K.M.W.), NIH grant DA057084 (to K.M.W. and M.J.S.), NSF GRFP (to A.C.S.), NSF CAREER 2143910 (to M.J.S.), the Staglin Center for Behavior and Brain Sciences, and the Wendell Jeffrey and Bernice Wenzel Term Chair in Behavioral Neuroscience (to K.M.W.).
Author information
Authors and Affiliations
Contributions
K.M.W. and A.C.S. designed the research and analyzed and interpreted the data. A.C.S. conducted the research with assistance from Y.J., N.K.G. and A.C.L. C.M.G. and T.M.W. conducted the behavioral blocking experiments. K.R.-A. contributed to the fiber photometry experiments. N.K.G. and K.P. assisted with histological verification. M.J.S. contributed to the design of the blocking experiments and advised on the project and paper. A.C.S. and K.M.W. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Neuroscience thanks Laura Bradfield and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 BLA neurons are active during cue-reward encoding.
To characterize the endogenous activity of BLA neurons, we used fiber photometry to record fluorescent activity of the genetically encoded calcium indicator GCaMP6f67 in the BLA of male and female rats. (a) Top: Representative fluorescent image of GCaMP6f expression and fiber placement in the BLA. Bottom: Fiber photometry approach for bulk calcium imaging in BLA neurons. (b) Schematic representation of GCaMP6f expression and placement of optical fiber tips in BLA for all subjects. (c) Pavlovian long-delay conditioning procedure schematic. CS, 30-s conditioned stimulus (aka, ‘cue’, white noise or click) followed immediately by reward outcome (O, sucrose solution or grain pellet). (d) Food-port entry rate during the cue relative to the preCue baseline period, averaged across the 2 cues for each Pavlovian conditioning session. Across training, rats developed a Pavlovian conditional approach response of entering the food-delivery port during cue presentation. Two-way RM ANOVA training x cue: F(2.44, 17.07) = 7.97, P = 0.002; training: F(3.30, 23.10) = 4.85, P = 0.008; cue: F(1, 7) = 80.33, P < 0.0001. *P < 0.05, **P < 0.01. N = 8, 4 male rats. (e-f) BLA neurons are active during the encoding of cue-reward memories. BLA neurons were robustly activated both at cue onset and offset when the outcome was delivered. Cue onset responses beginning on the first conditioning sessions have been detected previously2. These novelty responses rapidly attenuate if the stimuli are not associated with reward24. (e) Quantification of maximal (peak) GCaMP6f z-score ∆F/F during the 5-s period following cue onset or outcome delivery compared to the equivalent baseline period immediately prior to cue onset. Two-way RM ANOVA training x event: F(2.52, 17.61) = 3.94, P = 0.03; event: F(1.39, 9.71) = 58.63, P < 0.0001; training F(1.71, 11.97) = 2.30, P = 0.15. (f) GCaMP6f fluorescence changes (z-score ∆F/F) in response to cue presentation (blue) and outcome delivery across days of training. Tick marks represent time of outcome collection for each subject. Data from the last six sessions were averaged across 2-session bins (3/4, 5/6, and 7/8). N = 8, 4 male rats. Data presented as trial-averaged, between-subject mean ± s.e.m. with individual data points. *P < 0.05, **P < 0.01, ***P < 0.001 Bonferroni-corrected post-hoc comparisons. Consistent with prior evidence24, BLA neurons are activated by rewards and their predictors. BLA activation is particularly robust when the cues can become linked to the identifying features of the rewards they predict. Although these data likely reflect both somatic and non-somatic calcium activity, they are consistent with prior electrophysiological evidence that BLA neurons respond to reward during learning68,69,70,71.
Extended Data Fig. 2 Dopamine release in BLA during cue-reward learning across each of the 8 Pavlovian conditioning sessions.
(a) GRABDA2h fluorescence changes (z-score) in response to cue presentation (blue) and reward delivery across each of the 8 Pavlovian conditioning sessions. (b) Quantification of BLA GRABDA z-scored signal AUC during the 2-s period following cue onset or reward delivery compared to the equivalent baseline period immediately prior to cue onset. Two-way RM ANOVA event: F(1.85, 11.07) = 4.90, P = 0.03; training: F(2.34, 14.03) = 1.13, P = 0.36; training x event: F(3.45, 20.99) = 0.59, P = 0.65. *P < 0.05, relative to preCue baseline, Bonferroni correction. N = 7, 4 male rats. (c) GRABDA fluorescence changes (z-score) in response to cue presentation and reward delivery across each of the 8 Pavlovian conditioning sessions. (d) Quantification of BLA GRABDA z-scored signal AUC during the 1.5-s period following cue onset, cue offset (trace interval), or reward delivery compared to the equivalent baseline period immediately prior to cue onset. Two-way RM ANOVA event: F(2.06, 14.40) = 13.24, P = 0.0005; training: F(3.62, 25.33) = 2.43, P = 0.08; training x event: F(3.60, 25.17) = 2.60, P = 0.07. *P < 0.05, **P < 0.01, ***P < 0.001, relative to preCue baseline, Bonferroni correction. (GRABDA2h: N = 3, 2 male; GRABDA2m: N = 5, 3 male). The slope of the BLA dopamine reward response across training was significantly negative (β = −0.13, confidence interval −0.25 to −0.007; F(1,62) = 4.49, P = 0.04) and signifantly different (F(1,124) = 13.33, P = 0.0004) from the slope of the BLA dopamine cue-onset response across training, which was significantly positive (β = 0.13, confidence interval 0.06 to 0.20; F(1,62) = 13.53, P = 0.0005). Data presented as trial-averaged, between-subject mean ± s.e.m. with individual data points. *P < 0.05, **P < 0.01, ***P < 0.001 Bonferroni-corrected post-hoc comparisons.
Extended Data Fig. 3 GRABDA responses to reward collection.
(a) GRABDA2h fluorescence changes (z-score) in response to reward collection across Pavlovian long-delay conditioning. Data from the last six sessions were averaged across 2-session bins (3/4, 5/6, and 7/8). N = 9, 5 male rats. (b) GRABDA fluorescence changes (z-score) in response to reward collection across Pavlovian trace conditioning. Data from the last six sessions were averaged across 2-session bins (3/4, 5/6, and 7/8). GRABDA2h: N = 4, 3 male; GRABDA2m: N = 6, 3 male. Data presented as trial-averaged, between-subject mean ± s.e.m. with individual data points.
Extended Data Fig. 4 GRABDA responses to unpredicted rewarding and aversive events.
(a) GRABDA fluorescence changes (z-score) in response to unpredicted delivery of 1, 2, or 3 food pellets. (b) Quantification of BLA GRABDA z-scored signal AUC during the 20-s period following pellet delivery. Two-way RM ANOVA reward period x magnitude: F(1.92, 11.50) = 12.46, P = 0.001; magnitude: F(1.94, 11.66) = 11.04, P = 0.002; reward: F(1, 6) = 7.86, P = 0.03. GRABDA2h: N = 2, 2 male; GRABDA2m: N = 5, 3 male (c) GRABDA fluorescence changes (z-score) in response to unpredicted puff of air to the face. (d) Quantification of BLA GRABDA z-scored trace AUC during the 5-s period following airpuff delivery relative to 5-s preAirpuff baseline. Two-tailed paired sample t-test t(7) = 5.88, P = 0.0006. GRABDA2h: N = 2, 2 male; GRABDA2m: N = 6, 3 male. Data presented as trial-averaged, between-subject mean ± s.e.m. with individual data points. *P < 0.05, ***P < 0.001 Bonferroni-corrected post-hoc comparisons.
Extended Data Fig. 5 Inhibition of VTADA→BLA projections does not disrupt reward collection during Pavlovian conditioning.
There was no effect of optical inhibition of VTADA→BLA projections at reward delivery on collection of the food outcomes. (a) Entries into the food-delivery port during the 30-s periods before and after cue presentation during Pavlovian long-delay conditioning. Rats entered the food-delivery port during the 30-s postcue/reward-delivery period more than the preCue baseline period and similarly between groups. training x period: F(4.94,93.85) = 3.00, P = 0.02; training: F(3.13, 59.48) = 8.51, P < 0.0001; period: F(1,19) = 72.60, P < 0.0001; virus: F(1,19) = 0.47, P = 0.50; training x virus: F(7,133) = 0.65, P = 0.72; virus x period: F(1,19) = 0.87, P = 0.36; training x virus x period: F(7,133) = 0.71, P = 0.66. ArchT, N = 11, 6 male rats; tdTomato, N = 10, 5 male rats. (b) Percent time spent in the food-delivery port during the 10-s preCue baseline and 10-s postCue offset (including trace interval and reward delivery period) periods during Pavlovian trace conditioning. Rats entered the food-delivery port during the 10-s postCue period more than the preCue period and similarly between groups. training x period: F(1.93,19.27) = 9.68, P = 0.001; training: F(2.59, 25.88) = 9.28, P = 0.0004; period: F(1,10) = 138.50, P < 0.0001; virus: F(1,10) = 14.94, P = 0.003; training x virus: F(4, 40) = 1.35, P = 0.27; virus x period: F(1,10) = 1.37, P = 0.27; training x virus x period: F(4, 40) = 0.05, P = 0.996. ArchT, N = 5, 4 male rats; Control, N = 7, 4 male rats (3 WT/cre-dependent ArchT; 4 Th-cre/cre-dependent tdTomato). Data presented as trial-averaged, between-subject mean ± s.e.m. with individual data points. *P < 0.05, **P < 0.01, ***P < 0.001 Bonferroni-corrected post-hoc comparisons.
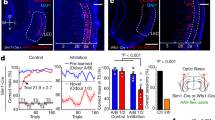
Extended Data Fig. 6 Optical inhibition of VTADA→BLA projections throughout cue and reward during learning attenuates the encoding of identity-specific cue-reward memories.
We cre-dependently expressed ArchT bilaterally in VTADA neurons of male and female Th-cre rats and implanted optical fibers bilaterally over BLA. (a) Bottom: Representative fluorescent image of cre-dependent ArchT-tdTomato expression in VTA cell bodies with coexpression of Th in Th-cre rats. Middle: Strategy for bilateral optogenetic inhibition of VTADA axons and terminals in the BLA of Th-cre rats. Top: Representative image of fiber placement in the vicinity of immunofluorescent ArchT-tdTomato-expressing VTADA axons and terminals in the BLA. (b) Schematic representation of cre-dependent ArchT-tdTomato expression in VTA and (c) placement of optical fiber tips in BLA for all subjects. For half of the control group, we expressed cre-dependent tdTomato in the VTA of Th-cre male and female rats. For the other half, wildtype rats were infused with cre-dependent ArchT (which did not express owing to the lack of cre recombinase) into the VTA. Both groups received bilateral optical fibers above the BLA. Thus, we control for light delivery, viral expression, and genotype. There were no significant behavioral differences between each type of control (lowest P: F(1, 6) = 1.61, P = 0.25). (d) Procedure. A, action (left or right lever press); CS, 30-s conditioned stimulus (aka, ‘cue’, white noise or click) followed immediately by reward outcome (O, sucrose solution or grain pellet). (e) Rats first received 11 sessions of instrumental conditioning, without manipulation, in which one of two different lever-press actions each earned one of two distinct food rewards (for example, left press→sucrose/right press→pellets). Lever-press rate averaged across levers and across the final 2 instrumental conditioning sessions. Two-tailed independent sample t-test t(13) = 1.20, P = 0.25. (f) Rats then received Pavlovian conditioning. During each of the 8 Pavlovian conditioning sessions, each of 2 distinct, 30-s, auditory cues was presented 8 times and terminated in the delivery of one of the food rewards (for example, white noiseꟷsucrose/clickꟷpellets). VTADA→BLA projections were optically inhibited (532 nm, 10 mW, 33 s) during the entirety of each cue-reward period. Light turned on at the onset of each cue and off 3 s following reward delivery. Optical inhibition of VTADA→BLA projections through the cue and reward period did not disrupt development of a Pavlovian conditional goal-approach response. Food-port entry rate during the cue relative to the preCue baseline period, averaged across trials and across the 2 cues for each Pavlovian conditioning session. Thin lines represent individual subjects. Three-way RM ANOVA training x cue: F(3.30, 42.87) = 20.69, P < 0.0001; cue: F(1, 13) = 295.60, P < 0.0001; training: F(3.03.,39.42) = 4.13, P = 0.01; virus: F(1,13) = 1.61, P = 0.23; training x virus: F(7,91) = 0.37, P = 0.92; virus x cue: F(1,13) = 3.05, P = 0.10; training x virus x cue: F(7,91) = 2.17, P = 0.04. By the end of training both groups showed similar elevation in food-port approach during the cues. (g-i) We next gave subjects an outcome-specific Pavlovian-to-instrumental transfer (PIT) test, without manipulation. Controls learned the identity-specific cue-reward memories as evidenced by their ability to use the cues to selectively elevate pressing on the lever associated with the same outcome as predicted by the cue. Conversely, the cues were not capable of guiding lever-press choice in the group for which VTADA→BLA projections were inhibited during Pavlovian conditioning. Rather, for these subjects, the cues caused a general increase in pressing across both levers. (g) Lever-press rates during the preCue baseline periods compared to press rates during the cue periods separated for presses on the lever that, in training, delivered the same outcome as predicted by the cue (Same) and pressing on the other available lever (Different). Three-way RM ANOVA virus x lever x cue: F(1, 13) = 7.35, P = 0.02; virus: F(1, 13) = 4.59, P = 0.05; lever: F(1, 13) = 5.76, P = 0.03; cue: F(1, 13) = 58.87, P < 0.0001; virus x lever: F(1, 13) = 1.91, P = 0.19; virus x cue: F(1, 13) = 12.00, P = 0.004; lever x cue : F(1, 13) = 7.56, P = 0.02. *P < 0.05, **P < 0.01, planned comparisons cue same presses v. preCue same presses and cue different presses v. preCue different presses. Inhibition of VTADA→BLA projections during cue-reward learning prevents subjects from learning identity-specific cue-reward memories, but does not prevent the assignment of general incentive properties to the cues that supports non-discriminate cue-induced motivation. (h) Elevation in lever presses on the Same lever [(Same lever presses during cue)/(Same presses during cue + Same presses during preCue)], relative to the elevation in pressing on the Different lever [(Different lever presses during cue)/(Different presses during cue + Different presses during preCue)], averaged across cues during the PIT test. Two-way RM ANOVA virus: F(1, 13) = 2.21, P = 0.16; lever: F(1, 13) = 1.67, P = 0.22; virus x lever: F(1, 13) = 1.14, P = 0.30. (i) As in training, during the PIT test the conditional goal-approach response was similar between groups, further indicating that even longer duration inhibition of VTADA→BLA projections during cue-reward learning does not disrupt development of conditional responses. Food-port entry rate during the cues relative to the preCue baseline periods, averaged across cues during the PIT test. Two-way RM ANOVA cue: F(1, 13) = 44.71, P < 0.0001; virus: F(1, 13) = 0.08, P = 0.79; virus x cue: F(1, 13) = 0.61, P = 0.45. *P < 0.05, **P < 0.01, ***P < 0.001, Bonferroni correction. ArchT, N = 7, 4 male rats; Control N = 8, 4 Th-cre/tdTomato 2 male rats, 4 wildtype cre-dependent ArchT 2 male rats. Data presented as trial-averaged, between-subject mean ± s.e.m. with individual data points. *P < 0.05, **P < 0.01, ***P < 0.001 Bonferroni-corrected post-hoc comparisons. These data confirm that VTADA→BLA projections are needed to link the identifying details of the reward to a predictive cue, but not to reinforce a conditional response or to assign general incentive properties to the cue to support general motivation.
Extended Data Fig. 7 Stimulation of VTADA→BLA projections does not affect reward collection during compound conditioning.
There was no effect of optical stimulation of VTADA→BLA projections paired with reward delivery on collection of the food outcomes. Rats entered the food-delivery port during the 30-s postCue/reward-delivery period more than the preCue baseline period and similarly between groups. Three-way RM ANOVA period: F(1, 22) = 46.80, P < 0.0001; training: F(1.50, 32.90) = 3.70, P = 0.047; virus: F(1, 22) = 1.89, P = 0.18; training x virus: F(3, 66) = 1.48, P = 0.23; training x period: F(2.55, 56.04) = 0.22, P = 0.85; virus x period: F(1, 22) = 0.04, P = 0.84; training x virus x period: F(3, 66) = 0.51, P = 0.68. *P < 0.05, **P < 0.01 relative to preCue baseline, Bonferroni correction. ChR2, N = 11, 6 male rats; eYFP, N = 13, 6 male rats. Data presented as trial-averaged, between-subject mean ± s.e.m. with individual data points. *P < 0.05, **P < 0.01, ***P < 0.001 Bonferroni-corrected post-hoc comparisons.
Extended Data Fig. 8 Stimulation of VTADA→BLA projections is not reinforcing.
To assess the reinforcing properties of VTADA→BLA activation, rats were given 2 sessions of intracranial self-stimulation (ICSS) in a context different from that of prior conditioning. Nose pokes in the active port triggered 1-s blue light delivery (473 nm; 10 mW; 25 ms pulse width; 20 Hz). Data show total active nose pokes compared to inactive nose pokes across 2, 1-hr ICSS sessions. Activation of VTADA→BLA projections was not reinforcing. Rats expressing ChR2 showed similar levels of active nose pokes as the eYFP control group in the first session and this decreased to the level of the inactive nose pokes in the second session. Three-way RM ANOVA session x virus x nose poke: F(1, 22) = 5.00, P = 0.04; virus x nose poke: F(1, 22) = 5.18, P = 0.03; session x virus: F(1,22) = 5.18, P = 0.03; session x nose poke: F(1, 22) = 1.24, P = 0.28; session: F(1, 22) = 3.05, P = 0.09; virus: F(1, 22) = 1.94, P = 0.18; nose poke: F(1, 22) = 54.66, P < 0.0001. Elevated active v. inactive port nose poking in both the eYFP and ChR2 groups could have resulted from the prior association formed between blue light and reward delivery during compound conditioning. If true, then this could have extinguished by the second session in the ChR2 group, potentially indicating that VTADA→BLA projection activity during either initial learning or online during the ICSS session may contribute to the reward expectation and/or learning processes that contribute to extinction. Alternatively, the nose poking in both groups could reflect salience of the light delivery, which could habituate more quickly in the ChR2 group. ChR2, N = 11, 6 male rats; eYFP, N = 13, 6 male rats. Data presented as trial-averaged, between-subject mean ± s.e.m. with individual data points. **P < 0.01, ***P < 0.001 Bonferroni-corrected post-hoc comparisons.
Supplementary information
Supplementary Table 1
Full statistical reporting for main text figures.
Source data
Source Data Fig. 1
Source data for Fig. 1.
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 3
Source data for Fig. 3.
Source Data Fig. 4
Source data for Fig. 4.
Source Data Extended Data Fig. 1
Source data for Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Source data for Extended Data Fig. 2.
Source Data Extended Data Fig. 3
Source data for Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Source data for Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Source data for Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Source data for Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Source data for Extended Data Fig. 7.
Source Data Extended Data Fig. 8
Source data for Extended Data Fig. 8.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sias, A.C., Jafar, Y., Goodpaster, C.M. et al. Dopamine projections to the basolateral amygdala drive the encoding of identity-specific reward memories. Nat Neurosci 27, 728–736 (2024). https://doi.org/10.1038/s41593-024-01586-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41593-024-01586-7