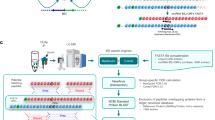
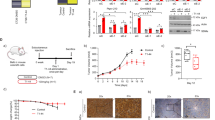
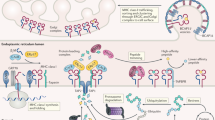
Abstract
Emerging data have shown that previously defined noncoding genomes might encode peptides that bind human leukocyte antigen (HLA) as cryptic antigens to stimulate adaptive immunity1,2. However, the significance and mechanisms of action of cryptic antigens in anti-tumour immunity remain unclear. Here mass spectrometry of the HLA class I (HLA-I) peptidome coupled with ribosome sequencing of human breast cancer samples identified HLA-I-binding cryptic antigenic peptides that were noncanonically translated by a tumour-specific circular RNA (circRNA): circFAM53B. The cryptic peptides efficiently primed naive CD4+ and CD8+ T cells in an antigen-specific manner and induced anti-tumour immunity. Clinically, the expression of circFAM53B and its encoded peptides was associated with substantial infiltration of antigen-specific CD8+ T cells and better survival in patients with breast cancer and patients with melanoma. Mechanistically, circFAM53B-encoded peptides had strong binding affinity to both HLA-I and HLA-II molecules. In vivo, administration of vaccines consisting of tumour-specific circRNA or its encoded peptides in mice bearing breast cancer tumours or melanoma induced enhanced infiltration of tumour-antigen-specific cytotoxic T cells, which led to effective tumour control. Overall, our findings reveal that noncanonical translation of circRNAs can drive efficient anti-tumour immunity, which suggests that vaccination exploiting tumour-specific circRNAs may serve as an immunotherapeutic strategy against malignant tumours.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon reasonable request. The data deposited and made public are compliant with the regulations of the Ministry of Science and Technology of the People’s Republic of China (2023BAT1050, 2023BAT0996). The data for RNA-seq, Ribo-seq and rRNA-depleted RNA-seq of clinical patient samples, RNA-seq data of human DCs and rRNA-depleted RNA-seq of mouse melanoma and breast cancer cell lines have been deposited into the GEO repository (www.ncbi.nlm.nih.gov/geo) under accession code GSE210793 and the GSA in NGDC (ngdc.cncb.ac.cn), CNCB (ngdc.cncb.ac.cn/gsa-human) (GSA-Human: HRA005200). Source data for WES have been deposited into the GSA in NGDC, CNCB (GSA-Human: HRA002820 andHRA004564,). MS raw data are available from iProX (www.iprox.cn) under accession number IPX0006186000. The human reference genome GRCh37/hg19 (genome.ucsc.edu) was used for sequencing data alignment. Original western blots and gating strategies are provided in the Supplementary Information. Source data are provided with this paper.
Change history
21 December 2023
In the version of the article initially published, there was an error in the colour key of Fig. 3d where “MUC1(12−20)” incorrectly appeared as “MUC1(112−20)”. This has now been updated in the HTML and PDF versions of the article.
References
Laumont, C. M. et al. Noncoding regions are the main source of targetable tumor-specific antigens. Sci. Transl. Med. 10, eaau5516 (2018).
Chong, C., Coukos, G. & Bassani-Sternberg, M. Identification of tumor antigens with immunopeptidomics. Nat. Biotechnol. 40, 175–188 (2022).
O’Donnell, J. S., Teng, M. W. L. & Smyth, M. J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 16, 151–167 (2019).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Galon, J. & Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 18, 197–218 (2019).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Shi, R. et al. Screening and identification of HLA-A2-restricted neoepitopes for immunotherapy of non-microsatellite instability-high colorectal cancer. Sci. China Life Sci. 65, 572–587 (2022).
Jhunjhunwala, S., Hammer, C. & Delamarre, L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 21, 298–312 (2021).
Jiang, T. et al. Tumor neoantigens: from basic research to clinical applications. J. Hematol. Oncol. 12, 93 (2019).
McGrail, D. J. et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 32, 661–672 (2021).
Cristescu, R. et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 362, eaar3593 (2018).
Abelin, J. G. et al. Mass spectrometry profiling of HLA-associated peptidomes in mono-allelic cells enables more accurate epitope prediction. Immunity 46, 315–326 (2017).
Bassani-Sternberg, M. et al. Direct identification of clinically relevant neoepitopes presented on native human melanoma tissue by mass spectrometry. Nat. Commun. 7, 13404 (2016).
Nesvizhskii, A. I. Proteogenomics: concepts, applications and computational strategies. Nat. Methods 11, 1114–1125 (2014).
Starck, S. R. & Shastri, N. Nowhere to hide: unconventional translation yields cryptic peptides for immune surveillance. Immunol. Rev. 272, 8–16 (2016).
Laumont, C. M. et al. Global proteogenomic analysis of human MHC class I-associated peptides derived from non-canonical reading frames. Nat. Commun. 7, 10238 (2016).
Thermes, V. et al. Medaka simplet (FAM53B) belongs to a family of novel vertebrate genes controlling cell proliferation. Development 133, 1881–1890 (2006).
Pei, J., Dou, H. & Deng, X. CircFAM53B promotes the proliferation and metastasis of glioma through activating the c-MET/PI3K/AKT pathway via sponging miR-532-3p. Cell Cycle 21, 462–476 (2022).
Pan, H. et al. Enhanced expression of circ_0000267 in hepatocellular carcinoma indicates poor prognosis and facilitates cell progression by sponging miR-646. J. Cell. Biochem. 120, 11350–11357 (2019).
Sun, D., Liu, J. & Zhou, L. Upregulation of circular RNA circ-FAM53B predicts adverse prognosis and accelerates the progression of ovarian cancer via the miR-646/VAMP2 and miR-647/MDM2 signaling pathways. Oncol. Rep. 42, 2728–2737 (2019).
Zhang, C. et al. The circ_FAM53B–miR-183-5p–CCDC6 axis modulates the malignant behaviors of papillary thyroid carcinoma cells. Mol. Cell. Biochem. 477, 2627–2641 (2022).
Batool, H. et al. Prediction of putative epitope-based vaccine against all corona virus strains for the Chinese population: approach toward development of vaccine. Microbiol. Immunol. 65, 154–160 (2021).
Hei, A. L. et al. Analysis of high-resolution HLA-A, -B, -Cw, -DRB1, and -DQB1 alleles and haplotypes in 718 Chinese marrow donors based on donor-recipient confirmatory typings. Int. J. Immunogenet. 36, 275–282 (2009).
Tailor, P., Tamura, T. & Ozato, K. IRF family proteins and type I interferon induction in dendritic cells. Cell Res. 16, 134–140 (2006).
Huang, D. et al. NKILA lncRNA promotes tumor immune evasion by sensitizing T cells to activation-induced cell death. Nat. Immunol. 19, 1112–1125 (2018).
Huang, D. et al. Targeting regulator of G protein signaling 1 in tumor-specific T cells enhances their trafficking to breast cancer. Nat. Immunol. 22, 865–879 (2021).
Liu, C. X. & Chen, L. L. Circular RNAs: characterization, cellular roles, and applications. Cell 185, 2016–2034 (2022).
Lei, M., Zheng, G., Ning, Q., Zheng, J. & Dong, D. Translation and functional roles of circular RNAs in human cancer. Mol. Cancer 19, 30 (2020).
Legnini, I. et al. Circ-ZNF609 is a circular RNA that can be translated and functions in myogenesis. Mol. Cell 66, 22–37.e29 (2017).
Dersh, D., Holly, J. & Yewdell, J. W. A few good peptides: MHC class I-based cancer immunosurveillance and immunoevasion. Nat. Rev. Immunol. 21, 116–128 (2021).
Roche, P. A. & Furuta, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 15, 203–216 (2015).
Chen, X. et al. circRNADb: a comprehensive database for human circular RNAs with protein-coding annotations. Sci. Rep. 6, 34985 (2016).
Gao, T., Cen, Q. & Lei, H. A review on development of MUC1-based cancer vaccine. Biomed. Pharmacother. 132, 110888 (2020).
Sibener, L. V. et al. Isolation of a structural mechanism for uncoupling T cell receptor signaling from peptide–MHC binding. Cell 174, 672–687.e27 (2018).
Friedrichs, K., Gluba, S., Eidtmann, H. & Jonat, W. Overexpression of p53 and prognosis in breast cancer. Cancer 72, 3641–3647 (1993).
Zhang, L. et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 527, 100–104 (2015).
Nagata, Y. et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6, 117–127 (2004).
Sinn, B. V. et al. Evaluation of mucin-1 protein and mRNA expression as prognostic and predictive markers after neoadjuvant chemotherapy for breast cancer. Ann. Oncol. 24, 2316–2324 (2013).
Li, Y. et al. METTL3 acetylation impedes cancer metastasis via fine-tuning its nuclear and cytosolic functions. Nat. Commun. 13, 6350 (2022).
Ott, P. A. et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 547, 217–221 (2017).
Keskin, D. B. et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 565, 234–239 (2019).
Qu, L. et al. Circular RNA vaccines against SARS-CoV-2 and emerging variants. Cell 185, 1728–1744.e16 (2022).
Shultz, L. D. et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 154, 180–191 (1995).
Li, J., Sun, D., Pu, W., Wang, J. & Peng, Y. Circular RNAs in cancer: biogenesis, function, and clinical significance. Trends Cancer 6, 319–336 (2020).
Zhang, J., Huang, D., Saw, P. E. & Song, E. Turning cold tumors hot: from molecular mechanisms to clinical applications. Trends Immunol. 43, 523–545 (2022).
Saxena, M., van der Burg, S. H., Melief, C. J. M. & Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 21, 360–378 (2021).
Hu, Z., Ott, P. A. & Wu, C. J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 18, 168–182 (2018).
Koboldt, D. C. et al. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Aran, D. et al. Comprehensive analysis of normal adjacent to tumor transcriptomes. Nat. Commun. 8, 1077 (2017).
Zhou, F. et al. Deep sequencing of the MHC region in the Chinese population contributes to studies of complex disease. Nat. Genet. 48, 740–746 (2016).
Bray, R. A. Flow cytometry in human leukocyte antigen testing. Semin. Hematol. 38, 194–200 (2001).
Wang, Q. et al. Direct detection and quantification of neoantigens. Cancer Immunol. Res. 7, 1748–1754 (2019).
UniProt, C. UniProt: the universal protein knowledgebase in 2023. Nucleic Acids Res. 51, D523–D531 (2023).
Rusch, M. et al. Clinical cancer genomic profiling by three-platform sequencing of whole genome, whole exome and transcriptome. Nat. Commun. 9, 3962 (2018).
Kent, W. J. et al. The human genome browser at UCSC. Genome Res. 12, 996–1006 (2002).
Ingolia, N. T., Brar, G. A., Rouskin, S., McGeachy, A. M. & Weissman, J. S. The ribosome profiling strategy for monitoring translation in vivo by deep sequencing of ribosome-protected mRNA fragments. Nat. Protoc. 7, 1534–1550 (2012).
Aeschimann, F., Xiong, J., Arnold, A., Dieterich, C. & Großhans, H. Transcriptome-wide measurement of ribosomal occupancy by ribosome profiling. Methods 85, 75–89 (2015).
Rombel, I. T., Sykes, K. F., Rayner, S. & Johnston, S. A. ORF-FINDER: a vector for high-throughput gene identification. Gene 282, 33–41 (2002).
Wang, L. et al. CPAT: coding-potential assessment tool using an alignment-free logistic regression model. Nucleic Acids Res. 41, e74 (2013).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
van Heesch, S. et al. The translational landscape of the human heart. Cell 178, 242–260.e29 (2019).
Vita, R. et al. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 47, D339–D343 (2019).
Reynisson, B., Alvarez, B., Paul, S., Peters, B. & Nielsen, M. NetMHCpan-4.1 and NetMHCIIpan-4.0: improved predictions of MHC antigen presentation by concurrent motif deconvolution and integration of MS MHC eluted ligand data. Nucleic Acids Res. 48, W449–W454 (2020).
Buckley, P. R. et al. Evaluating performance of existing computational models in predicting CD8+ T cell pathogenic epitopes and cancer neoantigens. Brief. Bioinform. 23, bbac141 (2022).
Pogorelyy, M. V. et al. Resolving SARS-CoV-2 CD4+ T cell specificity via reverse epitope discovery. Cell Rep. Med. 3, 100697 (2022).
Rammensee, H., Bachmann, J., Emmerich, N. P., Bachor, O. A. & Stevanović, S. SYFPEITHI: database for MHC ligands and peptide motifs. Immunogenetics 50, 213–219 (1999).
Liu, B. et al. A cytoplasmic NF-κB interacting long noncoding RNA blocks IκB phosphorylation and suppresses breast Cancer metastasis. Cancer Cell 27, 370–381 (2015).
Zheng, F. et al. The HIF-1α antisense long non-coding RNA drives a positive feedback loop of HIF-1α mediated transactivation and glycolysis. Nat. Commun. 12, 1341 (2021).
Fan, C. et al. LncRNA LITATS1 suppresses TGF-β-induced EMT and cancer cell plasticity by potentiating TβRI degradation. EMBO J. 42, e112806 (2023).
Luo, M. L. et al. The Role of APAL/ST8SIA6-AS1 lncRNA in PLK1 activation and mitotic catastrophe of tumor cells. J. Natl Cancer Inst. 112, 356–368 (2020).
Zhao, Q. et al. Targeting mitochondria-located circRNA SCAR alleviates NASH via reducing mROS output. Cell 183, 76–93.e22 (2020).
Su, S. et al. Blocking the recruitment of naive CD4+ T cells reverses immunosuppression in breast cancer. Cell Res. 27, 461–482 (2017).
Yang, W. et al. Immunogenic neoantigens derived from gene fusions stimulate T cell responses. Nat. Med. 25, 767–775 (2019).
Anderson, A. E. et al. Differential regulation of naïve and memory CD4+ T cells by alternatively activated dendritic cells. J. Leukoc. Biol. 84, 124–133 (2008).
Märten, A. et al. Generation of activated and antigen-specific T cells with cytotoxic activity after co-culture with dendritic cells. Cancer Immunol. Immunother. 51, 25–32 (2002).
Karpf, L. et al. A multivariate modeling framework to quantify immune checkpoint context-dependent stimulation on T cells. Cell Discov 8, 1 (2022).
Nguyen, H. H. et al. Naïve CD8+ T cell derived tumor-specific cytotoxic effectors as a potential remedy for overcoming TGF-β immunosuppression in the tumor microenvironment. Sci. Rep. 6, 28208 (2016).
Lin, Y. et al. Gallic acid alleviates gouty arthritis by inhibiting NLRP3 inflammasome activation and pyroptosis through enhancing Nrf2 signaling. Front. Immunol. 11, 580593 (2020).
Rapoport, A. P. et al. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 21, 914–921 (2015).
Panoskaltsis-Mortari, A., Taylor, P. A., Riddle, M. J., Shlomchik, M. A. & Blazar, B. R. In situ identification of allospecific B cells using pentamers. Blood 111, 3904–3905 (2008).
Skinner, P. J., Daniels, M. A., Schmidt, C. S., Jameson, S. C. & Haase, A. T. Cutting edge: in situ tetramer staining of antigen-specific T cells in tissues. J. Immunol. 165, 613–617 (2000).
Remmele, W. & Schicketanz, K. H. Immunohistochemical determination of estrogen and progesterone receptor content in human breast cancer. Computer-assisted image analysis (QIC score) vs. subjective grading (IRS). Pathol. Res. Pract. 189, 862–866 (1993).
Kreiter, S. et al. Mutant MHC class II epitopes drive therapeutic immune responses to cancer. Nature 520, 692–696 (2015).
Silvestrini, R. et al. Prognostic significance of proliferative activity and ploidy in node-negative breast cancers. Ann. Oncol. 4, 213–219 (1993).
Paik, S. et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N. Engl. J. Med. 351, 2817–2826 (2004).
Goldhirsch, A. et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann. Oncol. 20, 1319–1329 (2009).
Eiermann, W. et al. The 21-gene recurrence score assay impacts adjuvant therapy recommendations for ER-positive, node-negative and node-positive early breast cancer resulting in a risk-adapted change in chemotherapy use. Ann. Oncol. 24, 618–624 (2013).
Soliman, N. A. & Yussif, S. M. Ki-67 as a prognostic marker according to breast cancer molecular subtype. Cancer Biol. Med. 13, 496–504 (2016).
Liang, Q., Ma, D., Gao, R.-F. & Yu, K.-D. Effect of Ki-67 expression levels and histological grade on breast cancer early relapse in patients with different immunohistochemical-based subtypes. Sci. Rep. 10, 7648 (2020).
Engstrøm, M. J. et al. Molecular subtypes, histopathological grade and survival in a historic cohort of breast cancer patients. Breast Cancer Res. Treat. 140, 463–473 (2013).
Billgren, A. M., Tani, E., Liedberg, A., Skoog, L. & Rutqvist, L. E. Prognostic significance of tumor cell proliferation analyzed in fine needle aspirates from primary breast cancer. Breast Cancer Res. Treat. 71, 161–170 (2002).
Acknowledgements
This work was supported by grants from the Natural Science Foundation of China (82330056 (to E.S.), 92159303 (to E.S.), 81930081 (to E.S.), 82125017 (to S.S.), 92057210 (to S.S.), 32000430 (to X. Zhu) and 82222029 (to D.H.)), the National Key Research and Development Program of China (2021YFA1300502 (to S.S.)), Guangdong Science and Technology Department (2020B1212060018 (to E.S.), 2020B1212030004 (to E.S.)), the Department of Natural Resources of Guangdong Province (GDNRC[2021]51 (to E.S.)), the Bureau of Science and Technology of Guangzhou (20212200003 (to E.S.)), the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (2019BT02Y198 (to E.S.)), the Science and Technology Program of Guangzhou (202103000070 (to S.S.), 202201020479 (to S.S.)), the New Cornerstone Science Foundation through the XPLORER PRIZE (to S.S.), and the Guangdong Basic and Applied Basic Research Foundation (2022B1515020101 (to D.H.)). We thank L. Ling from the Clinical Research Design Division, Clinical Research Centre of Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University and Department of Medical Statistics, School of Public Health, Sun Yat-Sen University; C. Fan from the Department of Medical Statistics, School of Public Health, Sun Yat-sen University; Y. Zhu from the Clinical Research Design Division, Clinical Research Centre of Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University for their assistance in statistical analyses; and staff at the Disease Registry Department of Sun Yat-Sen Memorial Hospital, Sun Yat-Sen University for their assistance. Schematics in Fig. 1a and Fig. 5a were created using BioRender (www.biorender.com).
Author information
Authors and Affiliations
Contributions
D.H., X. Zhu, S.S. and E.S. conceived ideas and designed experiments. S.S. and E.S. conducted experiments. D.H. and X. Zhu carried out most of the experiments, analysed the data and prepared the figures. S.Y. and J.Z. carried out RT–qPCR, IHC and ISH detection on clinical samples and survival analysis. J.L. contributed to analyses of Ribo-seq experiments. N.Z. contributed to sample preparation for MS. X. Zeng and J.W. carried out PDX transplantation, identification and storage. B.Y. and Y.Z. contributed to bioinformatics analysis. L.L., J.C. and M.X. carried out primary cell isolation. Y.N., S.S. and E.S. provided patient samples for clinical data analysis and the PDX model. D.H., P.E.S., S.S. and E.S. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Alexandre Harari, George Calin and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 circFAM53B is a cytoplasmic circRNA.
(a) Relative quantitation of indicated circRNAs in breast tumour tissues and the paired adjacent normal breast tissues, evaluated by RT-qPCR (n = 6). *P = 0.0464. Ns, P = 0.7532 (circCAP1), 0.9165 (circCTTN). (b) Relative expression of polysome-bound circRNAs in breast tumour tissues and the paired normal breast tissues, evaluated by RT-qPCR. circCAP1: **P = 0.0079 (P4), 0.0079 (P7); circCTTN: **P = 0.0079 (P2), 0.0079 (P9). (c) Heatmap of Z-score normalized log2(count+1) expression of the selected circRNA transcripts between breast tumour (T) and adjacent normal tissues (N) (n = 6). (d) Relative quantitation of indicated circRNAs in tumour tissues and the paired adjacent normal breast tissues by RT-qPCR is shown (n = 6). *P = 0.0277. Ns, P = 0.4631. (e) Relative expression of polysome-bound circRNAs in another cohort of breast tumour tissues and the paired normal breast tissues, evaluated by RT-qPCR. circFAM53B: **P = 0.0079 (P1), 0.0079 (P5), 0.0079 (P6); circVDAC3: **P = 0.0079 (P6), 0.0079 (P11). (f) Relative quantitation of circular and linear FAM53B levels by RT-qPCR is shown. ***P = 0.0002, *P = 0.0102, **P = 0.0025. (g) Head-to-tail junction of circFAM53B was confirmed by Sanger sequencing (n = 3 independent experiments). (h) Relative abundance by RT-qPCR of circFAM53B in different cell fractions of MCF-7 cells. (i) FISH staining with junction-specific probes indicates the cellular localization of circFAM53B (green) in MCF-10A and MCF-7 cells. Scale bars, 5 µm. Representative images of n = 3 independent experiments. (j) circFAM53B expression in normal breast epithelial and breast cancer cell lines, evaluated by RT-qPCR. *P = 0.0462 (MDA-MB-231), 0.0380 (MDA-MB-468). (k) Representative images of circFAM53B expression in normal breast epithelial and breast cancer cell lines, are shown by northern blotting (n = 3 independent experiments). For gel source data, see Supplementary Fig. 2. (l) Representative images of circFAM53B expression in 6 cases of primary breast tumour tissues (T) and the paired normal adjacent tissues (N) (n = 3 independent experiments), are shown by northern blotting. For gel source data, see Supplementary Fig. 2. (m) circFAM53B expression in a variety of human tissues from circAtlas database. FPKM, Fragments Per Kilobase per Million. (n) FAM53B expression, normalized to ACTB expression, in breast tumour and adjacent normal breast tissues, evaluated by RT-qPCR (n = 18). Results are mean ± s.d. of n = 5 (b, e), n = 3 (f, h), n = 6 (j) independent experiments producing similar results. Ns, no significance. ****P < 0.0001. P values, compared with normal breast tissue (a, b, d, e, n), MCF-10A cells (j), indicated group (f), were determined by two-tailed Wilcoxon signed-rank tests (a, d, n), two-tailed Wilcoxon rank-sum tests (b, e), two-tailed one-way ANOVA with Tukey’s multiple-comparisons test (f) or with Dunnett’s multiple-comparisons test (j).
Extended Data Fig. 2 circFAM53B did not influence tumour cell activities.
MCF-7 cells were transfected with siGFP, circFAM53B siRNA-1 and siRNA-2 (sicirc-1 and sicirc-2, respectively) and MDA-MB-231 cells were transfected with empty vectors (vec) and circFAM53B overexpressing plasmid. (a, b) Relative quantitation of circular (a) and linear FAM53B levels (b) by RT-qPCR is shown. (c) CCK-8 assays were used to detect viability of MCF-7 and MDA-MB-231 cells after transfection. (d) Flow cytometric analysis of Annexin V/ PI staining was used to detect apoptosis of MCF-7 and MDA-MB-231 cells after transfection. Representative flow cytometric plots. Numbers represent the proportion of annexin V+ cells. (e, f) Migration and invasion assays of MCF-7 (e) and MDA-MB-231 cells (f). Scale bars, 100 μm. (g) The levels of epithelial-mesenchymal transition markers (E-cadherin and vimentin) in MCF-7 and MDA-MB-231 cells after transfection, as detected using western blotting (n = 3 independent experiments). For gel source data, see Supplementary Fig. 3. Results are mean ± s.d. of n = 3 (a-f) independent experiments producing similar results. ****P < 0.0001 compared with untreated cells (-) (a). P values were determined by two-tailed one-way ANOVA with Dunnett’s multiple-comparisons test (a-f).
Extended Data Fig. 3 circFAM53B-transfected DCs were capable of eliciting anti-tumour immune response in vitro.
(a) Heatmap of Z-score normalized log2(count+1) expression of the selected differential expressed genes in untransfected (-), mock-, linFAM53B- and circFAM53B-transfected DCs (n = 3 independent experiments). (b) Relative quantitation of indicated genes in DCs, as evaluated by RT-qPCR. **P = 0.0088 (IL12A), 0.0012 (CCL3); CD86: ***P = 0.0005 ((-) vs linFAM53B), 0.0006 (linFAM53B vs circFAM53B). (c) The migration of DCs towards PBS, CCL21 and CXCL7 was determined by the Transwell assay. ***P = 0.0001. (d, e) Representative histograms and quantitation of CD86, CD80, HLA-DR (d) and IL-12 expression (e) in DCs, determined by flow cytometry. HLA-DR: **P = 0.0063. CD86: **P = 0.0022. CD80: **P = 0.0098. IL-12: *P = 0.0416, **P = 0.0066, ***P = 0.0002. MFI: mean fluorescence intensity. (f) HLA-I immunoprecipitation followed by MS analysis identified circFAM53B(192-200) as the HLA-I binding peptide from DCs transfected with circFAM53B (n = 3 independent experiments). (g) Percentages of the in vitro primed T cells stained for CD45RO and CCR7 expression, evaluated by flow cytometry. ***P = 0.0002. (h) Quantification of the spot count per 5 × 104 T cells determined by IFNγ ELISpot. (i-k) Flow cytometric analysis for indicated intracellular cytokines (i, j), as well as perforin or GZMB (k) immunostaining, in the in vitro primed T cells of HLA-A*02+ (i, k) or HLA-A*11+ (j, k) patients. Percentages of the stained CD8+ or CD4+ T cells are shown. (i) ***P = 0.0008 ((-) vs circFAM53B), 0.0005 ((-) vs tumour lysates). (j) TNF: ***P = 0.0008, **P = 0.0022. IL-2: ***P = 0.0003 ((-) vs circFAM53B), 0.0003 ((-) vs tumour lysates). (k) Perforin: ***P = 0.0002 ((-) vs circFAM53B), 0.0005 ((-) vs tumour lysates). (l) The autologous breast cancer cells were transduced with polybrene only (mock), empty vector (shvec), shGFP, circFAM53B shRNA-1 and shRNA−2 (shcirc-1 and shcirc-2, respectively) and then co-cultured with in vitro primed CTLs. Percentages of CTLs stained for intracellular IFNγ, perforin and GZMB are shown, analysed by flow cytometry. (m) Tumour cell death induced by the in vitro primed T cells of HLA-A*11+ patients was examined by PI uptake through flow cytometry. Percentages of the dead tumour cells are shown. (n, o) The CTLs primed by circFAM53B-transfected DCs were rechallenged by circFAM53B WT, circFAM53B KD and “rescued” breast tumour cells MCF-7. The “rescued” breast tumour cells were established by transfecting circFAM53B KD cells with empty vector (vec), full length of circFAM53B (circFAM53Bfl)and truncated circFAM53BΔ1-117 RNAs, respectively. (n) Percentages of IFNγ stained CTLs are shown, evaluated by flow cytometry. ***P = 0.0003 (mock vs circFAM53Bfl), 0.0005 (circFAM53Bfl vs circFAM53BΔ1-117). (o) Percentages of the PI+ dead tumour cells induced by in vitro primed CTLs are shown. ***P = 0.0001 (circFAM53B WT vs mock), 0.0002 (mock vs circFAM53Bfl), 0.0006 (circFAM53Bfl vs circFAM53BΔ1-117). Results are mean ± s.d. of n = 3 (b-e, g-o) independent experiments producing similar results. ****P < 0.0001. P values, compared with DCs with indicated treatment (b-e), unprimed T cells (-) (g-k, m), mock-transduced tumour cells (mock) (l), tumour cells with indicated transfection (n, o), were determined by two-tailed one-way ANOVA with Dunnett’s multiple-comparisons test (g-m) and Tukey’s multiple-comparisons test (b-e, n, o).
Extended Data Fig. 4 circFAM53B-transfected DCs were capable of eliciting anti-tumour immune response in breast cancer PDXs.
(a) Tumour volumes were monitored weekly following the infusion of DCs and T cells (SYMH178: n = 4 per group; SYMH187: n = 4 per group). (b, c) Representative immunofluorescent images and quantitation of CD8 and GZMB co-staining (b), as well as TUNEL and CK (c) co-staining in collected PDXs. Scale bars, 50 μm. ND, not detected. (b) ***P = 0.0005 (SYMH169), 0.0006 (SYMH178), 0.0002 (SYMH187). (c) ***P = 0.0005 (SYMH178), 0.0002 (SYMH187). Results are mean ± s.d. of n = 3 mice per group per PDX case (b, c) producing similar results. ****P < 0.0001. P values, compared with mice without cell transfusion (no transfusion) (a, c), mice transfused with mock-transfected DCs and T cells (b), were determined by two-tailed one-way ANOVA with Dunnett’s multiple-comparisons test (a-c).
Extended Data Fig. 5 circFAM53B encodes a unique peptide.
(a) The putative IRES activity of circFAM53B, determined by relative luciferase activity of Luc/ Rluc, in the vectors was tested. **P = 0.0016. (b) Immunoblotting for Flag expression in HEK293T cells transfected with P-circ vector carrying an expression cassette for circFAM53B with a 3×Flag-coding sequence. For gel source data, see Supplementary Fig. 4. (c) Immunoblotting for circFAM53B-219 in HEK293T cells transfected with empty vector (vec), linFAM53B or circFAM53B. For gel source data, see Supplementary Fig. 4. (d) MS analysis identified circFAM53B-219 unique sequences in HEK293T cells transfected with circFAM53B. (e) HLA-I immunoprecipitation followed by MS analysis identified circFAM53B(192-200) as the HLA binding peptide in HEK293T cells transfected with circFAM53B. (f) Immunoblotting for circFAM53B-219 in 34 cases of primary breast tumour tissues (T) and the paired normal breast tissues (N). For gel source data, see Supplementary Fig. 5. (g) Immunoblotting for circFAM53B-219 in normal breast epithelial cells and several breast cancer cell lines. For gel source data, see Supplementary Fig. 4. (h-m) DCs from HLA-A*02+ breast cancer patients (h-j) or healthy donors (k-m) were pulsed with linFAM53B(264-302), circFAM53B(181-219) and then co-cultured with autologous T cells. The in vitro primed T cells were rechallenged by autologous breast cancer cells (h-j) or circFAM53B(192-200)-pulsed T2 cells, MCF-7 and MDA-MB-231 cells, respectively (k-m). (h, k) Flow cytometric analysis for the markers of effector T cells (CD45RO+CCR7−) in the primed T cells and the percentages of the stained T cells are shown. (i, l) Quantification of the spot count per 5 × 104 T cells determined by IFNγ ELISpot is shown. (l) ***P = 0.0003. (j, m) Percentages of the PI+ dead target cells, determined by flow cytometry, are shown. (j) ***P = 0.0002 (day 6), 0.0002 (day 8), 0.0001 (day 21). (m) ***P = 0.0009 (MCF-7), 0.0006 (MDA-MB-231). Results are mean ± s.d. of n = 3 (a, h-m) independent experiments producing similar results. Representative image of n = 3 independent experiments (b-g). ****P < 0.0001. P values, compared with HEK293T cells transfected with empty vector (vec) (a), untreated T cells (UT) (h-m), were determined by two-tailed one-way ANOVA with Dunnett’s multiple-comparisons test.
Extended Data Fig. 6 circFAM53B-encoded peptide elicits anti-tumour immunity via binding to HLA.
(a) The binding predictions for circFAM53B-encoded peptides to HLA-A*02:01 or HLA-A*11:01 using the IEDB algorithm (Rank, Score1) and SYFPEITHI (Score2). The circFAM53B-encoded unique amino acid sequences are shown in red. (b) Quantification of the spot count per 5 × 104 T cells, determined by IFNγ ELISpot. (c) Percentages of IFNγ-stained cells in the in vitro primed CD8+ T cells are shown, evaluated by flow cytometry. **P = 0.0014 (circFAM53B(181-219)), 0.0011 (MUC1(12-20)). (d) Representative flow cytometric plots of tumour death induced by the in vitro primed CTLs and the quantitation of PI+ tumour cells are shown. ***P = 0.0003 (circFAM53B(181-219)), 0.0003 (circFAM53B(192-200)), 0.0004 (MUC1(12-20)). (e) Percentages of CD8+ T cells stained for intracellular perforin or GZMB are shown, evaluated by flow cytometry. (f-i) The CTLs primed by circFAM53B(181-219)-pulsed DCs were rechallenged by circFAM53B WT, circFAM53B KD and “rescued” breast tumour cells MCF-7. The “rescued” breast tumour cells were established by transfecting circFAM53B KD cells with liposome only (mock), empty vector (vec), full length of circFAM53B (circFAM53Bfl), truncated circFAM53BΔ1-117, circFAM53BΔ60-117 RNAs, and mutated circFAM53B RNAs, respectively. (f) Scheme for circFAM53B mutation and truncation. (g) Immunoblotting for Flag and circFAM53B-219 expression in indicated cells (n = 3 independent experiments). For gel source data, see Supplementary Fig. 6. (h) Percentages of T cells stained for intracellular IFNγ, perforin and GZMB are shown, evaluated by flow cytometry. *P = 0.0141, **P = 0.0056. (i) Percentages of the PI+ dead tumour cells induced by in vitro primed CTLs are shown, evaluated by flow cytometry. ***P = 0.0003 (mock), 0.0004 (vec), 0.0004 (circFAM53BΔ1-117). (j) Quantification of the spot count per 5 × 104 T cells determined by IFNγ ELISpot. (k) Percentages of T cells stained for the indicated intracellular cytokines, evaluated by flow cytometry. (l) Percentages of the PI+ dead tumour cells induced by in vitro primed CTLs are shown, evaluated by flow cytometry. (m) MHC peptide binding predictions for circFAM53B-encoded peptides to HLA-DRB1*01:01 using the IEDB algorithm (Rank) and SYFPEITHI (Score2). The circFAM53B-encoded unique amino acid sequences are shown in red. (n) Percentages of T cells stained for the indicated intracellular cytokines, evaluated by flow cytometry. IFNγ: *P = 0.0116 (circFAM53B(191-204)), 0.0105 (circFAM53B(192-205)). TNF: ***P = 0.0002 (circFAM53B(191-204)), 0.0008 (circFAM53B(192-205)). IL-2: *P = 0.0416 (circFAM53B(192-205)). (o) Representative flow cytometric plots and quantitation of circFAM53B(192-200)-pentamer staining in the in vitro primed T cells. **P = 0.0014. (p) Clonotyping comparison between circFAM53B(192-200)-pentamer+ versus circFAM53B(192-200)-pentamer− CTLs. The non-overlapping TCR repertoires are shown. Results are mean ± s.d. of n = 3 (b-e, h-l, n, o) independent experiments producing similar results. ****P < 0.0001. P values, compared with T cells primed by unloaded DCs (0 µg/ml (b, c) or (-) (d, o)), untreated T cells (UT) (e, j-l, n), CTLs rechallenged by circFAM53B WT tumour cells (h), circFAM53B WT tumour cells (i), were determined by two-tailed one-way ANOVA with Dunnett’s multiple-comparisons test.
Extended Data Fig. 7 circFAM53B-encoded peptides elicit anti-tumour immune response in breast cancer PDXs.
Breast cancer PDXs were implanted in immunocompromised NOD/SCID mice, followed by autologous DC and T cell infusion. The infused DCs were pre-pulsed with linFAM53B(264-302) and circFAM53B(181-219) peptides, respectively. (a) Tumour volume was monitored weekly following cell infusion for five consecutive weeks (n = 6 mice per group per PDX case). (b) Representative images and quantitation of PDX growth monitored by PET-CT (mean ± s.d., n = 5 mice per group). %ID/g, the percentage of injected dose per gram of tissue. SUV-bw, Standardized Uptake Value-body weight. (c) Representative immunofluorescent images and quantitation of CD8 and circFAM53B(192-200)-pentamer co-staining in collected PDXs (mean ± s.d., n = 3 mice per group per PDX case). Arrows denote pentamer+ CD8+ T cells. Scale bars, 20 μm. ND, not detected. *P = 0.0119 (SYMH158), 0.0280 (SYMH168). **P = 0.0013 (SYMH151). (d-f) PDX-infiltrating T cells were immunostained with CD4, CD8 and circFAM53B(192-200)-pentamer (d) and intracellular IFNγ (e, f), GZMB and perforin (f). Representative flow cytometric plots (d) and quantitation of the gated CD4+ or CD8+ T cells immunostained with circFAM53B(192-200)-pentamer (d), IFNγ (e, f), GZMB and perforin (f) (mean ± s.d., n = 4 (d, e) or n = 3 (f) mice per group per PDX case). (f) IFNγ+CD8+ %: ***P = 0.0002 (SYMH158); IFNγ+CD4+ %: **P = 0.0026 (SYMH151), 0.0030 (SYMH168), ***P = 0.0002 (SYMH158). GZMB: ***P = 0.0001 (SYMH158), 0.0008 (SYMH168); Perforin: ***P = 0.0009 (SYMH158); ****P < 0.0001. P values, compared with PDX mice without cell infusion (no cell infusion) (a, b), PDX mice infused with unpulsed DCs and T cells (c-f), were determined by two-tailed one-way ANOVA with Dunnett’s multiple-comparisons test.
Extended Data Fig. 8 circFAM53B correlates with better survival and anti-tumour immunity in breast cancer and melanoma patients.
(a) The correlation between circFAM53B-219 expression, determined by IHC, and tumour-infiltrating circFAM53B(192-200)-pentamer+ CTLs in human breast cancer patients (n = 212 patients) (Spearman’s correlation coefficient r and two-tailed P value). (b) Representative flow cytometric plots and quantitation of circFAM53B(192-200)-pentamer+ CTLs in the peripheral blood of breast cancer patients with circFAM53Bhigh and circFAM53Blow expression (mean ± s.d., healthy donor: n = 15, circFAM53Blow: n = 20, circFAM53Bhigh: n = 20). ****P < 0.0001 by two-tailed one-way ANOVA with Tukey’s multiple-comparisons test. (c) Kaplan-Meier survival curves for overall survival in breast cancer patients with high (> 0.00219, n = 469) or low (≤ 0.00219, n = 469) circFAM53B expression in the tumours. Log rank P, hazard ratio (HR) and 95% confidence interval (95% CI) are shown. (d) Representative ISH and IHC images for the RNA and protein levels of circFAM53B, as well as representative immunofluorescence images of circFAM53B(192-200)-pentamer+ CTLs in the paraffin-embedded tissues of melanoma (n = 56). Scale bars, 50 µm. Correlation between circFAM53B, circFAM53B-219 expression and tumour-infiltrating circFAM53B(192-200)-pentamer+ CTLs of melanoma are shown (n = 56) (Spearman’s correlation coefficient r and two-tailed P value). (e, f) Kaplan-Meier survival curves for disease-free survival in melanoma patients with high (SI > 3, n = 28) or low (SI ≤ 3, n = 28) circFAM53B expression (e) or patients with high (IRS > 4, n = 29) or low (IRS ≤ 4, n = 27) circFAM53B-219 expression in the tumours. Log rank P, HR and 95% CI are shown.
Extended Data Fig. 9 circFam53b RNA elicits anti-tumour immune response against mouse melanoma via encoding cryptic antigenic peptides.
(a) Heatmap of Z-score normalized log2(count+1) expression of the selected differentially expressed circRNAs between normal melanocyte cell line Melan-a and melanoma cell line B16F10 (n = 3). (b) Flowcharts indicating key steps involved in TSA discovery for details. Numbers in the charts indicate the number of circRNAs upregulated in B16F10 cells. (c) The expression of indicated circRNAs, normalized to Actb expression, in Melan-a and B16F10 cells, as evaluated by RT-qPCR. **P = 0.0079 (circAsh1l, circCbfb, circSlco3a1, circFam53b). (d) Relative quantitation of circFam53b and linFam53b levels evaluated by RT-qPCR is shown. *P = 0.0125, **P = 0.0015. (e) Relative abundance by RT-qPCR of circFam53b in different cell fractions of B16F10 cells. (f) FISH with junction-specific probes indicates the cellular localization of circFam53b in Melan-a and B16F10 cells. Scale bars, 5 μm. (g-k) B16F10 cells were transfected with siGFP, circFam53b siRNA-1 and siRNA-2 (sicirc-1 and sicirc-2, respectively). Relative quantitation of circFam53b (g) and linFam53b levels (h) by RT-qPCR is shown. (i) CCK-8 assays were used to detect cell viability. Abs, Absorbance. (j) Percentages of annexin V+ cells are shown, evaluated by flow cytometry. (k) Migrated cell counts per field were shown, determined by Transwell migration assays. (l) The efficiency of in vitro circularization and purification of circFam53b and linFam53b were examined by RNase R digestion and denaturing PAGE gel. (m) Cytotoxic effect on B16F10 cells induced by splenic T cells from immunized mice was assessed by LDH assay. (n) The putative IRES activity in circFam53b was determined by the relative luciferase activity of Luc/ Rluc. (o, p) Immunoblotting for Flag expression (o) and circFam53b-221 (p) in HEK293T cells with indicated transfection. (q) MS identified circFam53b-encoded unique peptide sequences in HEK293T cells transfected with circFam53b (n = 3). (r) Immunoblotting for circFam53b-221 in Melan-a and B16F10 cells. (s) MHC peptide binding predictions for circFam53b-encoded peptides to H-2-Db or H-2-Kb using IEDB algorithm (Rank, Score1) and SYFPEITHI (Score2). The circFam53b-encoded unique amino acid sequences are shown in red. (t) MS analysis identified circFam53b(187-196) as the MHC-I binding peptide from B16F10 cells pulled down by H-2-Db. (u, v) C57BL/6 mice were immunized with peptides encoded by circFam53b and linFam53b in combination with adjuvant, poly(I:C). (u) Percentages of PI+ B16F10 cells induced by the splenic T cells of immunized mice are shown, evaluated by flow cytometry. (v) Cytotoxic effect on B16F10 cells was assessed by LDH assay. Representative image of n = 3 independent experiments (f, l, o, p, r, t). For gel source data of Extended Data Fig. 9l, o, p, r and t, see Supplementary Fig. 7. Results are mean ± s.d. of n = 5 (c), n = 3 (d, e, g-k, m, n, u, v) independent experiments producing similar results. ****P < 0.0001. P values, compared with Melan-a cells (c), cells with indicated treatment (d), untreated B16F10 cells (-) (g-k, m, u, v), HEK293T cells transfected with IRES-WT (n), were determined by two-tailed Wilcoxon rank-sum tests (c), two-tailed one-way ANOVA with Dunnett’s multiple-comparisons test (g-k, m, n, u, v) or with Tukey’s multiple-comparisons test (d).
Extended Data Fig. 10 circGigyf2 elicits anti-tumour immunity against mouse breast cancer via encoding cryptic antigenic peptides.
(a) Heatmap of Z-score normalized log2(count+1) expression of the selected differentially expressed circRNAs between mouse normal breast epithelial cell line EpH4-Ev and breast cancer cell line 4T1 (n = 3). (b) Flowcharts indicating key steps involved in TSA discovery for details. Numbers in the charts indicate the number of circRNAs upregulated in 4T1 cells. (c) The expression of circGigyf2, normalized to Actb expression, in EpH4-Ev and 4T1 cells, as evaluated by RT-qPCR. **P = 0.0079. (d) Relative quantitation of circGigyf2 and linGigyf2 levels by RT-qPCR is shown. **P = 0.0022, ***P = 0.0003. (e) Relative abundance by RT-qPCR of circGigyf2 in different cell fractions of 4T1 cells. (f) FISH with junction-specific probes indicates the cellular localization of circGigyf2 in EpH4-Ev and 4T1 cells. Scale bars, 5 μm. (g-k) 4T1 cells were transfected with siGFP, circGigyf2 siRNA-1 and siRNA-2 (sicirc-1 and sicirc-2, respectively). Relative quantitation of circGigyf2 (g) and linGigyf2 levels (h) by RT-qPCR is shown. ***P = 0.0003. (i) CCK-8 assays were used to detect 4T1 cell viability. Abs, Absorbance. (j) Percentages of Annexin V+ cells are shown, evaluated by flow cytometry. (k) Migrated cell counts per field are shown, determined by Transwell migration assays. (l, m) BALB/c mice were immunized with liposome-encapsulated in vitro circularized circGigyf2 or linGigyf2 RNA, respectively. (l) The splenic T cells from immunized mice were rechallenged with BMDCs transfected with linGigyf2 and circGigyf2 in vitro, respectively. Quantitation of the spot count per 5 × 105 T cells determined by IFNγ ELISpot. (m) The cytotoxic effect on 4T1 cells induced by splenic T cells was assessed by LDH assay. (n) The putative IRES activity in circGigyf2 was determined by relative luciferase activity of Luc/ Rluc. (o) Immunoblotting for Flag expression in HEK293T cells with indicated transfection. (p) MS identified circGigyf2-encoded unique peptide sequences in HEK293T cells transfected with circGigyf2. (q) Immunoblotting for circGigyf2-104 in EpH4-Ev and 4T1 cells. (r) MS analysis identified circGigyf2(95-103) as the MHC-I binding peptide from 4T1 cells pulled down by H-2-Kd. (s) MHC peptide binding predictions for circGigyf2 peptides to H-2-Dd, H-2-Kd or H-2-Ld using IEDB algorithm (Rank, Score1) and SYFPEITHI (Score2). The unique amino acid sequence encoded by circGigyf2 are shown in red. (t-w) BALB/c mice were immunized with peptides encoded by circGigyf2 and linGigyf2 in combination with adjuvant, poly(I:C). (t, u) The splenic T cells were re-challenged with BMDCs pulsed with peptides encoded by circGigyf2 and linGigyf2 in vitro. (t) Quantitation of the spot count per 5 × 105 T cells is shown, determined by IFNγ ELISpot. (u) Percentages of T cells stained for IFNγ, IL-2 and TNF are shown, evaluated by flow cytometry. (v) Percentages of PI+ 4T1 cells induced by the splenic T cells were determined by flow cytometry. (w) Cytotoxic effect on 4T1 cells was assessed by LDH assay. Representative image of n = 3 independent experiments (f, o-r). For gel source data of Extended Data Fig. 10o, q, and r, see Supplementary Fig. 8. Results are mean ± s.d. of n = 5 (c), n = 3 (d, e, g-n, t-w) independent experiments producing similar results. ****P < 0.0001. P values, compared with Eph4-Ev cells (c), cells with indicated treatment (d), untreated 4T1 cells (-) (g-k, m, w), T cells from mice injected with PBS (l, t-v), HEK293T cells transfected with empty vector (vec) (n), were determined by two-tailed Wilcoxon rank-sum tests (c), two-tailed one-way ANOVA with Tukey’s multiple-comparisons test (d) or Dunnett’s multiple-comparisons test (g-n, t-w).
Extended Data Fig. 11 Immunogenic circRNAs-encoded peptide vaccines inhibit mouse tumour growth and metastasis.
(a) C57BL/6 mice inoculated with B16F10 melanoma transduced with empty vector (shvec), GFP shRNA (shGFP), circFam53b shRNA-1 and shRNA-2 (shcirc-1 and shcirc-2, respectively) were immunized with circFam53b(181-203) peptides along with adjuvant poly(I:C). Tumour volumes were monitored every 3 days after tumour inoculation (n = 6 mice per group). (b) Representative images and quantitation of tumour growth monitored by PET-CT. **P = 0.0015. (c, d) BALB/c mice inoculated with 4T1 breast cancer were immunized with circGigyf2(82-104) peptides along with adjuvant poly(I:C). (c) Quantification of the percentage of mice with lung metastases (Met). (d) Quantification of the number of metastatic nodules per lung section is shown. (e) BALB/c mice inoculated with 4T1 breast cancer cells transduced with empty vector (shvec), GFP shRNA (shGFP), circGigyf2 shRNA-1 and shRNA-2 (shcirc-1 and shcirc-2, respectively) were immunized with circGigyf2(82-104) peptides along with adjuvant poly(I:C). Tumour volumes were monitored every 3 days after tumour inoculation (n = 6 mice per group). (f) C57BL/6 mice inoculated with B16F10 melanoma cells transduced with empty vector (circFam53b WT ) and circFam53b shRNA-1 (circFam53b KD) were immunized with circFam53b(181-203) peptides along with adjuvant poly(I:C). Quantification of the number of tumour-infiltrating NK cells (NK1.1+CD3−) and inflammatory myeloid cells (CD11b+), determined by immunofluorescent staining, is shown. (g) Flow cytometric analysis for circGigyf2(95-103)-tetramer staining of CD8+ T cells in tumours, spleens and lung metastases (lung met.). Numbers in plots denote percentages of the gated CD8+ cells with tetramer staining. (h) C57BL/6 mice inoculated with B16F10 melanoma cells transduced with empty vector (circFam53b WT) and circFam53b shRNA-1 (circFam53b KD) were immunized with circFam53b(181-203) peptides along with adjuvant poly(I:C). BALB/c mice inoculated with 4T1 breast cancer cells transduced with empty vector (circGigyf2 WT) and circGigyf2 shRNA-1 (circGigyf2 KD) were immunized with circGigyf2(82-104) peptides along with adjuvant poly(I:C). Percentages of CTLs with circFam53b(187-196)-pentamer or circGigyf2(95-103)-tetramer staining in tumours and spleens are shown. B16F10: *P = 0.0147. 4T1: spleen: ***P = 0.0003; Tumour: *P = 0.0366, ***P = 0.0002. (i-k) CD8+ T cells isolated from the tumours, spleens or lung metastases of the mice were then re-stimulated by BMDCs pulsed with circFam53b(187-196), circGigyf2(95-103) or VSV-NP. (i, j) Quantitation of the spot count per 2 × 105 T cells determined by IFNγ ELISpot is shown. (k) Percentages of IFNγ-releasing CD8+ T cells analysed by flow cytometry are shown. Results are mean ± s.d. of n = 5 (b, k), n = 6 (d), n = 4 (f, h), n = 3 (g, i, j) independent experiments producing similar results. ****P < 0.0001. P values, compared with mice bearing shvec-transduced tumour (shvec) (a, e), mice without vaccination (-) (b, d, g, i-k), indicated treatment (h), were determined by two-tailed one-way ANOVA with Dunnett’s multiple-comparisons test (a, b, d, e, g, i-k) or with Tukey’s multiple-comparisons test (f, h).
Extended Data Fig. 12 Immunogenic circRNA vaccines inhibit mouse tumour growth and metastasis.
(a-f) C57BL/6 mice with B16F10 melanoma and BALB/c mice with 4T1 breast cancer were immunized with the circRNAs and their linear versions encapsulated in liposomal delivery systems. (a) Quantification of the percentage of mice with lung metastases (Met). (b) Quantification of the number of metastatic nodules per lung are shown. ***P = 0.0001. (c, d) Flow cytometric analysis for intracellular IFNγ in the spleen, tumour and lung metastases. Percentages of the stained CD8+ or CD4+ T cells are shown. (e) Quantitation of the spot count per 2 × 105 T cells isolated from tumours and spleens and restimulated with tumour cells is shown, determined by IFNγ ELISpot. (f) Quantitation of the number of pentamer/tetramer+ CD8+ T cells in tumours per field is shown, evaluated by immunofluorescent co-staining. (g) C57BL/6 mice inoculated with B16F10 melanoma cells transduced with empty vector (circFam53b WT) and circFam53b shRNA-1 (circFam53b KD) were immunized with liposome-encapsulated circFam53b RNA. Quantification of the number of tumour-infiltrating NK cells (NK1.1+CD3−), inflammatory myeloid cells (CD11b+) and circFam53b(187-196)-specific CTLs (circFam53b(187-196)-pentamer+ CD8+), determined by immunofluorescent staining. (h) C57BL/6 mice inoculated with B16F10 melanoma transduced with shvec, shGFP, circFam53b shRNA-1 and shRNA-2 (shcirc-1 and shcirc-2, respectively) were immunized with circFam53b RNA encapsulated in liposomal delivery systems. Tumour volumes were monitored every 3 days after tumour inoculation (n = 6 mice per group). (i) BALB/c mice inoculated with 4T1 breast cancer cells transduced with shvec, shGFP, circGigyf2 shRNA-1 and shRNA-2 (shcirc-1 and shcirc-2, respectively) were immunized with circGigyf2 RNA encapsulated in liposomal delivery systems. Tumour volumes were monitored every 3 days after tumour inoculation (n = 6 mice per group). (j, k) NOD/SCID mice bearing B16F10 melanoma (j) or 4T1 breast cancer (k) were immunized with circRNAs encapsulated in liposomal delivery systems and the circRNA-encoded peptides along with adjuvant poly(I:C), respectively. Tumour volumes were monitored every 3 days after tumour inoculation. Results are mean ± s.d. of n = 6 (b, j, k), n = 3 (c-e), n = 4 (f, g) independent experiments producing similar results. ****P < 0.0001. P values, compared with mice bearing shvec-transduced tumour (shvec) (h, i), mice without vaccination ((-) (b-f) or no vaccine (j, k)), indicated treatment (g), were determined by two-tailed one-way ANOVA with Dunnett’s multiple-comparisons test (b-f, h-k) or with Tukey’s multiple-comparisons test (g).
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1 and 6–19, gating strategies for flow cytometry analysis (Supplementary Fig. 1) and uncropped source data (Supplementary Figs. 2–8).
Supplementary Table 2
The screening of canonical antigens in six cases of patients with breast cancer.
Supplementary Table 3
The screening of MS-identified cryptic peptides from the Ribo-seq data of six cases of patients with breast cancer.
Supplementary Table 4
The screening of MS-identified cryptic peptides from the rRNA-depleted RNA-seq data of six cases of patients with breast cancer.
Supplementary Table 5
The proteomics of the differentially expressed proteins in DCs.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, D., Zhu, X., Ye, S. et al. Tumour circular RNAs elicit anti-tumour immunity by encoding cryptic peptides. Nature 625, 593–602 (2024). https://doi.org/10.1038/s41586-023-06834-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06834-7
This article is cited by
-
CircPDE5A-encoded novel regulator of the PI3K/AKT pathway inhibits esophageal squamous cell carcinoma progression by promoting USP14-mediated de-ubiquitination of PIK3IP1
Journal of Experimental & Clinical Cancer Research (2024)
-
Circular RNA vaccines expose cryptic peptides
Nature Reviews Drug Discovery (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.