Abstract
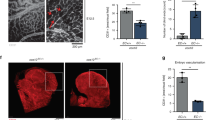
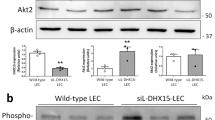
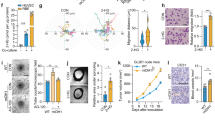
Angiogenic programming in the vascular endothelium is a tightly regulated process for maintaining tissue homeostasis and is activated in tissue injury and the tumor microenvironment. The metabolic basis of how gas signaling molecules regulate angiogenesis is elusive. Here, we report that hypoxic upregulation of ·NO in endothelial cells reprograms the transsulfuration pathway to increase biogenesis of hydrogen sulfide (H2S), a proangiogenic metabolite. However, decreased H2S oxidation due to sulfide quinone oxidoreductase (SQOR) deficiency synergizes with hypoxia, inducing a reductive shift and limiting endothelial proliferation that is attenuated by dissipation of the mitochondrial NADH pool. Tumor xenografts in whole-body (WBCreSqorfl/fl) and endothelial-specific (VE-cadherinCre-ERT2Sqorfl/fl) Sqor-knockout mice exhibit lower mass and angiogenesis than control mice. WBCreSqorfl/fl mice also exhibit decreased muscle angiogenesis following femoral artery ligation compared to control mice. Collectively, our data reveal the molecular intersections between H2S, O2 and ·NO metabolism and identify SQOR inhibition as a metabolic vulnerability for endothelial cell proliferation and neovascularization.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated and analyzed in this study are included in the main text and Supplementary Information file. Source data are provided with this paper.
Code availability
The code used to analyze tip cells in the microfluidic device is available for download at https://github.com/hirakih/Sulfide-oxidation-promotes-hypoxic-angiogenesis-and-neovascularization-MATLAB-Code.
References
Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 9, 653–660 (2003).
Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 438, 932–936 (2005).
Folkman, J. Angiogenesis. Annu. Rev. Med. 57, 1–18 (2006).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342 (2004).
Ribatti, D., Annese, T., Ruggieri, S., Tamma, R. & Crivellato, E. Limitations of anti-angiogenic treatment of tumors. Transl. Oncol. 12, 981–986 (2019).
Szabo, C. & Papapetropoulos, A. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br. J. Pharmacol. 164, 853–865 (2011).
Rushing, A. M. et al. Effects of a novel hydrogen sulfide prodrug in a porcine model of acute limb ischemia. J. Vasc. Surg. 69, 1924–1935 (2019).
Coletta, C. et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl Acad. Sci. USA 109, 9161–9166 (2012).
Papapetropoulos, A. et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl Acad. Sci. USA 106, 21972–21977 (2009).
Longchamp, A. et al. Amino acid restriction triggers angiogenesis via GCN2/ATF4 regulation of VEGF and H2S production. Cell 173, 117–129 (2018).
Olson, K. R. et al. Hydrogen sulfide as an oxygen sensor/transducer in vertebrate hypoxic vasoconstriction and hypoxic vasodilation. J. Exp. Biol. 209, 4011–4023 (2006).
Morikawa, T. et al. Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc. Natl Acad. Sci. USA 109, 1293–1298 (2012).
Singh, S. & Banerjee, R. PLP-dependent H2S biogenesis. Biochim. Biophys. Acta 1814, 1518–1527 (2011).
Zou, C.-G. & Banerjee, R. Homocysteine and redox signaling. Antioxid. Redox Signal. 7, 547–559 (2005).
Vitvitsky, V. et al. Perturbations in homocysteine-linked redox homeostasis in a murine model for hyperhomocysteinemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R39–R46 (2004).
Kumar, R. & Banerjee, R. Regulation of the redox metabolome and thiol proteome by hydrogen sulfide. Crit. Rev. Biochem. Mol. Biol. 56, 221–235 (2021).
Hanna, D., Kumar, R. & Banerjee, R. A metabolic paradigm for hydrogen sulfide signaling via electron transport chain plasticity. Antioxid. Redox Signal. 38, 57–67 (2023).
Banerjee, R. Catalytic promiscuity and heme-dependent redox regulation of H2S synthesis. Curr. Opin. Chem. Biol. 37, 115–121 (2017).
Kozich, V. et al. Human ultrarare genetic disorders of sulfur metabolism demonstrate redundancies in H2S homeostasis. Redox Biol. 58, 102517 (2022).
Chiku, T. et al. H2S biogenesis by human cystathionine γ-lyase leads to the novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J. Biol. Chem. 284, 11601–11612 (2009).
Weeks, C. L., Singh, S., Madzelan, P., Banerjee, R. & Spiro, T. G. Heme regulation of human cystathionine β-synthase activity: insights from fluorescence and Raman spectroscopy. J. Am. Chem. Soc. 131, 12809–12816 (2009).
Singh, S., Madzelan, P. & Banerjee, R. Properties of an unusual heme cofactor in PLP-dependent cystathionine β-synthase. Nat. Prod. Rep. 24, 631–639 (2007).
Kabil, O., Yadav, V. & Banerjee, R. Heme-dependent metabolite switching regulates H2S synthesis in response to ER stress. J. Biol. Chem. 291, 16418–16423 (2016).
Furne, J., Saeed, A. & Levitt, M. D. Whole tissue hydrogen sulfide concentrations are orders of magnitude lower than presently accepted values. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R1479–R1485 (2008).
Vitvitsky, V., Kabil, O. & Banerjee, R. High turnover rates for hydrogen sulfide allow for rapid regulation of its tissue concentrations. Antioxid. Redox Signal. 17, 22–31 (2012).
Libiad, M. et al. Hydrogen sulfide perturbs mitochondrial bioenergetics and triggers metabolic reprogramming in colon cells. J. Biol. Chem. 294, 12077–12090 (2019).
Banerjee, R. & Kumar, R. Gas regulation of complex II reversal via electron shunting to fumarate in the mammalian ETC. Trends Biochem. Sci. 47, 689–698 (2022).
Vitvitsky, V. et al. The mitochondrial NADH pool is involved in hydrogen sulfide signaling and stimulation of aerobic glycolysis. J. Biol. Chem. 296, 100736 (2021).
Hanna, D. A., Vitvitsky, V. & Banerjee, R. A growth chamber for chronic exposure of mammalian cells to H2S. Anal. Biochem. 673, 115191 (2023).
Carballal, S. et al. Hydrogen sulfide stimulates lipid biogenesis from glutamine that is dependent on the mitochondrial NAD(P)H pool. J. Biol. Chem. 297, 100950 (2021).
De Bock, K. et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154, 651–663 (2013).
Diebold, L. P. et al. Mitochondrial complex III is necessary for endothelial cell proliferation during angiogenesis. Nat. Metab. 1, 158–171 (2019).
Kumar, R. et al. A redox cycle with complex II prioritizes sulfide quinone oxidoreductase-dependent H2S oxidation. J. Biol. Chem. 298, 101435 (2022).
Spinelli, J. B. et al. Fumarate is a terminal electron acceptor in the mammalian electron transport chain. Science 374, 1227–1237 (2021).
Marutani, E. et al. Sulfide catabolism ameliorates hypoxic brain injury. Nat. Commun. 12, 3108 (2021).
Jackson, M. R. et al. Discovery of a first-in-class inhibitor of sulfide:quinone oxidoreductase that protects against adverse cardiac remodeling and heart failure. Cardiovasc. Res. 118, 1771–1784 (2022).
Mansfield, K. D., Simon, M. C. & Keith, B. Hypoxic reduction in cellular glutathione levels requires mitochondrial reactive oxygen species. J. Appl. Physiol. 97, 1358–1366 (2004).
Singhal, R. et al. HIF-2α activation potentiates oxidative cell death in colorectal cancers by increasing cellular iron. J. Clin. Invest. 131, e143691 (2021).
Maclean, K. N. et al. Cystathionine β-synthase is coordinately regulated with proliferation through a redox-sensitive mechanism in cultured human cells and Saccharomyces cerevisiae. J. Cell. Physiol. 192, 81–92 (2002).
Shi, Y. et al. Chronic hypoxia increases endothelial nitric oxide synthase generation of nitric oxide by increasing heat shock protein 90 association and serine phosphorylation. Circ. Res. 91, 300–306 (2002).
Hampl, V., Cornfield, D. N., Cowan, N. J. & Archer, S. L. Hypoxia potentiates nitric oxide synthesis and transiently increases cytosolic calcium levels in pulmonary artery endothelial cells. Eur. Respir. J. 8, 515–522 (1995).
Justice, J. M., Tanner, M. A. & Myers, P. R. Endothelial cell regulation of nitric oxide production during hypoxia in coronary microvessels and epicardial arteries. J. Cell. Physiol. 182, 359–365 (2000).
Min, J. et al. Hypoxia-induced endothelial NO synthase gene transcriptional activation is mediated through the tax-responsive element in endothelial cells. Hypertension 47, 1189–1196 (2006).
Ida, T. et al. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl Acad. Sci. USA 111, 7606–7611 (2014).
Yadav, P. K. et al. Biosynthesis and reactivity of cysteine persulfides in signaling. J. Am. Chem. Soc. 138, 289–299 (2016).
Wedmann, R. et al. Improved tag–switch method reveals that thioredoxin acts as depersulfidase and controls the intracellular levels of protein persulfidation. Chem. Sci. 7, 3414–3426 (2016).
Wang, W. Y., Lin, D., Jarman, E. H., Polacheck, W. J. & Baker, B. M. Functional angiogenesis requires microenvironmental cues balancing endothelial cell migration and proliferation. Lab Chip 20, 1153–1166 (2020).
Titov, D. V. et al. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science 352, 231–235 (2016).
Cherepanova, O. A. & Byzova, T. V. Pentose phosphate pathway drives vascular maturation. Nat. Metab. 4, 15–16 (2022).
Vizan, P. et al. Characterization of the metabolic changes underlying growth factor angiogenic activation: identification of new potential therapeutic targets. Carcinogenesis 30, 946–952 (2009).
Pugh, C. W. & Ratcliffe, P. J. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat. Med. 9, 677–684 (2003).
Li, X., Sun, X. & Carmeliet, P. Hallmarks of endothelial cell metabolism in health and disease. Cell Metab. 30, 414–433 (2019).
Kabil, O. & Banerjee, R. Characterization of patient mutations in human persulfide dioxygenase (ETHE1) involved in H2S catabolism. J. Biol. Chem. 287, 44561–44567 (2012).
Libiad, M., Yadav, P. K., Vitvitsky, V., Martinov, M. & Banerjee, R. Organization of the human mitochondrial hydrogen sulfide oxidation pathway. J. Biol. Chem. 289, 30901–30910 (2014).
Massari, S., Bosel, A. & Wrigglesworth, J. M. The variation of Km for oxygen of cytochrome oxidase with turnover under de-energized and energized conditions. Biochem. Soc. Trans. 24, 464S (1996).
Ast, T. & Mootha, V. K. Oxygen and mammalian cell culture: are we repeating the experiment of Dr. Ox? Nat. Metab. 1, 858–860 (2019).
Petersen, L. C. The effect of inhibitors on the oxygen kinetics of cytochrome c oxidase. Biochim. Biophys. Acta 460, 299–307 (1977).
Intlekofer, A. M. et al. Hypoxia induces production of l-2-hydroxyglutarate. Cell Metab. 22, 304–311 (2015).
Oldham, W. M., Clish, C. B., Yang, Y. & Loscalzo, J. Hypoxia-mediated increases in l-2-hydroxyglutarate coordinate the metabolic response to reductive stress. Cell Metab. 22, 291–303 (2015).
Berger, R. S. et al. Lactonization of the oncometabolite d-2-hydroxyglutarate produces a novel endogenous metabolite. Cancers 13, 1756 (2021).
Pan, X. et al. A genetically encoded tool to increase cellular NADH/NAD+ ratio in living cells. Nat. Chem. Biol. https://doi.org/10.1038/s41589-023-01460-w (2023).
Ji, M. et al. Methionine restriction-induced sulfur deficiency impairs antitumour immunity partially through gut microbiota. Nat. Metab. 5, 1526–1543 (2023).
Hine, C. et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 160, 132–144 (2015).
Jia, J. et al. SQR mediates therapeutic effects of H2S by targeting mitochondrial electron transport to induce mitochondrial uncoupling. Sci. Adv. 6, eaaz5752 (2020).
Wang, Y. et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 465, 483–486 (2010).
Mosharov, E., Cranford, M. R. & Banerjee, R. The quantitatively important relationship between homocysteine metabolism and glutathione synthesis by the transsulfuration pathway and its regulation by redox changes. Biochemistry 39, 13005–13011 (2000).
Vitvitsky, V., Thomas, M., Ghorpade, A., Gendelman, H. E. & Banerjee, R. A functional transsulfuration pathway in the brain links to glutathione homeostasis. J. Biol. Chem. 281, 35785–35793 (2006).
Carpentier, G. et al. Angiogenesis analyzer for ImageJ—a comparative morphometric analysis of ‘endothelial tube formation assay’ and ‘fibrin bead assay’. Sci. Rep. 10, 11568 (2020).
Doyle, A. D. Generation of 3D collagen gels with controlled diverse architectures. Curr. Protoc. Cell Biol. 72, 10 20 1–10 20 16 (2016).
Solanki, S. et al. Dysregulated amino acid sensing drives colorectal cancer growth and metabolic reprogramming leading to chemoresistance. Gastroenterology 164, 376–391 (2023).
Kerk, S. A. et al. Metabolic requirement for GOT2 in pancreatic cancer depends on environmental context. eLife 11, e73245 (2022).
Brenes, R. A. et al. Toward a mouse model of hind limb ischemia to test therapeutic angiogenesis. J. Vasc. Surg. 56, 1669–1679 (2012).
Acknowledgements
This work was supported, in part, by grants from the National Institutes of Health (GM130183 to R.B., R01CA248160 to C.A.L. and R01CA148828 and R01CA245546 to Y.M.S.), the American Heart Association (826245 to R.K. and 19POST34380588 to S.S.), the American Physiology Society and Crohn’s and Colitis Foundation (1003279 to R.S. and 623914 to S.S.) and the National Institute of Dental and Cranofacial Research (T32DE00705745 to H.L.H.) and by T32 GM 132046 (National Institutes of Health) support to S.A. We acknowledge A. Landry and W. Huang (University of Michigan) for their technical help with generating the SQOR KD in EA.hy926 cells and with collecting mouse samples, respectively. We acknowledge S. Whitesall in the Physiology Phenotyping Core at the University of Michigan for hind limb ischemia surgery and laser doppler perfusion imaging. We thank M. Mattea at the University of Michigan Center for Gastrointestinal Research for histology studies.
Author information
Authors and Affiliations
Contributions
R.K., Y.M.S. and R.B. conceptualized the study, and R.K. performed and analyzed the majority of the experiments, with assistance from V.V. ([35S]methionine flux, glucose consumption assays and NAD+:NADH estimation), A.S. (proliferation assays), R.S. (HIF-1/HIF-2 western blots, YUMM5.2 culture, lung endothelial cell isolation FACS and real-time quantitative PCR), S.S. (tumor xenograft (experiment 2) and immunohistochemistry), S.A. (GSH, GSSG, cysteine quantitation and HIF-1 stabilization in KD cells), H.L.H. and B.M.B. (tip sprouting assay), H.N.B. (tumor xenograft (experiment 1)) and A.A. and C.A.L. (metabolomics data generation and analysis). R.K. and R.B. drafted the manuscript, and all authors edited and approved the final version.
Corresponding author
Ethics declarations
Competing interests
C.A.L. has received consulting fees from Astellas Pharmaceuticals, Odyssey Therapeutics and T-Knife Therapeutics and is an inventor on patents pertaining to Kras-regulated metabolic pathways, redox control pathways in pancreatic cancer and targeting the GOT1 pathway as a therapeutic approach (US Patent number 2015126580-A1, 5 July 2015; US Patent number 20190136238, 9 May 2019; International Patent number WO2013177426-A2, 23 April 2015). The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Christopher Hine, Peter Nagy and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8 and uncropped blots for Supplementary Fig. 3.
Supplementary Table 1
Comparison of metabolite levels in normoxia (N; 21% O2) versus hypoxia (H; 2% O2) in quadruplicates.
Supplementary Table 2
Comparison of metabolite levels in normoxia (21% O2) versus hypoxia (2% O2) in quadruplicates in scrambled and SQOR-KD EA.hy926 cells.
Supplementary Data 1
Source data for Supplementary Fig. 1.
Supplementary Data 2
Source data for Supplementary Fig. 2.
Supplementary Data 3
Source data for Supplementary Fig. 3.
Supplementary Data 4
Source data for Supplementary Fig. 4.
Supplementary Data 5
Source data for Supplementary Fig. 5.
Supplementary Data 6
Source data for Supplementary Fig. 6.
Supplementary Data 7
Source data for Supplementary Fig. 7.
Supplementary Data 8
Source data for Supplementary Fig. 8.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Uncropped western blots.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, R., Vitvitsky, V., Sethaudom, A. et al. Sulfide oxidation promotes hypoxic angiogenesis and neovascularization. Nat Chem Biol (2024). https://doi.org/10.1038/s41589-024-01583-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41589-024-01583-8