Abstract
The stabilization of stalled forks has emerged as a crucial mechanism driving resistance to poly(ADP-ribose) polymerase (PARP) inhibitors in BRCA1/2-deficient tumors. Here, we identify UFL1, a UFM1-specific E3 ligase, as a pivotal regulator of fork stability and the response to PARP inhibitors in BRCA1/2-deficient cells. On replication stress, UFL1 localizes to stalled forks and catalyzes the UFMylation of PTIP, a component of the MLL3/4 methyltransferase complex, specifically at lysine 148. This modification facilitates the assembly of the PTIP–MLL3/4 complex, resulting in the enrichment of H3K4me1 and H3K4me3 at stalled forks and subsequent recruitment of the MRE11 nuclease. Consequently, loss of UFL1, disruption of PTIP UFMylation or overexpression of the UFM1 protease UFSP2 protects nascent DNA strands from extensive degradation and confers resistance to PARP inhibitors in BRCA1/2-deficient cells. These findings provide mechanistic insights into the processes underlying fork instability in BRCA1/2-deficient cells and offer potential therapeutic avenues for the treatment of BRCA1/2-deficient tumors.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article, the accompanying source data files and the Supplementary Information. Source data are provided with this paper.
References
Gudmundsdottir, K. & Ashworth, A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene 25, 5864–5874 (2006).
Moynahan, M. E., Pierce, A. J. & Jasin, M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol. Cell 7, 263–272 (2001).
Jensen, R. B., Carreira, A. & Kowalczykowski, S. C. Purified human BRCA2 stimulates RAD51-mediated recombination. Nature 467, 678–683 (2010).
Schlacher, K. et al. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell 145, 529–542 (2011).
Ray Chaudhuri, A. et al. Replication fork stability confers chemoresistance in BRCA-deficient cells. Nature 535, 382–387 (2016).
Ying, S., Hamdy, F. C. & Helleday, T. Mre11-dependent degradation of stalled DNA replication forks is prevented by BRCA2 and PARP1. Cancer Res. 72, 2814–2821 (2012).
Ding, X. et al. Synthetic viability by BRCA2 and PARP1/ARTD1 deficiencies. Nat. Commun. 7, 12425 (2016).
Raghunandan, M., Chaudhury, I., Kelich, S. L., Hanenberg, H. & Sobeck, A. FANCD2, FANCJ and BRCA2 cooperate to promote replication fork recovery independently of the Fanconi anemia core complex. Cell Cycle 14, 342–353 (2015).
Halder, S. et al. Double-stranded DNA binding function of RAD51 in DNA protection and its regulation by BRCA2. Mol. Cell 82, 3553–3565 e5 (2022).
Schlacher, K., Wu, H. & Jasin, M. A distinct replication fork protection pathway connects Fanconi anemia tumor suppressors to RAD51-BRCA1/2. Cancer Cell 22, 106–116 (2012).
Pathania, S. et al. BRCA1 haploinsufficiency for replication stress suppression in primary cells. Nat. Commun. 5, 5496 (2014).
Tian, T. et al. The ZATT-TOP2A-PICH axis drives extensive replication fork reversal to promote genome stability. Mol. Cell 81, 198–211 e6 (2021).
Zellweger, R. et al. Rad51-mediated replication fork reversal is a global response to genotoxic treatments in human cells. J. Cell Biol. 208, 563–579 (2015).
Poole, L. A. & Cortez, D. Functions of SMARCAL1, ZRANB3, and HLTF in maintaining genome stability. Crit. Rev. Biochem. Mol. Biol. 52, 696–714 (2017).
Neelsen, K. J. & Lopes, M. Replication fork reversal in eukaryotes: from dead end to dynamic response. Nat. Rev. Mol. Cell Biol. 16, 207–220 (2015).
Quinet, A., Lemacon, D. & Vindigni, A. Replication fork reversal: players and guardians. Mol. Cell 68, 830–833 (2017).
Betous, R. et al. SMARCAL1 catalyzes fork regression and Holliday junction migration to maintain genome stability during DNA replication. Genes Dev. 26, 151–162 (2012).
Ciccia, A. et al. Polyubiquitinated PCNA recruits the ZRANB3 translocase to maintain genomic integrity after replication stress. Mol. Cell 47, 396–409 (2012).
Kile, A. C. et al. HLTF’s ancient HIRAN domain binds 3′ DNA ends to drive replication fork reversal. Mol. Cell 58, 1090–1100 (2015).
Blastyak, A., Hajdu, I., Unk, I. & Haracska, L. Role of double-stranded DNA translocase activity of human HLTF in replication of damaged DNA. Mol. Cell. Biol. 30, 684–693 (2010).
Taglialatela, A. et al. Restoration of replication fork stability in BRCA1- and BRCA2-deficient cells by inactivation of SNF2-family fork remodelers. Mol. Cell 68, 414–430 e8 (2017).
Kolinjivadi, A. M. et al. Smarcal1-mediated fork reversal triggers Mre11-dependent degradation of nascent DNA in the absence of Brca2 and stable Rad51 nucleofilaments. Mol. Cell 67, 867–881 e7 (2017).
Mijic, S. et al. Replication fork reversal triggers fork degradation in BRCA2-defective cells. Nat. Commun. 8, 859 (2017).
Thangavel, S. et al. DNA2 drives processing and restart of reversed replication forks in human cells. J. Cell Biol. 208, 545–562 (2015).
Lemacon, D. et al. MRE11 and EXO1 nucleases degrade reversed forks and elicit MUS81-dependent fork rescue in BRCA2-deficient cells. Nat. Commun. 8, 860 (2017).
Rondinelli, B. et al. EZH2 promotes degradation of stalled replication forks by recruiting MUS81 through histone H3 trimethylation. Nat. Cell Biol. 19, 1371–1378 (2017).
Kim, J. J. et al. PCAF-mediated histone acetylation promotes replication fork degradation by MRE11 and EXO1 in BRCA-deficient cells. Mol. Cell 80, 327–344 e8 (2020).
Cho, Y. W. et al. PTIP associates with MLL3- and MLL4-containing histone H3 lysine 4 methyltransferase complex. J. Biol. Chem. 282, 20395–20406 (2007).
Bryant, H. E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005).
Farmer, H. et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
Lord, C. J. & Ashworth, A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat. Med. 19, 1381–1388 (2013).
Edwards, S. L. et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature 451, 1111–1115 (2008).
Sakai, W. et al. Functional restoration of BRCA2 protein by secondary BRCA2 mutations in BRCA2-mutated ovarian carcinoma. Cancer Res. 69, 6381–6386 (2009).
Bunting, S. F. et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. Cell 141, 243–254 (2010).
Norquist, B. et al. Secondary somatic mutations restoring BRCA1/2 predict chemotherapy resistance in hereditary ovarian carcinomas. J. Clin. Oncol. 29, 3008–3015 (2011).
Jaspers, J. E. et al. Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors. Cancer Discov. 3, 68–81 (2013).
Xu, G. et al. REV7 counteracts DNA double-strand break resection and affects PARP inhibition. Nature 521, 541–544 (2015).
Guillemette, S. et al. Resistance to therapy in BRCA2 mutant cells due to loss of the nucleosome remodeling factor CHD4. Genes Dev. 29, 489–494 (2015).
Komatsu, M. et al. A novel protein-conjugating system for Ufm1, a ubiquitin-fold modifier. EMBO J. 23, 1977–1986 (2004).
Tatsumi, K. et al. A novel type of E3 ligase for the Ufm1 conjugation system. J. Biol. Chem. 285, 5417–5427 (2010).
Daniel, J. & Liebau, E. The UFM1 cascade. Cells 3, 627–638 (2014).
Liang, Q. et al. Human UFSP1 translated from an upstream near-cognate initiation codon functions as an active UFM1-specific protease. J. Biol. Chem. 298, 102016 (2022).
Kang, S. H. et al. Two novel ubiquitin-fold modifier 1 (UFM1)-specific proteases, UfSP1 and UfSP2. J. Biol. Chem. 282, 5256–5262 (2007).
Tatsumi, K. et al. The Ufm1-activating enzyme Uba5 is indispensable for erythroid differentiation in mice. Nat. Commun. 2, 181 (2011).
Zhang, M. et al. RCAD/Ufl1, a Ufm1 E3 ligase, is essential for hematopoietic stem cell function and murine hematopoiesis. Cell Death Differ. 22, 1922–1934 (2015).
Cai, Y. et al. UFBP1, a key component of the Ufm1 conjugation system, is essential for Ufmylation-mediated regulation of erythroid development. PLoS Genet. 11, e1005643 (2015).
Liu, J. et al. A critical role of DDRGK1 in endoplasmic reticulum homoeostasis via regulation of IRE1alpha stability. Nat. Commun. 8, 14186 (2017).
Li, J. et al. Ufm1-Specific Ligase Ufl1 regulates endoplasmic reticulum homeostasis and protects against heart failure. Circ. Heart Fail. 11, e004917 (2018).
Wang, Z. et al. MRE11 UFMylation promotes ATM activation. Nucleic Acids Res. 47, 4124–4135 (2019).
Qin, B. et al. UFL1 promotes histone H4 ufmylation and ATM activation. Nat. Commun. 10, 1242 (2019).
Qin, B. et al. STK38 promotes ATM activation by acting as a reader of histone H4 ufmylation. Sci. Adv. 6, eaax8214 (2020).
Liu, J. et al. UFMylation maintains tumour suppressor p53 stability by antagonizing its ubiquitination. Nat. Cell Biol. 22, 1056–1063 (2020).
Lee, L. et al. UFMylation of MRE11 is essential for telomere length maintenance and hematopoietic stem cell survival. Sci. Adv. 7, eabc7371 (2021).
Dungrawala, H. et al. The replication checkpoint prevents two types of fork collapse without regulating replisome stability. Mol. Cell 59, 998–1010 (2015).
Patel, S. R., Kim, D., Levitan, I. & Dressler, G. R. The BRCT-domain containing protein PTIP links PAX2 to a histone H3, lysine 4 methyltransferase complex. Dev. Cell 13, 580–592 (2007).
Meszaros, B., Erdos, G. & Dosztanyi, Z. IUPred2A: context-dependent prediction of protein disorder as a function of redox state and protein binding. Nucleic Acids Res. 46, W329–W337 (2018).
Lord, C. J., Tutt, A. N. & Ashworth, A. Synthetic lethality and cancer therapy: lessons learned from the development of PARP inhibitors. Annu. Rev. Med. 66, 455–470 (2015).
Noordermeer, S. M. & van Attikum, H. PARP inhibitor resistance: a tug-of-war in BRCA-mutated cells. Trends Cell Biol. 29, 820–834 (2019).
Zellweger, R. & Lopes, M. Dynamic architecture of eukaryotic DNA replication forks in vivo, visualized by electron microscopy. Methods Mol. Biol. 1672, 261–294 (2018).
Nabet, B. et al. The dTAG system for immediate and target-specific protein degradation. Nat. Chem. Biol. 14, 431–441 (2018).
Acknowledgements
We thank all members of the Liu and Huang groups for insightful discussions. This work was supported by National Key Research and Development Program of China (grants 2022YFA1302800 and 2021YFA1101000 to T.L.), National Natural Science Foundation of China (grants 32270769, 31970664 and 31822031 to T.L., 32200582 to T.T. and 32170730 to J. Han) and the fundamental research funds for the central universities (grant 226-2024-00001 to J. Huang).
Author information
Authors and Affiliations
Contributions
T.T., J.C., H.Z., Y.L. and F.X. performed the cell biology and biochemistry experiments. J. Huang and J. Han participated in experimental design and data analysis. T.L. designed and supervised the project and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Zhenkun Lou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
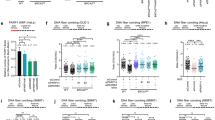
Extended Data Fig. 1 Depletion of UFL1 induces PARPi resistance in BRCA1/2-deficient cells.
(a-b) Knockdown efficiency was confirmed by western blotting. (c-d) HCC1937 or Capan-1 cells were transfected with the indicated siRNAs/plasmids and treated with the PARP inhibitor Veliparib, as indicated. Cells were then allowed to grow for 14 days before staining. The data represent the means ± SEM from three independent experiments. Representative images are shown in the lower panels. (e-g) Knockdown efficiency was confirmed by western blotting. A-B, E-G, Data were independently replicated at least three times, with similar results.
Extended Data Fig. 2 UFL1 deficiency does not restore HR in BRCA1/2-deficient cells.
(a-c) HeLa cells were transfected with the indicated siRNAs and exposed to 10 Gy of IR. Six hours later, cells were immunostained with a RAD51 antibody. Representative images showing RAD51 foci (a). The scale bar represents 10 μm. The percentage of cells with more than 10 RAD51 foci per nucleus was quantified (b). The results represent the mean ± SEM from four independent experiments. P values were derived from a one-way ANOVA with Tukey’s multiple comparisons test. Knockdown efficiency was confirmed by western blotting (c). (d-e) U2OS DR-GFP cells were transfected with the indicated siRNAs and then electroporated with an I-SceI expression plasmid. After 48 hours, cells were harvested and subjected to FACS analysis to measure GFP expression. The data represent the means ± SEM from three independent experiments (d). P values were derived from a one-way ANOVA with Tukey’s multiple comparisons test. Knockdown efficiency was confirmed by western blotting (e). C, E, Data were independently replicated at least three times, with similar results.
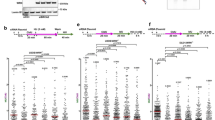
Extended Data Fig. 3 UFL1 promotes nascent DNA degradation in BRCA1/2-deficient cells.
(a, c) Top: Schematic representation of the DNA fiber assay. Bottom: Representative images of DNA fibers. (b, d) Dot plot showing the CldU to IdU track length ratios for individual replication forks. The data represent the mean ± SD from three independent experiments (n=300 fibers for each sample). P values were derived from a one-way ANOVA with Tukey’s multiple comparisons test. (e) Schematic of the generation of UFL1-dTAG cells. (f, i) Verification of UFL1 degradation in UFL1-dTAG HeLa (f) or UFL1-dTAG PEO-1 (i) cells by treatment with dTAG13 (100 nM) for 12 hours. (g, j) Top: Schematic representation of the DNA fiber assay. UFL1-dTAG cells were treated with DMSO or dTAG13 for 12 hours and subjected to sequential pulse-labeling with 50 μM IdU and 250 μM CldU for 30 (g) or 20 (j) minutes, followed by a 5-hour treatment with 4 mM HU. Bottom: Representative images of DNA fibers in UFL1-dTAG cells treated with DMSO or dTAG13. (h) Dot plot showing the CldU to IdU track length ratios for individual replication forks. The data represent the mean ± SD from three independent experiments (from left: n=300, 300, 303, 302 fibers). P values were derived from a one-way ANOVA with Dunnett’s multiple comparisons test. (k) Dot plot showing the CldU to IdU track length ratios for individual replication forks. The data represent the mean ± SD from three independent experiments (from left: n=302, 320 fibers). Statistical analysis was calculated with two-sided, unpaired t-test. F, I, Data were independently replicated at least three times, with similar results.
Extended Data Fig. 4 UFL1 is not required for replication fork reversal and DNA2 accumulation at stalled forks.
(a) Top: Schematic representation of the DNA fiber assay. Bottom: Representative images of DNA fibers. (b) Dot plot showing the CldU to IdU track length ratios for individual replication forks. The data represent the mean ± SD of three independent experiments (from left: n=221, 254, 194 fibers). P values were derived from a one-way ANOVA with Dunnett’s multiple comparisons test. (c) Electron micrograph of a representative reversed fork from HeLa cells. P indicates the parental duplex, D indicates daughter duplexes, and R indicates the regressed arm. (d) Frequency of reversed forks in HU-treated wild-type or UFL1-depleted HeLa cells. The total number of analyzed replication intermediates from two independent experiments is provided in brackets. P values were derived from a one-way ANOVA with Dunnett’s multiple comparisons test. (e) Knockdown efficiency was confirmed by western blotting. (f-h) Representative images of PLA foci (f). The scale bar represents 10 μm. Quantification of the average number of PLA foci per focus-positive cell (g). The data shown represent the mean ± SD from three independent experiments (n=300 cells for each sample). P values were derived from a one-way ANOVA with Tukey’s multiple comparisons test. Knockdown efficiency was confirmed by western blotting (h). (i-k) Representative images of PLA foci (i). The scale bar represents 10 μm. Quantification of the average number of PLA foci per focus-positive cell (j). The results represent the mean ± SD of three independent experiments (n=300 cells for each sample). P values were derived from a one-way ANOVA with Tukey’s multiple comparisons test. Knockdown efficiency was confirmed by western blotting (k). E, H, K, Data were independently replicated at least three times, with similar results.
Extended Data Fig. 5 MRE11 promotes fork degradation independently of its UFMylation.
(a, e) Top: Schematic representation of the DNA fiber assay. Bottom: Representative images of DNA fibers. (b, f) Dot plot showing the CldU to IdU track length ratios for individual replication forks. The data represent the mean ± SD of three independent experiments (from left: n=300, 300, 300, 300, 300, 300, 300, 295 fibers in B; n=303, 302, 303, 303, 300, 277, 300 fibers in F). P values were derived from a one-way ANOVA with Dunnett’s multiple comparisons test. (c, g) Knockdown efficiency was confirmed by western blotting. (d) Schematic representation of the MRE11-WT-UFM1 or the MRE11-K282R-UFM1 fusion construct. C, G, Data were independently replicated at least three times, with similar results.
Extended Data Fig. 6 UFL1 promotes MRE11 recruitment to stalled forks by UFMylating PTIP at lysine 148.
(a) Top: Schematic representation of the DNA fiber assay. Bottom: Representative images of DNA fibers. (b) Dot plot showing the CldU to IdU track length ratios for individual replication forks. The results represent the mean ± SD of three independent experiments (from left: n=305, 303, 302, 306, 302, 302, 307 fibers). P values were derived from a one-way ANOVA with Dunnett’s multiple comparisons test. (c) Knockdown efficiency was confirmed by western blotting. (d, g) Top: Schematic representation of the DNA fiber assay. HCC1937 (d) or Capan-1 (g) cells were transfected with the indicated siRNAs/plasmids and subjected to sequential pulse-labeling with 50 μM IdU and 250 μM CldU for 30 minutes, followed by a 5-hour treatment with 4 mM HU. Bottom: Representative images of DNA fibers. (e, h) Dot plot showing the CldU to IdU track length ratios for individual replication forks in HCC1937 (e) or Capan-1 (h) cells transfected with the indicated siRNAs/plasmids. The data represent the mean ± SD from three independent experiments (n=300 fibers for each sample). P values were derived from a one-way ANOVA with Tukey’s multiple comparisons test. (f, i) Knockdown efficiency was confirmed by western blotting. C, F, I, Data were independently replicated at least three times, with similar results.
Extended Data Fig. 7 PTIP UFMylation facilitates nascent DNA degradation in BRCA1/2-deficient cells.
(a) The strategy and sequencing verification of PTIP K148R knockin (K148R-KI) by CRISPR/Cas9-mediated gene editing. (b) Parental PEO-1 cells or PEO-1 cells with the PTIP K148R knockin were transfected with HA-UFM1-WT or HA-UFM1-ΔG plasmids for 24 hours. The cells were then harvested and lysed in a buffer containing 1% SDS, and the total cell lysates were denatured by boiling for 5 minutes. Subsequently, the lysates were diluted to a final concentration of 0.1% SDS and subjected to immunoprecipitation using protein A-Sepharose beads coupled with anti-PTIP antibody. The resulting immunoprecipitates were analyzed by western blotting. (c-d) Parental PEO-1 cells or PEO-1 cells with the PTIP K148R knockin were labeled with 10 μM EdU for 15 minutes, and then left untreated or treated with 4 mM HU for 3 hours. Representative images of PLA foci (c). The scale bar represents 10 μm. Quantification of the average number of PLA foci per focus-positive cell (d). The data shown represent the mean ± SD from three independent experiments (n=300 cells for each sample). P values were derived from a one-way ANOVA with Tukey’s multiple comparisons test. (e) Top: Schematic representation of the DNA fiber assay. Parental PEO-1 cells or PEO-1 cells with the PTIP K148R knockin were subjected to sequential pulse-labeling with 50 μM IdU and 250 μM CldU for 20 minutes, followed by a 5-hour treatment with 4 mM HU or left untreated. Bottom: Representative images of DNA fibers in cells. (f) Dot plot showing the CldU to IdU track length ratios for individual replication forks in parental PEO-1 cells or PEO-1 cells with the PTIP K148R knockin. The data represent the mean ± SD from three independent experiments (from left: n=301, 313, 314, 300 fibers). P values were derived from a one-way ANOVA with Dunnett’s multiple comparisons test. (g-h) Knockdown efficiency was confirmed by western blotting. B, G, H, Data were independently replicated at least three times, with similar results.
Extended Data Fig. 8 UFL1 is not required for PTIP recruitment to stalled forks.
(a-c) Representative images of PLA foci (a). The scale bar represents 10 μm. Quantification of the average number of PLA foci per focus-positive cell (b). The data represent the mean ± SD of three independent experiments (from left: n=300, 300, 300, 288 fibers). P values were derived from a one-way ANOVA with Dunnett’s multiple comparisons test. Western blot analysis of PTIP expression (c). (d-f) Representative images of PLA foci (d). The scale bar represents 10 μm. Quantification of the average number of PLA foci per focus-positive cell (e). The data represent the mean ± SD of three independent experiments (n=300 cells for each sample). P values were derived from a one-way ANOVA with Tukey’s multiple comparisons test. Knockdown efficiency was confirmed by western blotting (f). (g-i) Representative images of PLA foci (g). The scale bar represents 10 μm. Quantification of the average number of PLA foci per focus-positive cell (h). The results represent the mean ± SD of three independent experiments (from left: n=300, 291, 293, 299 cells). P values were derived from a one-way ANOVA with Dunnett’s multiple comparisons test. Knockdown efficiency was confirmed by western blotting (i). (j) Western blot analysis of iPOND and input samples. C, F, I, J, Data were independently replicated at least three times, with similar results.
Extended Data Fig. 9 PTIP UFMylation facilitates MLL4 recruitment to stalled forks.
(a-b, d-e) Representative images of PLA foci (a, d). The scale bar represents 10 μm. Quantification of the average number of PLA foci per focus-positive cell (b, e). The data shown represent the mean ± SD from three independent experiments (n=300 cells for each sample). P values were derived from a one-way ANOVA with Tukey’s multiple comparisons test. (c) Western blot analysis of iPOND and input samples. (f) Knockdown efficiency was confirmed by western blotting. (g-h) Representative images of PLA foci (g). The scale bar represents 10 μm. Quantification of the average number of PLA foci per focus-positive cell (h). The data represent the mean ± SD of three independent experiments (from left: n=300, 300, 300, 300, 300, 289 cells). P values were derived from a one-way ANOVA with Dunnett’s multiple comparisons test. (i-j) Representative images of PLA foci (i). The scale bar represents 10 μm. Quantification of the average number of PLA foci per focus-positive cell (j). The results represent the mean ± SD of three independent experiments (from left: n=300, 300, 300, 300, 286, 300 fibers). P values were derived from a one-way ANOVA with Dunnett’s multiple comparisons test. C, F, Data were independently replicated at least three times, with similar results.
Extended Data Fig. 10 PTIP UFMylation confers PARPi sensitivity in BRCA1/2-deficient cells.
(a) Parental PEO-1 cells or PEO-1 cells with the PTIP K148R knockin were treated with the PARP inhibitor Veliparib, as indicated. Cells were then allowed to grow for 14 days before staining. The data represent the means ± SEM from three independent experiments. Representative images are shown in the lower panels. (b)Western blot analysis showing the expression levels of wild-type UFSP2 or the C302S mutant in the indicated cells. (c-d) HeLa cells were transfected with the indicated siRNAs/plasmids and treated with the PARP inhibitor Veliparib, as indicated. Cells were then allowed to grow for 14 days before staining. The presented data represent the means ± SEM from three independent experiments. Representative images are displayed in the lower panels. (e-f) Western blot analysis of BRCA1, BRCA2, or UFSP2 expression in the indicated cells. B, E, F, Data were independently replicated at least three times, with similar results.
Supplementary Information
Supplementary Information
Supplementary Figs. 1–3 and source data for Supplementary Figs. 2 and 3.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blots.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed western blots.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 9
Unprocessed western blots.
Source Data Extended Data Fig. 10
Statistical source data.
Source Data Extended Data Fig. 10
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, T., Chen, J., Zhao, H. et al. UFL1 triggers replication fork degradation by MRE11 in BRCA1/2-deficient cells. Nat Chem Biol (2024). https://doi.org/10.1038/s41589-024-01611-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41589-024-01611-7