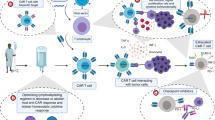
Abstract
Antibody and chimeric antigen receptor (CAR) T cell-mediated targeted therapies have improved survival in patients with solid and haematologic malignancies1,2,3,4,5,6,7,8,9. Adults with T cell leukaemias and lymphomas, collectively called T cell cancers, have short survival10,11 and lack such targeted therapies. Thus, T cell cancers particularly warrant the development of CAR T cells and antibodies to improve patient outcomes. Preclinical studies showed that targeting T cell receptor β-chain constant region 1 (TRBC1) can kill cancerous T cells while preserving sufficient healthy T cells to maintain immunity12, making TRBC1 an attractive target to treat T cell cancers. However, the first-in-human clinical trial of anti-TRBC1 CAR T cells reported a low response rate and unexplained loss of anti-TRBC1 CAR T cells13,14. Here we demonstrate that CAR T cells are lost due to killing by the patient’s normal T cells, reducing their efficacy. To circumvent this issue, we developed an antibody–drug conjugate that could kill TRBC1+ cancer cells in vitro and cure human T cell cancers in mouse models. The anti-TRBC1 antibody–drug conjugate may provide an optimal format for TRBC1 targeting and produce superior responses in patients with T cell cancers.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The anti-TRBC1 CAR amino acid sequence, sequences of reagents used for CRISPR editing of T cells and sequence of the anti-TRBC1 chimeric antibody are available in Supplementary Tables 1–3. Plasmids generated for this study can be accessed through GenBank (PP212885 and PP212886). Source data are provided with this paper.
References
June, C. H. & Sadelain, M. Chimeric antigen receptor therapy. N. Engl. J. Med. 379, 64–73 (2018).
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020).
Zahavi, D. & Weiner, L. Monoclonal antibodies in cancer therapy. Antibodies https://doi.org/10.3390/antib9030034 (2020).
Fu, Z., Li, S., Han, S., Shi, C. & Zhang, Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 7, 93 (2022).
Majzner, R. G. et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 603, 934–941 (2022).
Roschewski, M., Longo, D. L. & Wilson, W. H. CAR T-cell therapy for large B-cell lymphoma—who, when, and how? N. Engl. J. Med. 386, 692–696 (2022).
Del Bufalo, F. et al. GD2-CART01 for relapsed or refractory high-risk neuroblastoma. N. Engl. J. Med. 388, 1284–1295 (2023).
Mikkilineni, L. & Kochenderfer, J. N. CAR T cell therapies for patients with multiple myeloma. Nat. Rev. Clin. Oncol. 18, 71–84 (2021).
Esfandiari, A., Cassidy, S. & Webster, R. M. Bispecific antibodies in oncology. Nat. Rev. Drug. Discov. 21, 411–412 (2022).
Fielding, A. K. et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 109, 944–950 (2007).
Bellei, M. et al. The outcome of peripheral T-cell lymphoma patients failing first-line therapy: a report from the prospective, International T-Cell Project. Haematologica 103, 1191–1197 (2018).
Maciocia, P. M. et al. Targeting the T cell receptor β-chain constant region for immunotherapy of T cell malignancies. Nat. Med. 23, 1416–1423 (2017).
Cwynarski, K. et al. First in Human Study of AUTO4, a TRBC1-Targeting CAR T-Cell Therapy in R/R TRBC1-Positive Peripheral T Cell Lymphoma (Autolus Therapeutics, 2022); www.autolus.com/media/1zsbaddr/4634-auto4-poster.pdf.
Cwynarski, K. et al. First in human study of AUTO4, a TRBC1-targeting CAR T-cell therapy in relapsed/refractory TRBC1-positive peripheral T-cell lymphoma. Blood 140, 10316–10317 (2022).
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Went, P. et al. Marker expression in peripheral T-cell lymphoma: a proposed clinical-pathologic prognostic score. J. Clin. Oncol. 24, 2472–2479 (2006).
Kirsch, I. R. et al. TCR sequencing facilitates diagnosis and identifies mature T cells as the cell of origin in CTCL. Sci. Transl. Med. 7, 308ra158 (2015).
Asnafi, V. et al. Analysis of TCR, pTα, and RAG-1 in T-acute lymphoblastic leukemias improves understanding of early human T-lymphoid lineage commitment. Blood 101, 2693–2703 (2003).
Asnafi, V. et al. Age-related phenotypic and oncogenic differences in T-cell acute lymphoblastic leukemias may reflect thymic atrophy. Blood 104, 4173–4180 (2004).
Sims, J. E., Tunnacliffe, A., Smith, W. J. & Rabbitts, T. H. Complexity of human T-cell antigen receptor β-chain constant- and variable-region genes. Nature 312, 541–545 (1984).
Cwynarski, K. et al. First in human study of AUTO4, a TRBC1-tragetting CAR T cell therapy in relapsed/refractory TRBC1-positive peripheral T-cell lymphoma. Hematol. Oncol. 41, 80–81 (2023).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Rodriguez-Otero, P. et al. Ide-cel or standard regimens in relapsed and refractory multiple myeloma. N. Engl. J. Med. 388, 1002–1014 (2023).
Berdeja, J. G. et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 398, 314–324 (2021).
Fry, T. J. et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat. Med. 24, 20–28 (2018).
Lee, D. W. et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385, 517–528 (2015).
Kung, P., Goldstein, G., Reinherz, E. L. & Schlossman, S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science 206, 347–349 (1979).
Van Wauwe, J. P., De Mey, J. R. & Goossens, J. G. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J. Immunol. 124, 2708–2713 (1980).
Schlitt, H. J., Kurrle, R. & Wonigeit, K. T cell activation by monoclonal antibodies directed to different epitopes on the human T cell receptor/CD3 complex: evidence for two different modes of activation. Eur. J. Immunol. 19, 1649–1655 (1989).
Levine, B. L. et al. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J. Immunol. 159, 5921–5930 (1997).
Paul, S. et al. TCR β chain-directed bispecific antibodies for the treatment of T cell cancers. Sci. Transl. Med. 13, eabd3595 (2021).
Wawrzyniecka, P. A., Ibrahim, L., Gritti, G., Pule, M. A. & Maciocia, P. M. Chimeric antigen receptor T cells for gamma-delta T cell malignancies. Leukemia 36, 577–579 (2022).
Long, A. H. et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat. Med. 21, 581–590 (2015).
Gargett, T. et al. GD2-specific CAR T cells undergo potent activation and deletion following antigen encounter but can be protected from activation-induced cell death by PD-1 blockade. Mol. Ther. 24, 1135–1149 (2016).
Krangel, M. S. Endocytosis and recycling of the T3-T cell receptor complex. The role of T3 phosphorylation. J. Exp. Med. 165, 1141–1159 (1987).
Liu, H., Rhodes, M., Wiest, D. L. & Vignali, D. A. On the dynamics of TCR:CD3 complex cell surface expression and downmodulation. Immunity 13, 665–675 (2000).
Ogitani, Y. et al. DS-8201a, a novel HER2-targeting ADC with a novel DNA topoisomerase I inhibitor, demonstrates a promising antitumor efficacy with differentiation from T-DM1. Clin. Cancer Res. 22, 5097–5108 (2016).
Francisco, J. A. et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood 102, 1458–1465 (2003).
Zammarchi, F. et al. ADCT-402, a PBD dimer-containing antibody drug conjugate targeting CD19-expressing malignancies. Blood 131, 1094–1105 (2018).
Lewis Phillips, G. D. et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 68, 9280–9290 (2008).
Xiao, A. et al. WSTF regulates the H2A.X DNA damage response via a novel tyrosine kinase activity. Nature 457, 57–62 (2009).
Rogakou, E. P., Pilch, D. R., Orr, A. H., Ivanova, V. S. & Bonner, W. M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858–5868 (1998).
Hristov, A. C., Vonderheid, E. C. & Borowitz, M. J. Simplified flow cytometric assessment in mycosis fungoides and Sezary syndrome. Am. J. Clin. Pathol. 136, 944–953 (2011).
DiJoseph, J. F. et al. Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood 103, 1807–1814 (2004).
Castaigne, S. et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet 379, 1508–1516 (2012).
Caimi, P. F. et al. Loncastuximab tesirine in relapsed or refractory diffuse large B-cell lymphoma (LOTIS-2): a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 22, 790–800 (2021).
Hamadani, M. et al. Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma. Blood 137, 2634–2645 (2021).
Roth, T. L. et al. Reprogramming human T cell function and specificity with non-viral genome targeting. Nature 559, 405–409 (2018).
Nicholson, I. C. et al. Construction and characterisation of a functional CD19 specific single chain Fv fragment for immunotherapy of B lineage leukaemia and lymphoma. Mol. Immunol. 34, 1157–1165 (1997).
Paul, S. et al. T cell receptor signals to NF-κB are transmitted by a cytosolic p62-Bcl10-Malt1-IKK signalosome. Sci. Signal. 7, ra45 (2014).
Paul, S., Kashyap, A. K., Jia, W., He, Y. W. & Schaefer, B. C. Selective autophagy of the adaptor protein Bcl10 modulates T cell receptor activation of NF-κB. Immunity 36, 947–958 (2012).
Zhang, Z. M., Chen, S. & Liang, Y. Z. Baseline correction using adaptive iteratively reweighted penalized least squares. Analyst 135, 1138–1146 (2010).
Marty, M. T. et al. Bayesian deconvolution of mass and ion mobility spectra: from binary interactions to polydisperse ensembles. Anal. Chem. 87, 4370–4376 (2015).
Acknowledgements
We thank R. Ambinder, M. Popoli, J. Cohen, A. Tam, C. Blair, K. Judge, K. Helwig and L. Wang for scientific and technical support; and the patients and the biorepositories at the Johns Hopkins, Dana-Farber Cancer Institute and St Jude Children’s Research Hospital. The illustrations were generated using BioRender and ChemDraw. This work was supported by grants from The Virginia and D.K. Ludwig Fund for Cancer Research, Lustgarten Foundation for Pancreatic Cancer Research, Commonwealth Fund, Bloomberg~Kimmel Institute for Cancer Immunotherapy, Bloomberg Philanthropies, NIH Cancer Center Support Grant P30 CA006973. S.P. was supported by NCI grant K08CA270403, the Leukemia Lymphoma Society Translation Research Program award, the American Society of Hematology Scholar award and the Swim Across America Translational Cancer Research Award; B.J.M., S.R.D. and A.H.P. by NIH grant T32 GM136577; T.D.N. by NCI grant T32 CA153952; M.F.K. by NIH/NIAID grant 1R21AI176764-01, the Jerome Greene Foundation and the Cupid Foundation; and C.B. by NCI grant R37 CA230400.
Author information
Authors and Affiliations
Contributions
S.P., T.D.N., K.W.K., B.V., S.Z. and N.P. conceived the study. S.P., T.D.N., J.G., S.S., B.J.M. and M.S.H. developed the methods. T.D.N., J.G. and S.P. generated the CAR T cells, ADCs and conducted in vitro assays. T.D.N., J.G., E.W., B.S.L., K.G. and S.P. conducted in vivo studies. T.D.N., J.G., B.J.M., A.H.P., M.S.H., S.R.D., N.W., N.M., S.G., M.F.K., S.B.G., C.S., N.W.-J., S.R., L.S., E.F., D.M.P., N.P., C.B., K.G., K.W.K., S.Z., S.S., B.V. and S.P. assisted with analysis and interpretation of data. S.P., T.D.N. and B.V. wrote the original draft. All of the authors reviewed and edited the final manuscript. S.P. and B.V. supervised the study.
Corresponding author
Ethics declarations
Competing interests
The Johns Hopkins University has filed patent applications related to technologies described in this paper on which S.P., T.D.N., B.V., K.W.K., N.P. and S.Z. are listed as inventors. S.P. is a consultant to Merck, owns equity in Gilead and received payment from IQVIA and Curio Science. B.V., K.W.K. and N.P. are founders of Thrive Earlier Detection, an Exact Sciences Company. K.W.K. and N.P. are consultants to Thrive Earlier Detection. B.V., K.W.K., N.P. and S.Z. hold equity in Exact Sciences. B.V., K.W.K., N.P. and S.Z. are founders of or consultants to and own equity in ManaT Bio, Neophore and Personal Genome Diagnostics. B.V., K.W.K. and N.P. hold equity in Haystack Oncology and CAGE Pharma. N.P. is a consultant to Vidium. M.F.K. received personal fees from Argenx, Atara Biotherapeutics, Revel Pharmaceuticals, Sana Biotechnology, Sanofi and Doximity, all unrelated to this work. B.V. is a consultant to and holds equity in Catalio Capital Management. S.Z. has a research agreement with BioMed Valley Discoveries. C.B. is a consultant to Depuy-Synthes, Bionaut Labs, Haystack Oncology, Privo Technologies and Galectin Therapeutics; a co-founder of OrisDx; and a co-founder of Belay Diagnostics. D.M.P. reports grant and patent royalties through institution from BMS, a grant from Compugen, stock from Trieza Therapeutics and Dracen Pharmaceuticals and founder equity from Potenza; being a consultant for Aduro Biotech, Amgen, Astra Zeneca (Medimmune/Amplimmune), Bayer, DNAtrix, Dynavax Technologies Corporation, Ervaxx, FLX Bio, Rock Springs Capital, Janssen, Merck, Tizona and Immunomic Therapeutics; being on the scientific advisory board of Five Prime Therapeutics, Camden Nexus II, WindMil; and being on the board of directors for Dracen Pharmaceuticals. The companies named above, as well as other companies, have licensed previously described technologies related to the work described in this paper from Johns Hopkins University. B.V., K.W.K. and N.P. are listed as inventors of some of these technologies. Licences to these technologies are or will be associated with equity or royalty payments to the inventors as well as to Johns Hopkins University. Patent applications on the work described in this paper may be filed by Johns Hopkins University. The terms of all of these arrangements are being managed by Johns Hopkins University according to its conflict of interest policies.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Generation and testing of anti-TRBC1 CAR T cells.
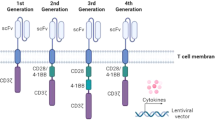
a, b, Illustration depicting the anti-TRBC1 CAR construct c, CAR T cells were stained with anti-mouse scFv-PE antibody or anti-NGFR-APC antibody followed by analysis using flow cytometry. Control T cells indicate staining in unedited T cells. d, The aggregate data of the experiment is shown in Fig. 1a. CAR T cells were incubated with cancer cells (SUP-T1 or H9 or Jurkat cells) for 48 h. The percentage of surviving cancer cells is shown in the bar graphs. Bar graphs represent mean ± standard error of mean using three technical replicates. Number of biological replicates, n = 2. e, The aggregate data of the experiment is shown in Fig. 1c. CAR T cells were incubated with normal T cells. Flow cytometry was used to assess NGFR and TRBC1 expression after 48 h. The percentage of surviving normal TRBC1+ or TRBC2+ T cells and CAR T cells are shown in the bar graphs. Bar graphs represent mean ± standard error of mean using three technical replicates. Number of biological replicates, n = 3. f, g, Anti-TRBC1 CAR T cells (2.5 × 104) were incubated with 2.5 × 104 (1:1) or 5 × 104 (1:2) or 12.5 × 104 (1:5) Jurkat cells, in the presence or absence of normal T cells. After 48 h, flow cytometry was used to assess GFP and NGFR expression. Numbers beside flow plots show the percentage of surviving cells in each condition (f) and aggregate data from three technical replicates are shown in (g). Bar graphs represent mean ± standard error of mean. Number of biological replicates, n = 3. In (d, e, g) p values obtained by one-way ANOVA with Šidák’s multiple comparison test. The diagrams in a and b were created using BioRender.
Extended Data Fig. 2 Synthesis and characterization of anti-TRBC1 ADCs.
a, Schematic of the anti-TRBC1 antibody conjugation to SG3249. b, Hydrophobic interaction chromatography of anti-TRBC1 antibody, SG3249 and anti-TRBC1-SG3249 ADC. Number of repeated experiments, n = 4. c, The deconvoluted mass spectra of the anti-TRBC1 antibody and the anti-TRBC1-SG3249 ADC. HC = heavy chain and LC = light chain. HC+1 and HC+2 indicate heavy chains conjugated with one or two molecules of SG3249. LC+1 indicates light chain conjugated with one molecule of SG3249. Number of repeated experiments, n = 2. d, Size exclusion chromatography (SEC) of anti-TRBC1-SG3249 and anti-TRBC1 antibody. SEC standards include: A, thyroglobulin (MW 670 kDa); B, gamma globulin (MW 158 kDa); C, ovalbumin (MW 44 kDa); D, myoglobin (MW 17 kDa); E, vitamin B12 (MW 1.35 kDa). e, Schematic representation of the anti-TRBC1 antibody conjugation to MC-VC-MMAE. f, Hydrophobic interaction chromatography analysis of anti-TRBC1 antibody, MC-VC-MMAE and anti-TRBC1-MMAE ADC. Number of repeated experiments, N = 2.
Extended Data Fig. 3 Anti-TRBC1-SG3249 stability and binding epitope.
a, Anti-TRBC1-SG3249 ADC was incubated in human serum at 7 µg/mL concentration for 0, 3, and 7 days at 37 °C. After incubation with human serum, anti-TRBC1-ADC (at 100 ng/mL) was added to TRBC1+ cells (Jurkat and H9) and TRBC1- cells (HPB-ALL and SUP-T1). After 5 days, cancer cell viability was assessed using luminescence. Bar graphs represent mean ± standard error of mean using three technical replicates. Number of biological replicates n = 3. b, TRBC1 and TRBC2 amino acid sequence alignment. The distinct amino acid residues are highlighted in red. The anti-TRBC1 antibody binding epitope at position 3,4 are shown inside a box. c, Jurkat, Jurkat TCR-KO, Jurkat TRBC2+, HPBALL or HPB-ALL TRBC1+ cancer cell lines were stained with anti-TRBC1-PE antibody. The histograms of the anti-TRBC1-PE stain of the indicated cell lines are shown on the left. The nucleotide and amino acid sequences of the anti-TRBC1-antibody binding epitope are shown on the right.
Extended Data Fig. 4 Anti-TRBC1-SG3249 kills TRBC1+ cells in vivo.
a, b, Flow cytometry to assess Jurkat cells (CD3 + , GFP + , top right quadrant) on day 205, from bone marrow of mice treated with anti-TRBC1-SG3249 in Fig. 5a. For positive control, 5 mice were injected with 1 × 106 Jurkat cells followed flow cytometry from bone marrow on day 21 “Jurkat injected NSG mice no ADC”. Data from 5 mice in each group shown in (b). c, TapeStation gel image of PCR product from mouse cells (black arrowhead shows amplified mouse β2 microglobulin sequence, 343 base pairs) and Jurkat cells (grey arrowhead shows amplified IVISbrite Red-F-luc-GFP sequence in Jurkat cells, 199 base pairs). Negative control “neg control” indicates sample with no input DNA, positive control “pos control” indicates sample with input DNA from mouse EMT6 cells and Red-F-luc-GFP transduced Jurkat cells. Data from 5 mice in each group shown in (c). d, Weights of 5 mice in each group as means ± S.E.M from Fig. 5a. e, Representative sections of hematoxylin and eosin-stained skin and liver from the 5 mice treated with anti-TRBC1-SG3249 ADC as in Fig. 5a. Untreated mice used as control (NSG mice, no ADC). Number of biological replicates, n = 5. f, Bioluminescence imaging of H9-injected NSG mice used in experiment Fig. 5g. g, Weights of 5 mice in each group as means ± S.E.M from Fig. 5g. h, Timeline of in vivo experiment using NSG mice injected with Jurkat cells and normal human T cells. On day 12, mice received mIgG2a-SG3249 or anti-TRBC1-SG3249 ADC. i, j, Flow cytometry on day 20 to assess Jurkat cells (CD3 + , GFP + , top right quadrant), normal human T cells (CD3 + , GFP-, top left quadrant), and aggregate data from 3 mice shown in (f). In (b) p value by two-tailed Mann-Whitney test. In (j) p values by one-way ANOVA with Šidák’s multiple comparison test. The diagram in h was created using BioRender.
Extended Data Fig. 5 Anti-TRBC1-SG3249 kills TRBC1+ patient-derived xenografts.
a, Jurkat, Jurkat TCR-KO, and T cell cancer PDX samples were stained with anti-TCRαβ-APC and anti-TRBC1-PE antibodies. b, Timeline of in vivo experiment using NSG mice injected with PDX cells. On day 25, mice were intravenously injected with either mIgG2a-SG3249 or anti-TRBC1-SG3249 ADC. c, d, Flow cytometry on day 21 and day 33 to assess circulating PDX cells (gated on the top right CD3 + , and TRBC1+ cells), and aggregate data from 4 mice are shown in (d). In (d) p value obtained by one-way ANOVA with Šidák’s multiple comparison test. The diagram in b was created using BioRender.
Extended Data Fig. 6 Chimeric anti-TRBC1-SG3249 ADC has comparable cytotoxicity.
a, Schematic representation of the mouse IgG2a anti-TRBC1 antibody and chimeric IgG1 anti-TRBC1 antibody. The mouse IgG2a heavy chain (HC) and kappa light chain (LC) constant regions were replaced with human IgG1 HC and human kappa LC constant regions. b, Hydrophobic interaction chromatography analysis of chimeric anti-TRBC1 antibody and chimeric anti-TRBC1-SG3249 ADC. Number of repeated experiments n = 2. c, H9 or Jurkat cells were incubated with indicated concentrations of anti-TRBC1-SG3249 ADC or chimeric anti-TRBC1-SG3249 ADC for 5 days. The H9 and Jurkat cells expressed luciferase and luminescence was used to assess cell viability. Data plotted as means ± standard error of mean from three technical replicates. The calculated IC50 for anti-TRBC1-SG3249 and chimeric anti-TRBC1-SG3249 ADC is shown at the right of the graphs. Number of repeated experiments n = 3. The diagram in a was created using BioRender.
Supplementary information
Supplementary Information
Supplementary Figs. 1–2 (flow cytometry gating strategies) and Supplementary Tables 1–3.
Supplementary Video 1
Live-cell imaging of anti-TRBC1–pHrodo antibody added to Jurkat cells.
Supplementary Video 2
Live-cell imaging of anti-TRBC1–pHrodo antibody added to Jurkat TCR-KO cells.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nichakawade, T.D., Ge, J., Mog, B.J. et al. TRBC1-targeting antibody–drug conjugates for the treatment of T cell cancers. Nature 628, 416–423 (2024). https://doi.org/10.1038/s41586-024-07233-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07233-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.