Abstract
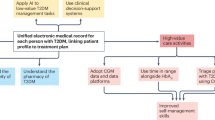
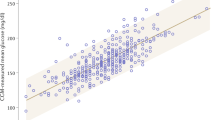
Few young people with type 1 diabetes (T1D) meet glucose targets. Continuous glucose monitoring improves glycemia, but access is not equitable. We prospectively assessed the impact of a systematic and equitable digital-health-team-based care program implementing tighter glucose targets (HbA1c < 7%), early technology use (continuous glucose monitoring starts <1 month after diagnosis) and remote patient monitoring on glycemia in young people with newly diagnosed T1D enrolled in the Teamwork, Targets, Technology, and Tight Control (4T Study 1). Primary outcome was HbA1c change from 4 to 12 months after diagnosis; the secondary outcome was achieving the HbA1c targets. The 4T Study 1 cohort (36.8% Hispanic and 35.3% publicly insured) had a mean HbA1c of 6.58%, 64% with HbA1c < 7% and mean time in the range (70–180 mg dl−1) of 68% at 1 year after diagnosis. Clinical implementation of the 4T Study 1 met the prespecified primary outcome and improved glycemia without unexpected serious adverse events. The strategies in the 4T Study 1 can be used to implement systematic and equitable care for individuals with T1D and translate to care for other chronic diseases. ClinicalTrials.gov registration: NCT04336969.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets include information that is protected health information; as it currently sits, it can lead to the identification of potential participants. Thus, the current institutional review board coverage for this study does not allow data sharing. However, the authors are willing to share non-privileged data on a case by case bases as appropriate and indicated. Per the NIH guidelines, de-identified datasets will be made available on completion of all phases of the study, which we anticipate to occur in mid-2025. Please address any data requests to prahalad@stanford.edu and data requests will be reviewed per National Institute of Diabetes and Digestive and Kidney Diseases guidelines and timelines.
Code availability
To ease adoption at other institutions, our RPM platform is open source (github.com/jferstad/SURF-TIDE). The custom code used to perform the analyses is available at github.com/qsuProjects/4T-Study1.
References
Chronic diseases in America. Centers for Disease Control and Prevention www.cdc.gov/chronicdisease/resources/infographic/chronic-diseases.htm (2022).
Management of chronic conditions in schools. American Academy of Pediatrics www.aap.org/en/patient-care/school-health/management-of-chronic-conditions-in-schools/(2023).
Wagner, E. H. Chronic disease management: what will it take to improve care for chronic illness? Eff. Clin. Pract. 1, 2–4 (1998).
Carrigan, A. et al. Mapping care provision for type 1 diabetes throughout Australia: a protocol for a mixed-method study. BMJ Open 12, e067209 (2022).
Charalampopoulos, D. et al. Exploring variation in glycemic control across and within eight high-income countries: a cross-sectional analysis of 64,666 children and adolescents with type 1 diabetes. Diabetes Care 41, 1180–1187 (2018).
Foster, N. C. et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol. Ther. 21, 66–72 (2019).
Miller, K. M. et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 38, 971–978 (2015).
Gerhardsson, P. et al. The SWEET Project 10-year benchmarking in 19 countries worldwide is associated with improved HbA1c and increased use of diabetes technology in youth with type 1 diabetes. Diabetes Technol. Ther. 23, 491–499 (2021).
ElSayed, N. A. et al. 14. Children and adolescents: standards of care in diabetes-2023. Diabetes Care 46, S230–S253 (2023).
de Bock, M. et al. ISPAD Clinical Practice Consensus Guidelines 2022: glycemic targets and glucose monitoring for children, adolescents, and young people with diabetes. Pediatr. Diabetes 23, 1270–1276 (2022).
Addala, A. et al. Uninterrupted continuous glucose monitoring access is associated with a decrease in HbA1c in youth with type 1 diabetes and public insurance. Pediatr. Diabetes 21, 1301–1309 (2020).
Burckhardt, M.-A. et al. The use of continuous glucose monitoring with remote monitoring improves psychosocial measures in parents of children with type 1 diabetes: a randomized crossover trial. Diabetes Care 41, 2641–2643 (2018).
Danne, T. et al. International consensus on use of continuous glucose monitoring. Diabetes Care 40, 1631–1640 (2017).
Laffel, L. M. et al. Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA 323, 2388–2396 (2020).
Barnard, K. D. et al. Closing the loop overnight at home setting: psychosocial impact for adolescents with type 1 diabetes and their parents. BMJ Open Diabetes Res. Care 2, e000025 (2014).
Weissberg‐Benchell, J. & Antisdel‐Lomaglio, J. Diabetes‐specific emotional distress among adolescents: feasibility, reliability, and validity of the problem areas in diabetes‐teen version. Pediatr. Diabetes 12, 341–344 (2011).
ElSayed, N. A. et al. 7. Diabetes technology: standards of care in diabetes-2023. Diabetes Care 46, S111–S127 (2023).
Sherr, J. L. et al. ISPAD Clinical Practice Consensus Guidelines 2022: diabetes technologies: insulin delivery. Pediatr. Diabetes 23, 1406–1431 (2022).
Tauschmann, M. et al. ISPAD Clinical Practice Consensus Guidelines 2022: diabetes technologies: glucose monitoring. Pediatr. Diabetes 23, 1390–1405 (2022).
Majidi, S. et al. Inequities in health outcomes in children and adults with type 1 diabetes: data from the T1D Exchange Quality Improvement Collaborative. Clin. Diabetes 39, 278–283 (2021).
Addala, A. et al. A decade of disparities in diabetes technology use and HbA(1c) in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care 44, 133–140 (2021).
Lipman, T. H. & Hawkes, C. P. Racial and socioeconomic disparities in pediatric type 1 diabetes: time for a paradigm shift in approach. Diabetes Care 44, 14–16 (2021).
Miller, K. M. et al. Longitudinal changes in continuous glucose monitoring use among individuals with type 1 diabetes: international comparison in the German and Austrian DPV and U.S. T1D Exchange registries. Diabetes Care 43, e1–e2 (2020).
Morris, Z. S., Wooding, S. & Grant, J. The answer is 17 years, what is the question: understanding time lags in translational research. J. R. Soc. Med. 104, 510–520 (2011).
Nathan, D. M. et al. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus. J. Pediatr. 125, 177–188 (1994).
Foster, C. et al. Remote monitoring of patient- and family-generated health data in pediatrics. Pediatrics 149, e2021054137 (2022).
Prahalad, P. et al. Teamwork, targets, technology, and tight control in newly diagnosed type 1 diabetes: the Pilot 4T study. J. Clin. Endocrinol. Metab. 107, 998–1008 (2022).
Prahalad, P. et al. Improving clinical outcomes in newly diagnosed pediatric type 1 diabetes: Teamwork, Targets, Technology, and Tight Control-The 4T Study. Front. Endocrinol. 11, 360 (2020).
Zaharieva, D. P., Bishop, F. K. & Maahs, D. M. Advancements and future directions in the teamwork, targets, technology, and tight control-the 4T study: improving clinical outcomes in newly diagnosed pediatric type 1 diabetes. Curr. Opin. Pediatr. 34, 423–429 (2022).
American Diabetes Association 12. Children and adolescents: standards of medical care in diabetes-2018. Diabetes Care 41, S126–S136 (2018).
Prahalad, P. et al. Hemoglobin A1c trajectory in pediatric patients with newly diagnosed type 1 diabetes. Diabetes Technol. Ther. 21, 456–461 (2019).
Addala, A. et al. Disparities in hemoglobin A1c levels in the first year after diagnosis among youths with type 1 diabetes offered continuous glucose monitoring. JAMA Netw. Open 6, e238881 (2023).
Tanenbaum, M. L. et al. ‘I was ready for it at the beginning’: parent experiences with early introduction of continuous glucose monitoring following their child’s type 1 diabetes diagnosis. Diabet. Med. 38, e14567 (2021).
Tanenbaum, M. L. et al. ‘Much more convenient, just as effective’: experiences of starting continuous glucose monitoring remotely following type 1 diabetes diagnosis. Diabet. Med. 39, e14923 (2022).
Boughton, C. K. et al. Closed-loop therapy and preservation of C-peptide secretion in type 1 diabetes. N. Engl. J. Med. 387, 882–893 (2022).
Forlenza, G. P. et al. Effect of verapamil on pancreatic beta cell function in newly diagnosed pediatric type 1 diabetes: a randomized clinical trial. JAMA 329, 990–999 (2023).
Phillip, M. et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N. Engl. J. Med. 368, 824–833 (2013).
Akturk, H. K., Agarwal, S., Hoffecker, L. & Shah, V. N. Inequity in racial-ethnic representation in randomized controlled trials of diabetes technologies in type 1 diabetes: critical need for new standards. Diabetes Care 44, e121–e123 (2021).
McVean, J. et al. Effect of tight glycemic control on pancreatic beta cell function in newly diagnosed pediatric type 1 diabetes: a randomized clinical trial. JAMA 329, 980–989 (2023).
Lipman, T. H., Smith, J. A., Patil, O., Willi, S. M. & Hawkes, C. P. Racial disparities in treatment and outcomes of children with type 1 diabetes. Pediatr. Diabetes 22, 241–248 (2021).
Miller, K. M., Beck, R. W., Foster, N. C. & Maahs, D. M. HbA1c levels in type 1 diabetes from early childhood to older adults: a deeper dive into the influence of technology and socio-economic status on HbA1c in the T1D Exchange Clinic Registry findings. Diabetes Technol. Ther. 22, 645–650 (2020).
Cengiz, E. et al. Severe hypoglycemia and diabetic ketoacidosis among youth with type 1 diabetes in the T1D Exchange Clinic Registry. Pediatr. Diabetes 14, 447–454 (2013).
Hilliard, M. E., Wu, Y. P., Rausch, J., Dolan, L. M. & Hood, K. K. Predictors of deteriorations in diabetes management and control in adolescents with type 1 diabetes. J. Adolesc. Health 52, 28–34 (2013).
Hassan, K., Loar, R., Anderson, B. J. & Heptulla, R. A. The role of socioeconomic status, depression, quality of life, and glycemic control in type 1 diabetes mellitus. J. Pediatr. 149, 526–531 (2006).
Zuijdwijk, C. S., Cuerden, M. & Mahmud, F. H. Social determinants of health on glycemic control in pediatric type 1 diabetes. J. Pediatr. 162, 730–735 (2013).
Saydah, S., Imperatore, G., Cheng, Y., Geiss, L. S. & Albright, A. Disparities in diabetes deaths among children and adolescents—United States, 2000–2014. MMWR Morb. Mortal. Wkly Rep. 66, 502–505 (2017).
Hill-Briggs, F. et al. Social determinants of health and diabetes: a scientific review. Diabetes Care 44, 258–279 (2020).
Scheinker, D. et al. New technology-enabled care model for pediatric type 1 diabetes. NEJM Catal. Innov. Care Deliv. https://doi.org/10.1056/CAT.21.0438 (2022).
Scheinker, D. et al. Algorithm-enabled, personalized glucose management for type 1 diabetes at the population scale: prospective evaluation in clinical practice. JMIR Diabetes 7, e27284 (2022).
Ferstad, J. O. et al. Population-level management of type 1 diabetes via continuous glucose monitoring and algorithm-enabled patient prioritization: precision health meets population health. Pediatr. Diabetes 22, 982–991 (2021).
Nirantharakumar, K., Mohammed, N., Toulis, K. A., Thomas, G. N. & Narendran, P. Clinically meaningful and lasting HbA1c improvement rarely occurs after 5 years of type 1 diabetes: an argument for early, targeted and aggressive intervention following diagnosis. Diabetologia 61, 1064–1070 (2018).
Prahalad, P. et al. Hemoglobin A1c trajectories in the first 18 months after diabetes diagnosis in the SWEET diabetes registry. Pediatr. Diabetes 23, 228–236 (2022).
Ebekozien, O. et al. Longitudinal trends in glycemic outcomes and technology use for over 48,000 people with type 1 diabetes (2016–2022) from the T1D Exchange Quality Improvement Collaborative. Diabetes Technol. Ther. 25, 765–773 (2023).
Bergenstal, R. M. et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care 41, 2275–2280 (2018).
Shah, V. N., Vigers, T., Pyle, L., Calhoun, P. & Bergenstal, R. M. Discordance between glucose management indicator and glycated hemoglobin in people without diabetes. Diabetes Technol. Ther. 25, 324–328 (2023).
Dupenloup, P. et al. A model to design financially sustainable algorithm-enabled remote patient monitoring for pediatric type 1 diabetes care. Front. Endocrinol. 13, 1021982 (2022).
Johnson, S. R. et al. Universal subsidized continuous glucose monitoring funding for young people with type 1 diabetes: uptake and outcomes over 2 years, a population-based study. Diabetes Care 45, 391–397 (2022).
Battelino, T. et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 42, 1593–1603 (2019).
Prahalad, P. et al. 4T Study: tighter glucose target settings with early CGM initiation further improves A1c in youth with T1D. Diabetes 71, 40-OR (2022).
Harris, P. A. et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 42, 377–381 (2009).
Zaharieva, D. P. et al. An evaluation of point-of-care HbA1c, HbA1c home kits, and glucose management indicator: potential solutions for telehealth glycemic assessments. Diabetology 3, 494–501 (2022).
van Buuren, S. & Groothuis-Oudshoorn, K. mice: multivariate imputation by chained equations in R. J. Stat. Softw. 45, 1–67 (2011).
R Development Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2023).
Acknowledgements
We thank all the young people who participated in the 4T Study. We thank the other members of the research team, including the research coordinators, clinical staff, students in the Systems Utilization for Stanford Medicine group, the Quantitative Sciences Unit and the T1D Working Group in Statistics and Informatics at Stanford Medicine Children’s Health. We especially thank all staff and team members who are involved in the 4T Study including: D. Naranjo, C. Guestrin, M. Tannenbaum, E. Fox, A. Cortes, E. Pang, R. Tam, I. Balistreri, A. Loyola, N. Alramahi, E. Frank, J. Leverenz, P. Sagan, A. Martinez-Singh, B. Conrad, A. Chmielewski, S. Lin, K. Clash,J. Senaldi, E. Hodgeson, K. Seeley, G. Keoung, G. Kim MS, P. Dupenloup, J. Kurtzig, R. Sesanayake, M. Petel, P.-A. Laforcade, V. Ritter, B. Shaw, B. Bunning, B. J. Zou, A. Wang, Y. Jeong, N. Pageler, S. Ghuman, C. Brown, B. Watkins and G. Loving. This work was supported in part by the NIH via the Stanford Diabetes Research Center (1P30DK11607401) and grant no. R18DK122422 to D.M.M. Funding support was also received from the Helmsley Charitable Trust (grant no. G-2002-04251-2 to D.P.Z.), the National Science Foundation (NSF) (grant no. 2205084 to R.J.), Stanford Human-Centered Artificial Intelligence (HAI) to D.M.M., D.S., P.P. and R.J. and Stanford Maternal & Child Health Research Institute (MCHRI) grants to P.P., R.J. and M.Y.L. Clinical and Translational Science Award, including Biostatistics, Epidemiology, and Research Design Program (UL1 TR003142) and the Stanford REDCap Platform (UL1 T001085) provided additional support. A.A. received support from grant no. K23 DK131342. M.Y.L. received support from grant no. T32DK007217. J.F. has received support from the Stanford Data Science Scholars Program. This manuscript was partially supported by the Biostatistics Shared Resource of the National Cancer Institute-sponsored Stanford Cancer Institute (P30CA124435). Funding for the iOS devices and some CGM supplies was provided by a grant through the Lucile Packard Children’s Hospital Auxiliaries Endowment to P.P. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
D.M.M., P.P., M.D., D.S. and K.H. designed the interventions. V.Y.D. and M.D. performed the data analyses. M.Y.L. and J.F. performed the analysis of RPM messaging. P.P. and V.Y.D. wrote the manuscript. D.M.M., M.D., K.H., D.S., D.P.Z., A.A., F.K.B. and R.J. contributed to the discussion and reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.M.M., D.S., P.P., K.H. and R.J. have received support from Stanford MCHRI, Stanford HAI and the NSF. D.S., R.J., D.M.M., K.H., D.P.Z. and A.A. have received funding from the Helmsley Charitable Trust. J.F. has received support from an NSF grant. D.P.Z. has received speakers honoraria from Medtronic Diabetes, Ascensia Diabetes, and Insulet Canada and Dexcom Canada, as well as research support from the ISPAD-Juvenile Diabetes Research Foundation Research Fellowship. D.M.M. has had research support from the NIH and his institution has received research support from Dexcom. D.M.M. has consulted for Abbott, the Helmsley Charitable Trust, Lifescan, Sanofi, Medtronic, Provention Bio, Kriya, Biospex and Bayer. K.H. has received research support from Dexcom for investigator-initiated research, and consultant fees from the Lilly Innovation Center, LifeScan Diabetes Institute and MedIQ. He has also received consulting fees from Sanofi Health and Cecelia Health. D.S. is an adviser to Carta Health. A.A. has received research support from the NIH. The other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Jennifer Sherr, Howard Wolpert and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Lorenzo Righetto, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–4 and Fig. 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prahalad, P., Scheinker, D., Desai, M. et al. Equitable implementation of a precision digital health program for glucose management in individuals with newly diagnosed type 1 diabetes. Nat Med (2024). https://doi.org/10.1038/s41591-024-02975-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41591-024-02975-y