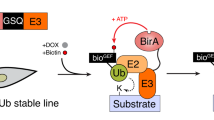
Abstract
Protein ubiquitylation controls diverse processes within eukaryotic cells, including protein degradation, and is often dysregulated in disease. Moreover, small-molecule degraders that redirect ubiquitylation activities toward disease targets are an emerging and promising therapeutic class. Over 600 E3 ubiquitin ligases are expressed in humans, but their substrates remain largely elusive, necessitating the development of new methods for their discovery. Here we report the development of E3-substrate tagging by ubiquitin biotinylation (E-STUB), a ubiquitin-specific proximity labeling method that biotinylates ubiquitylated substrates in proximity to an E3 ligase of interest. E-STUB accurately identifies the direct ubiquitylated targets of protein degraders, including collateral targets and ubiquitylation events that do not lead to substrate degradation. It also detects known substrates of E3 ligase CRBN and VHL with high specificity. With the ability to elucidate proximal ubiquitylation events, E-STUB may facilitate the development of proximity-inducing therapeutics and act as a generalizable method for E3-substrate mapping.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Raw data files of all MS studies in this study have been deposited in the PRIDE Archive, including PXD042673, PXD042691, PXD042687, PXD047583, PXD042693, PXD042674, PXD042697, PXD042700, PXD042698, PXD042702, PXD042694, PXD042706, PXD021255, PXD042657 and PXD047455. Processed protein abundance values and results from limma analyses are provided in Supplementary Data 1–4. Details on the experimental conditions, associated figures and corresponding PRIDE accession number can be also found in the ‘metadata’ tab in Supplementary Data 1–4. Source data are provided with this paper.
Code availability
The code necessary to reproduce the statistical analysis for quantitative proteomics can be found at: https://doi.org/10.5281/zenodo.10633173.
References
Hershko, A. & Ciechanover, A. The ubiquitin system. Annu. Rev. Biochem. 67, 425–479 (1998).
Harper, J. W. & Schulman, B. A. Cullin-RING ubiquitin ligase regulatory circuits: a quarter century beyond the F-box hypothesis. Annu. Rev. Biochem. 90, 403–429 (2021).
Zheng, N. & Shabek, N. Ubiquitin ligases: structure, function, and regulation. Annu. Rev. Biochem. 86, 129–157 (2017).
Popovic, D., Vucic, D. & Dikic, I. Ubiquitination in disease pathogenesis and treatment. Nat. Med. 20, 1242–1253 (2014).
Békés, M., Langley, D. R. & Crews, C. M. PROTAC targeted protein degraders: the past is prologue. Nat. Rev. Drug Discov. 21, 181–200 (2022).
Iconomou, M. & Saunders, D. N. Systematic approaches to identify E3 ligase substrates. Biochem. J. 473, 4083–4101 (2016).
Tan, M.-K., Lim, H.-J., Bennett, E. J., Shi, Y. & Harper, J. W. Parallel SCF adaptor capture proteomics reveals a role for SCFFBXL17 in NRF2 activation via BACH1 repressor turnover. Mol. Cell 52, 9–24 (2013).
Manford, A. G. et al. A cellular mechanism to detect and alleviate reductive stress. Cell 183, 46–61 (2020).
Yamanaka, S. et al. A proximity biotinylation-based approach to identify protein-E3 ligase interactions induced by PROTACs and molecular glues. Nat. Commun. 13, 183 (2022).
Coyaud, E. et al. BioID-based identification of Skp Cullin F-box (SCF) β-TrCP1/2 E3 ligase substrates. Mol. Cell. Proteom. 14, 1781–1795 (2015).
Zhuang, M., Guan, S., Wang, H., Burlingame, A. L. & Wells, J. A. Substrates of IAP ubiquitin ligases identified with a designed orthogonal E3 ligase, the NEDDylator. Mol. Cell 49, 273–282 (2013).
Lu, G. et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 343, 305–309 (2014).
Emanuele, M. J. et al. Global identification of modular cullin-RING ligase substrates. Cell 147, 459–474 (2011).
Yoshida, Y. et al. A comprehensive method for detecting ubiquitinated substrates using TR-TUBE. Proc. Natl Acad. Sci. USA 112, 4630–4635 (2015).
Sarraf, S. A. et al. Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature 496, 372–376 (2013).
Theurillat, J.-P. et al. Ubiquitylome analysis identifies dysregulation of effector substrates in SPOP-mutant prostate cancer. Science 346, 85–89 (2014).
Loveless, T. B. et al. DNA damage regulates translation through β-TRCP targeting of CReP. PLoS Genet. 11, e1005292 (2015).
Watanabe, M. et al. A substrate-trapping strategy to find E3 ubiquitin ligase substrates identifies Parkin and TRIM28 targets. Commun. Biol. 3, 592 (2020).
O’Connor, H. F. et al. Ubiquitin-activated interaction traps (UBAITs) identify E3 ligase binding partners. EMBO Rep. 16, 1699–1712 (2015).
Kumar, R., González-Prieto, R., Xiao, Z., Verlaan-de Vries, M. & Vertegaal, A. C. The STUbL RNF4 regulates protein group SUMOylation by targeting the SUMO conjugation machinery. Nat. Commun. 8, 1809 (2017).
Fernández-Suárez, M., Chen, T. S. & Ting, A. Y. Protein–protein interaction detection in vitro and in cells by proximity biotinylation. J. Am. Chem. Soc. 130, 9251–9253 (2008).
Jan, C. H., Williams, C. C. & Weissman, J. S. Principles of ER cotranslational translocation revealed by proximity-specific ribosome profiling. Science 346, 1257521 (2014).
Krönke, J. et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 343, 301–305 (2014).
Matyskiela, M. E. et al. A novel cereblon modulator recruits GSPT1 to the CRL4CRBN ubiquitin ligase. Nature 535, 252–257 (2016).
Winter, G. E. et al. BET bromodomain proteins function as master transcription elongation factors independent of CDK9 recruitment. Mol. Cell 67, 5–18 (2017).
Raina, K. et al. PROTAC-induced BET protein degradation as a therapy for castration-resistant prostate cancer. Proc. Natl Acad. Sci. USA 113, 7124–7129 (2016).
Popow, J. et al. Highly selective PTK2 proteolysis targeting chimeras to probe focal adhesion kinase scaffolding functions. J. Med. Chem. 62, 2508–2520 (2019).
Han, T. et al. Anticancer sulfonamides target splicing by inducing RBM39 degradation via recruitment to DCAF15. Science 356, eaal3755 (2017).
Uehara, T. et al. Selective degradation of splicing factor CAPERα by anticancer sulfonamides. Nat. Chem. Biol. 13, 675–680 (2017).
Donovan, K. A. et al. Mapping the degradable kinome provides a resource for expedited degrader development. Cell 183, 1714–1731 (2020).
Meier, F. et al. diaPASEF: parallel accumulation–serial fragmentation combined with data-independent acquisition. Nat. Methods 17, 1229–1236 (2020).
Xiong, Y. et al. Chemo-proteomics exploration of HDAC degradability by small molecule degraders. Cell Chem. Biol. 28, 1514–1527.e4 (2021).
Hsu, J. H.-R. et al. EED-targeted PROTACs degrade EED, EZH2, and SUZ12 in the PRC2 complex. Cell Chem. Biol. 27, 41–46 (2020).
Farnaby, W. et al. BAF complex vulnerabilities in cancer demonstrated via structure-based PROTAC design. Nat. Chem. Biol. 15, 672–680 (2019).
Archibald, L. J. et al. Hydroxamic acid-modified peptide library provides insights into the molecular basis for the substrate selectivity of HDAC corepressor complexes. ACS Chem. Biol. 17, 2572–2582 (2022).
Ivan, M. et al. HIFα targeted for VHL-Mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 (2001).
Jaakkola, P. et al. Targeting of HIF-α to the von Hippel–Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 (2001).
Van Nguyen, T. et al. Glutamine triggers acetylation-dependent degradation of glutamine synthetase via the thalidomide receptor cereblon. Mol. Cell 61, 809–820 (2016).
Song, T. et al. CRL4 antagonizes SCFFbxo7-mediated turnover of cereblon and BK channel to regulate learning and memory. PLoS Genet. 14, e1007165 (2018).
Ohh, M. et al. Synthetic peptides define critical contacts between elongin C, elongin B, and the von Hippel–Lindau protein. J. Clin. Invest. 104, 1583–1591 (1999).
Frost, J. et al. Potent and selective chemical probe of hypoxic signalling downstream of HIF-α hydroxylation via VHL inhibition. Nat. Commun. 7, 13312 (2016).
Riching, K. M. et al. CDK family PROTAC profiling reveals distinct kinetic responses and cell cycle-dependent degradation of CDK2. SLAS Discov. 26, 560–569 (2021).
Hanzl, A. et al. E3-specific degrader discovery by dynamic tracing of substrate receptor abundance. J. Am. Chem. Soc. 145, 1176–1184 (2023).
Słabicki, M. et al. The CDK inhibitor CR8 acts as a molecular glue degrader that depletes cyclin K. Nature 585, 293–297 (2020).
Xiong, Y. et al. Bridged proteolysis targeting chimera (PROTAC) enables degradation of undruggable targets. J. Am. Chem. Soc. 144, 22622–22632 (2022).
Barroso-Gomila, O. et al. BioE3 identifies specific substrates of ubiquitin E3 ligases. Nat. Commun. 14, 7656 (2023).
Mukhopadyay, U. et al. A ubiquitin-specific, proximity-based labeling approach for the identification of ubiquitin ligase substrates. Preprint at bioRxiv https://doi.org/10.1101/2023.09.04.556194 (2023).
Sowa, M. E., Bennett, E. J., Gygi, S. P. & Harper, J. W. Defining the human deubiquitinating enzyme interaction landscape. Cell 138, 389–403 (2009).
Teo, G. et al. SAINTexpress: improvements and additional features in significance analysis of INTeractome software. J. Proteom. 100, 37–43 (2014).
Donovan, K. A. et al. Thalidomide promotes degradation of SALL4, a transcription factor implicated in Duane Radial Ray syndrome. eLife 7, e38430 (2018).
R Core Team. R: A language and environment for statistical computing. (R Foundation for Statistical Computing, 2023).
Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015).
Demichev, V., Messner, C. B., Vernardis, S. I., Lilley, K. S. & Ralser, M. DIA-NN: neural networks and interference correction enable deep proteome coverage in high throughput. Nat. Methods 17, 41–44 (2020).
Acknowledgements
This work was supported by the Merkin Institute for Transformative Technologies in Healthcare and the Ludwig Center at Harvard. BI-0319 and BI-3663 were provided by Boehringer Ingelheim via its open innovation platform opnMe (available at https://opnme.com). SK-3-91 and SB1-G-187 were provided by N.S. Gray (Stanford University, CA, USA). DB0646 was provided by T. Sim (Yonsei University College of Medicine, Republic of Korea). WH-10417-099 was provided by S.J. Buhrlage (Dana-Farber Cancer Institute, MA, USA). We thank W.G. Kaelin for helpful comments; M. L. Meyerson (Dana-Farber Cancer Institute, MA, USA) for providing the pRK5-puroR and PX330 constructs; J.A. Cutler, M. Slabicki, H. Yoon and Y.-D. Li for helpful discussions; D. Kesar for advice on bioinformatic analysis; K. L. Shaw for proofreading the paper. W.R.S. was supported by R01CA233626 from the National Institutes of Health (NIH).
Author information
Authors and Affiliations
Contributions
H.-T.H. and W.R.S. conceived the project. H.-T.H., R.W.T. and S.S. performed the E-STUB experiments. K.A.D. and N.M. performed the total proteomics experiments. R.J.L. and K.A.D. acquired the MS data. R.J.L., K.A.D., H.-T.H. and X.Z. performed bioinformatic analysis. H.-T.H, R.T., S.S. and J.C. performed the biological validation and analysis. Y.X. synthesized the HDAC degraders. H.-T.H. prepared the figures for the main text and the extended data. H.-T.H. and W.R.S. wrote the paper with critical reading and feedback from the other coauthors. W.R.S. and E.S.F. supervised the project.
Corresponding author
Ethics declarations
Competing interests
W.R.S. and H.-T.H. are inventors on a filed patent describing E-STUB (PCT application publication WO/2023/235313). W.R.S. is a board or scientific advisory board (SAB) member and holds equity in Ideaya Biosciences, Civetta Therapeutics, Red Ridge Bio, Delphia Therapeutics and 2Seventy Bio. W.R.S. has consulted for Array, Astex, Epidarex Capital, Ipsen, PearlRiver Therapeutics, Merck Pharmaceuticals, Sanofi, Servier and Syndax Pharmaceuticals and receives research funding from Pfizer Pharmaceuticals, Merck Pharmaceuticals, Ideaya Biosciences, Calico, Boehringer Ingelheim, Bristol Myers Squibb, Novartis Institutes for Biomedical Research, Bayer Pharmaceuticals and Ridgeline Discovery. E.S.F. is a founder, SAB member and equity holder of Civetta Therapeutics, Lighthorse Therapeutics, Proximity Therapeutics and Neomorph (also board of directors). E.S.F. is an equity holder and SAB member for Avilar Therapeutics and Photys Therapeutics and a consultant to Novartis, Sanofi, EcoR1 Capital, Ajax Therapeutics and Deerfield. The Fischer Lab receives or has received research funding from Deerfield, Novartis, Ajax, Interline and Astellas. K.A.D. is a consultant to Kronos Bio and Neomorph. The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Wei Qin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Development and optimization of E-STUB.
a, Immunoblotting analysis of Avi-ubiquitylated and biotinylated proteins in 293T cells expressing Avi-ubiquitin, 3F-BirA-mCherry, and treated with biotin (50 μM), where indicated. Images from a single experiment. b, Immunoblotting analysis of GSPT1 abundance after 4-hour CC-885 (1 μM) treatment in 293T, 293TCRBN−/− and 293TCRBN−/− cells expressing CRBN-BirA or BirA-CRBN as indicated. Images from a single experiment. c, 293T cells expressing Avi-ubiquitin, CRBN-BirA and V5-IKZF1 were treated with carfilzomib (0.4 μM, 2 hours), and where indicated, POM (1 μM, 1 hour) and biotin (50 μM, 15 minutes). Right: ectopically expressed proteins detected by immunoblotting. Middle: Avi-ubiquitylation and biotinylation of immunoprecipitated V5-IKZF1 detected by immunoblotting. Left: V5-IKZF1 and biotinylated proteins enriched by streptavidin beads detected by immunoblotting. Representative of two independent measurements. POM, pomalidomide. IP, immunoprecipitation. AP, affinity purification. d, 293T cells expressing the indicated variant of BAP-ubiquitin, V5-IKZF1 and CRBN-BirA were treated with carfilzomib (0.4 μM, 2 hours), and where indicated, POM (1 μM, 1 hour) and biotin (50 μM, 15 minutes). Left: ectopically expressed and biotinylated proteins detected by immunoblotting. Right: BAP-ubiquitylation and biotinylation of immunoprecipitated V5-IKZF1 detected by immunoblotting. Representative of two independent measurements. e, 293TCRBN−/− cells expressing the indicated variant of BAP-ubiquitin and CRBN-BirA were treated with carfilzomib (0.4 μM, 3 hours), biotin (50 μM, 15 minutes), and where indicated, CC-885 (1 μM, 2 hours). GSPT1, ectopically expressed proteins, and biotinylated proteins enriched by streptavidin beads were detected by immunoblotting. Representative of two independent measurements.
Extended Data Fig. 2 Two-day biotin depletion does not affect cellular health and response.
a,b, Two-day growth curves of 293T (a) or A375 (b) cells grown in normal media versus biotin-depleted media. Individual data points were shown for two biological replicates. c,d, Scatter plot showing fold change (FC) in protein abundance of 293T cells treated with SK-3-91 (1 μM) (c) or XY-07-187 (1 μM) (d) for 5 hours when cultured in biotin-depleted media (2-day depletion) versus normal media. Proteins that are significantly degraded in both conditions (log2 FC < −0.585; p-value < 0.001) are highlighted in yellow; proteins only significantly degraded in the biotin-depleted condition are highlighted red; proteins only significantly degraded in the normal media condition are highlighted blue; significant hits that are detected in one condition but not the other are shown in the respective gray boxes labeled n.d. (not detected). e, FC in protein abundance of 293T cells grown in biotin-depleted media (2-day depletion) vs normal media. Total proteomics experiments were evaluated by label-free quantification (timsTOF Pro2) in biological triplicates. log2(FC) and P values were calculated by two-sided moderated t test implemented by the limma package. Adjusted P values of multiple comparisons are included in Supplementary Data 4.
Extended Data Fig. 3 Unbiased discovery of chemically induced substrates by E-STUB and mass spectrometry.
a, Schematic of E-STUB workflow for substrate identification by mass spectrometry. b. Left: rank-ordered abundance plot highlighting top 50 streptavidin-enriched proteins from an E-STUB experiment in 293TCRBN−/− cells expressing CRBN-BirA and A3-Ub after 2-hour CC-885 (1 μM) treatment. Right: heatmap showing the respective abundances of those top 50 streptavidin-enriched proteins in CC-885-treated versus DMSO-treated conditions. c, Immunoblotting analysis of protein inputs and those enriched by Ni-NTA resins from 293T cells expressing His-tagged ubiquitin, Flag-tagged CRBN and the indicated V5-tagged proteins. The cells were treated with carfilzomib (0.4 μM) and, where indicated, with CC-885 (1 μM) for 2 hours. Images from a single experiment. d,e, E-STUB data showing fold change (FC) in abundance of streptavidin-enriched proteins following 1-hour CC-885 (1 μM) treatment in U2OS cells expressing CRBN-BirA and A3-Ub (d) or 1-hour E7820 (1 μM) treatment in 293T cells expressing DCAF15-BirA and A3-Ub (e). All E-STUB experiments were evaluated by label-free quantification (Exploris 480) in biological triplicates. log2(FC) and P values were calculated by two-sided moderated t test implemented by the limma package. Adjusted P values of multiple comparisons are included in Supplementary Data 2.
Extended Data Fig. 4 E-STUB characterization of multikinase degrader SK-3-91.
a, Bubble chart showing fold change (FC) in abundance (represented by size of dots) and statistical significance (represented by saturation of color) of significant biotinylated proteins (log2 FC > 1; P value < 0.001) in an E-STUB experiment where 293TCRBN−/− cells expressing CRBN-BirA and A3-Ub were treated with SK-3-91 (1 μM) for 15 minutes, 1 hour and 4 hours. b, E-STUB data showing FC in abundance of streptavidin-enriched proteins following 1-hour SK-3-91 (1 μM) treatment in 293TCRBN−/− cells expressing CRBN-BirA and A3-Ub. c, FC in abundance (represented by size of dots) and statistical significance (represented by saturation of color) from b overlaid on top of a volcano plot displaying FC in protein abundance of 293T cells treated with SK-3-91 (1 μM) for 5 hours. Proteins identified in total proteomics that are not detected by E-STUB are shown as cross (×). d, Scatter plot showing FC in abundance of streptavidin-enriched proteins described in b versus FC in total protein abundance described in c. Protein that are both significantly biotinylated (log2(FC) > 1; P value < 0.001) and degraded (log2(FC) < −0.585; P value < 0.001) are highlighted in yellow; proteins only significantly biotinylated are highlighted in red; proteins only significantly degraded are highlighted in blue; significant hits that are detected by one assay but not the other are shown in the respective gray boxes labeled n.d. (not detected). The E-STUB experiment shown in a was evaluated by label-free quantification (Exploris 480) in biological triplicates. The E-STUB experiment shown in b–d was evaluated by label-free quantification (timsTOF Pro2) in biological triplicates. Total proteomics experiments were evaluated by TMT quantitative proteomics (Exploris 480) in at least biological duplicates30. log2(FC) and P values were calculated by two-sided moderated t test implemented by the limma package. Adjusted P values of multiple comparisons are included in Supplementary Data 2 and 4.
Extended Data Fig. 5 Targeted engagement does not predict induced ubiquitylation.
a–e, Scatterplots comparing induced bio-ubiquitylation (E-STUB) and kinase engagement (KiNativ) for SK-3-91 (a), SB1-G-187 (b), DB0646 (c), WH-10417-099 (d) and SK-3-91 (e; timsTOF Pro2). KiNativ data were previously published30. E-STUB data correspond to those shown in Fig. 3a (a), Fig. 3d (b), Fig. 3g (c), Fig. 3j (d) and Extended Data Fig. 4b (e). The coloring scheme for each data point also follows the rules specified in Fig. 3c,f,i,l and Extended Data Fig. 4d.
Extended Data Fig. 6 Protein degradation kinetics in response to multi-HDAC degrader XY-07-187.
Immunoblotting analysis of select HDACs, corepressor complex proteins and known short half-life proteins from 293T cells treated with CHX (50 μg/ml), XY-07-187 (1 μM) or both over a 6-hour time course. CHX, cycloheximide. Representative images of two independent measurements.
Extended Data Fig. 7 Substrate identification utilizing mutant E3 ubiquitin ligases.
a, E-STUB data showing fold change (FC) in abundance of streptavidin-enriched proteins in 293T cells expressing A3-Ub and VHL-BirA versus 293TCRBN−/− cells expressing A3-Ub and CRBN-BirA. b,c, E-STUB data showing FC in abundance of streptavidin-enriched proteins in 293TCRBN−/− cells expressing A3-Ub and CRBN(W386A)-BirA versus CRBN(WT)-BirA (b) or CRBN(D249Y)-BirA versus CRBN(WT)-BirA (c) after 1-hour CC-885 (1 μM) treatment. All E-STUB experiments were evaluated by label-free quantification (Exploris 480) in biological triplicates. log2(FC) and P values were calculated by two-sided moderated t test implemented by the limma package. Adjusted P values of multiple comparisons are included in Supplementary Data 2.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Supplementary Data 1
Quantification of streptavidin-enriched proteins of all E-STUB experiments in this study.
Supplementary Data 2
Differential analysis implemented by the limma package of all E-STUB experiments in this study.
Supplementary Data 3
Protein quantification of all total proteomics experiments in this study.
Supplementary Data 4
Differential analysis implemented by the limma package of all total proteomics experiments in this study.
Source data
Source Data Fig. 1
Uncropped images.
Source Data Fig. 2
Uncropped images.
Source Data Fig. 4
Uncropped images.
Source Data Extended Data Fig. 1
Uncropped images.
Source Data Extended Data Fig. 3
Uncropped images.
Source Data Extended Data Fig. 6
Uncropped images.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, HT., Lumpkin, R.J., Tsai, R.W. et al. Ubiquitin-specific proximity labeling for the identification of E3 ligase substrates. Nat Chem Biol (2024). https://doi.org/10.1038/s41589-024-01590-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41589-024-01590-9