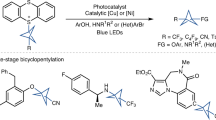
Abstract
The replacement of benzene rings with sp3-hybridized bioisosteres in drug candidates generally improves pharmacokinetic properties while retaining biological activity1,2,3,4,5. Rigid, strained frameworks such as bicyclo[1.1.1]pentane and cubane are particularly well suited as the ring strain imparts high bond strength and thus metabolic stability on their C–H bonds. Cubane is the ideal bioisostere as it provides the closest geometric match to benzene6,7. At present, however, all cubanes in drug design, like almost all benzene bioisosteres, act solely as substitutes for mono- or para-substituted benzene rings1,2,3,4,5,6,7. This is owing to the difficulty of accessing 1,3- and 1,2-disubstituted cubane precursors. The adoption of cubane in drug design has been further hindered by the poor compatibility of cross-coupling reactions with the cubane scaffold, owing to a competing metal-catalysed valence isomerization8,9,10,11. Here we report expedient routes to 1,3- and 1,2-disubstituted cubane building blocks using a convenient cyclobutadiene precursor and a photolytic C–H carboxylation reaction, respectively. Moreover, we leverage the slow oxidative addition and rapid reductive elimination of copper to develop C–N, C–C(sp3), C–C(sp2) and C–CF3 cross-coupling protocols12,13. Our research enables facile elaboration of all cubane isomers into drug candidates, thus enabling ideal bioisosteric replacement of ortho-, meta- and para-substituted benzenes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the main text or in the Supplementary information.
References
Subbaiah, M. A. M. & Meanwell, N. A. Bioisosteres of the phenyl ring: recent strategic applications in lead optimization and drug design. J. Med. Chem. 64, 14046–14128 (2021).
Mykhailiuk, P. K. Saturated bioisosteres of benzene: where to go next? Org. Biomol. Chem. 17, 2839–2849 (2019).
Stepan, A. F. et al. Application of the bicyclo[1.1.1]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active γ-secretase inhibitor. J. Med. Chem. 55, 3414–3424 (2012).
Gianatassio, R. et al. Strain-release amination. Science 351, 241–246 (2016).
Zhang, X. et al. Copper-mediated synthesis of drug-like bicyclopentanes. Nature 580, 220–226 (2020).
Eaton, P. E. Cubanes: starting materials for the chemistry of the 1990s and the new century. Angew. Chem. Int. Ed. 31, 1421–1436 (1992).
Reekie, T. A., Williams, C. M., Rendina, L. M. & Kassiou, M. Cubanes in medicinal chemistry. J. Med. Chem. 62, 1078–1095 (2019).
Cassar, L., Eaton, P. E. & Halpern, J. Silver(I)- and palladium(II)-catalyzed isomerizations of cubane. Synthesis and characterization of cuneane. J. Am. Chem. Soc. 92, 6366–6368 (1970).
Cassar, L., Eaton, P. E. & Halpern, J. Catalysis of symmetry-restricted reactions by transition metal compounds. The valence isomerization of cubane. J. Am. Chem. Soc. 92, 3515–3518 (1970).
Plunkett, S., Flanagan, K. J., Twamley, B. & Senge, M. O. Highly strained tertiary sp3 scaffolds: synthesis of functionalized cubanes and exploration of their reactivity under Pd(II) catalysis. Organometallics 34, 1408–1414 (2015).
Toriyama, F. et al. Redox-active esters in Fe-catalyzed C–C coupling. J. Am. Chem. Soc. 138, 11132–11135 (2016).
Le, C., Chen, T. Q., Liang, T., Zhang, P. & MacMillan, D. W. C. A radical approach to the copper oxidative addition problem: trifluoromethylation of bromoarenes. Science 360, 1010–1014 (2018).
Liang, Y., Zhang, X. & MacMillan, D. W. C. Decarboxylative sp3 C–N coupling via dual copper and photoredox catalysis. Nature 559, 83–88 (2018).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Feng, Y., Liu, L., Wang, J.-T., Zhao, S.-W. & Guo, Q.-X. Homolytic C–H and N–H bond dissociation energies of strained organic compounds. J. Org. Chem. 69, 3129–3138 (2004).
Levterov, V. V., Panasyuk, Y., Pivnytska, V. O. & Mykhailiuk, P. K. Water-soluble non-classical benzene mimetics. Angew. Chem. Int. Ed. 59, 7161–7167 (2020).
Denisenko, A., Garbuz, P., Shishkina, S. V., Voloshchuk, N. M. & Mykhailiuk, P. K. Saturated bioisosteres of ortho-substituted benzenes. Angew. Chem. Int. Ed. 59, 20515–20521 (2020).
Zhao, J.-X. et al. 1,2-Difunctionalized bicyclo[1.1.1]pentanes: long–sought-after mimetics for ortho/meta-substituted arenes. Proc. Natl Acad. Sci. USA 118, e2108881118 (2021).
Epplin, R. C. et al. [2]-Ladderanes as isosteres for meta-substituted aromatic rings and rigidified cyclohexanes. Nat. Commun. 13, 6056 (2022).
Iida, T. et al. Practical and facile access to bicyclo[3.1.1]heptanes: potent bioisosteres of meta-substituted benzenes. J. Am. Chem. Soc. 144, 21848–21852 (2022).
Kleinmans, R. et al. Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer. Nature 605, 477–482 (2022).
Frank, N. et al. Synthesis of meta-substituted arene bioisosteres from [3.1.1]propellane. Nature 611, 721–726 (2022).
Rigotti, T. & Bach, T. Bicyclo[2.1.1]hexanes by visible light-driven intramolecular crossed [2 + 2] photocycloadditions. Org. Lett. 24, 8821–8825 (2022).
Eaton, P. E. & Cole, T. W. Cubane. J. Am. Chem. Soc. 86, 3157–3158 (1964).
Falkiner, M. J., Littler, S. W., McRae, K. J., Savage, G. P. & Tsanaktsidis, J. Pilot-scale production of dimethyl 1,4-cubanedicarboxylate. Org. Process Res. Dev. 17, 1503–1509 (2013).
Biegasiewicz, K. F., Griffiths, J. R., Savage, G. P., Tsanaktsidis, J. & Priefer, R. Cubane: 50 years later. Chem. Rev. 115, 6719–6745 (2015).
Kassiou, M., Coster, M. & Gunosewoyo, H. Polycyclic molecular compounds. Patent WO2008064432A1 (2008).
Wlochal, J., Davies, R. D. M. & Burton, J. Cubanes in medicinal chemistry: synthesis of functionalized building blocks. Org. Lett. 16, 4094–4097 (2014).
Chalmers, B. A. et al. Validating Eaton’s hypothesis: cubane as a benzene bioisostere. Angew. Chem. Int. Ed. 55, 3580–3585 (2016).
Houston, S. D. et al. The cubane paradigm in bioactive molecule discovery: further scope, limitations and the cyclooctatetraene complement. Org. Biomol. Chem. 17, 6790–6798 (2019).
Bernhard, S. S. R. et al. Cubane cross-coupling and cubane–porphyrin arrays. Chem. Eur. J. 24, 1026–1030 (2018).
Okude, R., Mori, G., Yagi, A. & Itami, K. Programmable synthesis of multiply arylated cubanes through C–H metalation and arylation. Chem. Sci. 11, 7672–7675 (2020).
Barborak, J. C., Watts, L. & Pettit, R. A convenient synthesis of the cubane system. J. Am. Chem. Soc. 88, 1328–1329 (1966).
Brewer, C. R., Sheehan, N. C., Herrera, J., Walker, A. V. & McElwee-White, L. Photochemistry of (η4-diene)Ru(CO)3 complexes as precursor candidates for photoassisted chemical vapor deposition. Organometallics 41, 761–775 (2022).
Pettit, R. & Henery, J. Cyclobutadieneiron tricarbonyl. Org. Synth. 50, 57–59 (1970).
Masamune, S., Nakamura, N. & Sapadaro, J. 1,2-Bis(β-tosylethoxycarbonyl)diazene. Its application to the 2,3-diazabicyclo[2.2.0]hexene system. J. Am. Chem. Soc. 97, 918–919 (1975).
Britten, T. K., Akien, G. R., Kemmitt, P. D., Halcovitch, N. R. & Coote, S. C. An efficient preparation of 1,2-dihydropyridazines through a Diels–Alder/palladium-catalysed elimination sequence. Tetrahedron Lett. 60, 1498–1500 (2019).
Altman, L. J., Semmelhack, M. F., Hornby, R. B. & Vederas, J. C. Photochemical isomerisation of dimethyl 1,2-dihydropyridazine-1,2-dicarboxylate. Chem. Commun. 1968, 686–687 (1968).
Britten, T. K., Kemmitt, P. D., Halcovitch, N. R. & Coote, S. C. 4-π-Photocyclization of 1,2-dihydropyridazines: an approach to bicyclic 1,2-diazetidines with rich synthetic potential. Org. Lett. 21, 9232–9235 (2019).
Bashir-Hashemi, A. Photochemical carboxylation of cubanes. Angew. Chem. Int. Ed. 32, 612–613 (1993).
Collin, D. E., Kovacic, K., Light, M. E. & Linclau, B. Synthesis of ortho-functionalized 1,4-cubanedicarboxylate derivatives through photochemical chlorocarbonylation. Org. Lett. 23, 5164–5169 (2021).
Chan, A. Y. et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev. 122, 1485–1542 (2022).
Rodríguez, N. & Gooßen, L. J. Decarboxylative coupling reactions: a modern strategy for C–C-bond formation. Chem. Soc. Rev. 40, 5030–5048 (2011).
Ruiz-Castillo, P. & Buchwald, S. L. Applications of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 116, 12564–12649 (2016).
Hartwig, J. F. Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides. Acc. Chem. Res. 41, 1534–1544 (2008).
Zhao, W., Wurz, R. P., Peters, J. C. & Fu, G. C. Photoinduced, copper-catalyzed decarboxylative C−N coupling to generate protected amines: an alternative to the Curtius rearrangement. J. Am. Chem. Soc. 139, 12153–12156 (2017).
Sodano, T. M., Combee, L. A. & Stephenson, C. R. J. Recent advances and outlook for the isosteric replacement of anilines. ACS Med. Chem. Lett. 11, 1785–1788 (2020).
Sklyarova, A. S. et al. Preparation and testing of homocubyl amines as therapeutic NMDA receptor antagonists. Med. Chem. Res. 22, 360–366 (2013).
Sakai, H. A., Liu, W., Le, C. & MacMillan, D. W. C. Cross-electrophile coupling of unactivated alkyl chlorides. J. Am. Chem. Soc. 142, 11691–11697 (2020).
Acknowledgements
We thank Z. Dong, P. Sarver, Y. Liang, C. Oswood, W. Liu and M. Heilmann for discussions; I. Pelcer and K. Conover for assistance with NMR spectroscopy; R. Lambert for assistance with the preparation of this paper; J. Piesvaux, J. P. Imredy, R. L. Kraus and B. Lacey for help with biological profiling; and A. Beard, M. Darlak, S. McMinn, L. Nogle, M. Pietrafitta, D. Smith and Y. Ye (all Merck & Co., Inc.) for help with reverse-phase chromatography. The research was supported by the NIH National Institute of General Medical Sciences (NIGMS), the NIH (R35GM134897-03), the Princeton Catalysis Initiative, and kind gifts from Merck & Co., Inc., Bristol-Myers Squibb (BMS), Celgene, Genentech, Janssen Research and Development LLC, and Pfizer. M.P.W. was supported by the Deutsche Akademie der Naturforscher Leopoldina (LPDS 2018-16). F.B. was funded by the German Research Foundation (DFG) – 421436809, and J.D. was supported by an SNSF Early Postdoc.Mobility fellowship.
Author information
Authors and Affiliations
Contributions
M.P.W. and I.B.P. developed the route towards dimethyl cubane-1,3-dicarboxylate. O.L.G., M.P.W. and J.A.R.-A. developed the route towards 1-tert-butyl-2-methyl cubane-1,2-dicarboxylate. J.A.R.-A. and I.B.P. developed the amination reaction, J.D. and M.P.W. developed the alkylation reaction, M.P.W., F.B. and J.D. developed the arylation reaction, and J.A.R.-A. and F.B. developed the trifluoromethylation reaction. J.A.R.-A. applied the reactions to new cubane isomers and synthesized the drug analogues. Biological testing was conducted by X.M., C.S.Y. and D.J.B. D.W.C.M., S.C.C., X.M., C.S.Y. and D.J.B. provided advice. D.W.C.M., M.P.W., J.A.R.-A., I.B.P. and J.D. wrote the paper with contributions by all authors. D.W.C.M. directed the project.
Corresponding author
Ethics declarations
Competing interests
D.W.C.M. declares an ownership interest in the Penn PhD photoreactor, which is used to irradiate reactions in this work. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks Kaid Harper and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains general information, experimental procedures, characterization data, extended information, Supplementary Figs. 1–36, NMR spectra and references.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wiesenfeldt, M.P., Rossi-Ashton, J.A., Perry, I.B. et al. General access to cubanes as benzene bioisosteres. Nature 618, 513–518 (2023). https://doi.org/10.1038/s41586-023-06021-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06021-8
This article is cited by
-
Rapid reaction optimization by robust and economical quantitative benchtop 19F NMR spectroscopy
Nature Protocols (2024)
-
2-Oxabicyclo[2.2.2]octane as a new bioisostere of the phenyl ring
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.