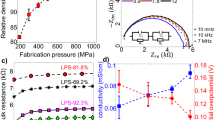
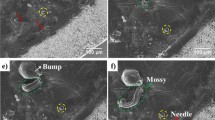
Abstract
All-solid-state batteries with a Li anode and ceramic electrolyte have the potential to deliver a step change in performance compared with today’s Li-ion batteries1,2. However, Li dendrites (filaments) form on charging at practical rates and penetrate the ceramic electrolyte, leading to short circuit and cell failure3,4. Previous models of dendrite penetration have generally focused on a single process for dendrite initiation and propagation, with Li driving the crack at its tip5,6,7,8,9. Here we show that initiation and propagation are separate processes. Initiation arises from Li deposition into subsurface pores, by means of microcracks that connect the pores to the surface. Once filled, further charging builds pressure in the pores owing to the slow extrusion of Li (viscoplastic flow) back to the surface, leading to cracking. By contrast, dendrite propagation occurs by wedge opening, with Li driving the dry crack from the rear, not the tip. Whereas initiation is determined by the local (microscopic) fracture strength at the grain boundaries, the pore size, pore population density and current density, propagation depends on the (macroscopic) fracture toughness of the ceramic, the length of the Li dendrite (filament) that partially occupies the dry crack, current density, stack pressure and the charge capacity accessed during each cycle. Lower stack pressures suppress propagation, markedly extending the number of cycles before short circuit in cells in which dendrites have initiated.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets generated and/or analysed during this study are available from the corresponding author on reasonable request.
Code availability
The computer code generated and used during this study is available from the corresponding author on reasonable request.
References
Janek, J. & Zeier, W. G. A solid future for battery development. Nat. Energy 1, 16141 (2016).
Famprikis, T., Canepa, P., Dawson, J. A., Islam, M. S. & Masquelier, C. Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 18, 1278–1291 (2019).
Ning, Z. et al. Visualizing plating-induced cracking in lithium-anode solid-electrolyte cells. Nat. Mater. 20, 1121–1129 (2021).
Kasemchainan, J. et al. Critical stripping current leads to dendrite formation on plating in lithium anode solid electrolyte cells. Nat. Mater. 18, 1105–1111 (2019).
Feldman, L. A. & De Jonghe, L. C. Initiation of mode I degradation in sodium-beta alumina electrolytes. J. Mater. Sci. 17, 517–524 (1982).
Porz, L. et al. Mechanism of lithium metal penetration through inorganic solid electrolytes. Adv. Energy Mater. 7, 1701003 (2017).
Bucci, G. & Christensen, J. Modeling of lithium electrodeposition at the lithium/ceramic electrolyte interface: the role of interfacial resistance and surface defects. J. Power Sources 441, 227186 (2019).
Klinsmann, M., Hildebrand, F. E., Ganser, M. & McMeeking, R. M. Dendritic cracking in solid electrolytes driven by lithium insertion. J. Power Sources 442, 227226 (2019).
Barroso-Luque, L., Tu, Q. & Ceder, G. An analysis of solid-state electrodeposition-induced metal plastic flow and predictions of stress states in solid ionic conductor defects. J. Electrochem. Soc. 167, 20534 (2020).
Zhou, L. et al. High areal capacity, long cycle life 4 V ceramic all-solid-state Li-ion batteries enabled by chloride solid electrolytes. Nat. Energy 7, 83–93 (2022).
Koç, T., Marchini, F., Rousse, G., Dugas, R. & Tarascon, J.-M. In search of the best solid electrolyte-layered oxide pairing for assembling practical all-solid-state batteries. ACS Appl. Energy Mater. 4, 13575–13585 (2021).
Liang, J. et al. A series of ternary metal chloride superionic conductors for high-performance all-solid-state lithium batteries. Adv. Energy Mater. 12, 2103921 (2022).
Tu, Q., Shi, T., Chakravarthy, S. & Ceder, G. Understanding metal propagation in solid electrolytes due to mixed ionic-electronic conduction. Matter 4, 3248–3268 (2021).
Kazyak, E. et al. Li penetration in ceramic solid electrolytes: operando microscopy analysis of morphology, propagation, and reversibility. Matter 2, 1025–1048 (2020).
Scharf, J. et al. Bridging nano- and microscale X-ray tomography for battery research by leveraging artificial intelligence. Nat. Nanotechnol. 17, 446–459 (2022).
De Jonghe, L. C., Feldman, L. & Beuchele, A. Slow degradation and electron conduction in sodium/beta-aluminas. J. Mater. Sci. 16, 780–786 (1981).
Han, F. et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 4, 187–196 (2019).
Masias, A., Felten, N., Garcia-Mendez, R., Wolfenstine, J. & Sakamoto, J. Elastic, plastic, and creep mechanical properties of lithium metal. J. Mater. Sci. 54, 2585–2600 (2019).
Sedlatschek, T. et al. Large-deformation plasticity and fracture behavior of pure lithium under various stress states. Acta Mater. 208, 116730 (2021).
Doltsinis, I. & Dattke, R. Modelling the damage of porous ceramics under internal pressure. Comput. Methods Appl. Mech. Eng. 191, 29–46 (2001).
Foulk, J. W. III, Johnson, G. C., Klein, P. A. & Ritchie, R. O. On the toughening of brittle materials by grain bridging: promoting intergranular fracture through grain angle, strength, and toughness. J. Mech. Phys. Solids 56, 2381–2400 (2008).
Fricker, H. S. Why does charge concentrate on points? Phys. Educ. 24 157 (1989).
Liu, G. et al. Densified Li6PS5Cl nanorods with high ionic conductivity and improved critical current density for all-solid-state lithium batteries. Nano Lett. 20, 6660–6665 (2020).
Begley, J. A. & Landes, J. D. in Proc. 1971 National Symposium on Fracture Mechanics—Part II, ASTM STP 514 1–20 (ASTM, 1972).
Huang, Z. & Li, X. Origin of flaw-tolerance in nacre. Sci. Rep. 3, 1693 (2013).
Kinzer, B. et al. Operando analysis of the molten Li|LLZO interface: understanding how the physical properties of Li affect the critical current density. Matter 4, 1947–1961 (2021).
Lewis, J. A. et al. Role of areal capacity in determining short circuiting of sulfide-based solid-state batteries. ACS Appl. Mater. Interfaces 14, 4051–4060 (2022).
Hänsel, C. & Kundu, D. The stack pressure dilemma in sulfide electrolyte based Li metal solid-state batteries: a case study with Li6PS5Cl solid electrolyte. Adv. Mater. Interfaces 8, 2100206 (2021).
Doux, J.-M. et al. Stack pressure considerations for room-temperature all-solid-state lithium metal batteries. Adv. Energy Mater. 10, 1903253 (2020).
Haslam, C. G., Wolfenstine, J. B. & Sakamoto, J. The effect of aspect ratio on the mechanical behavior of Li metal in solid-state cells. J. Power Sources 520, 230831 (2022).
Otto, S.-K. et al. In situ investigation of lithium metal–solid electrolyte anode interfaces with ToF-SIMS. Adv. Mater. Interfaces 9, 2102387 (2022).
Baranowski, L. L., Heveran, C. M., Ferguson, V. L. & Stoldt, C. R. Multi-scale mechanical behavior of the Li3PS4 solid-phase electrolyte. ACS Appl. Mater. Interfaces 8, 29573–29579 (2016).
Oliver, W. C. & Pharr, G. M. Measurement of hardness and elastic modulus by instrumented indentation: advances in understanding and refinements to methodology. J. Mater. Res. 19, 3–20 (2004).
Zhang, T., Feng, Y., Yang, R. & Jiang, P. A method to determine fracture toughness using cube-corner indentation. Scr. Mater. 62, 199–201 (2010).
Cuadrado, N., Casellas, D., Anglada, M. & Jiménez-Piqué, E. Evaluation of fracture toughness of small volumes by means of cube-corner nanoindentation. Scr. Mater. 66, 670–673 (2012).
Di Maio, D. & Roberts, S. G. Measuring fracture toughness of coatings using focused-ion-beam-machined microbeams. J. Mater. Res. 20, 299–302 (2005).
Chen, Y. et al. Measurements of elastic modulus and fracture toughness of an air plasma sprayed thermal barrier coating using micro-cantilever bending. Surf. Coat. Technol. 374, 12–20 (2019).
Acknowledgements
P.G.B. is indebted to the Faraday Institution SOLBAT (FIRG007, FIRG008, FIRG026), as well as the Engineering and Physical Sciences Research Council, Enabling Next Generation Lithium Batteries (EP/M009521/1), the University of Oxford experimental equipment upgrade (EP/M02833X/1) and the Henry Royce Institute for Advanced Materials (EP/R0066X/1, EP/S019367/1, EP/R010145/1) for financial support. We thank the Diamond Light Source for the provision of synchrotron radiation beam time (experiment no. MG23980-1) at the I13-2 beamline at the Diamond Light Source. We acknowledge technical and experimental support at the I13-2 beamline by A. J. Bodey.
Author information
Authors and Affiliations
Contributions
Z.N., G.L. and D.L.R.M. contributed to all aspects of the research. Z.N., D.L.R.M., D.S.-J., S.D.P., G.O.H. and A.J.B. carried out the operando synchrotron XCT. Z.N. and D.L.R.M. performed the preparation of electrolyte discs and cell assembly. Z.N., D.L.R.M, C.G. and X.G. performed the on-line mass spectrometry. Z.N., D.L.R.M., B.H., B.L. and J.B. performed the plasma FIB imaging. D.L.R.M. and J.B. performed plasma FIB imaging with SIMS. Z.N., D.L.R.M., J.P., J.L. and D.E.J.A. conducted the preparation of microcantilever and mechanical tests. G.L., Y.C. and C.W.M. conducted the modelling. Z.N., G.L., D.L.R.M., D.S.-J., R.I.T., P.S.G., D.E.J.A., T.J.M., C.W.M. and P.G.B. discussed the data. All authors contributed to the interpretation of data. Z.N., D.L.R.M., G.L., C.W.M. and P.G.B. wrote the manuscript, with contributions and revisions from all authors. The project was supervised by C.W.M., T.J.M. and P.G.B.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Kelsey Hatzell, Chen-Zi Zhao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains details of the dendrite initiation and propagation modelling, Supplementary Figs. 1–21 and Supplementary Tables 1–3.
Supplementary Video 1
Operando XCT imaging showing the development of a dendrite crack from initiation through propagation to short circuit.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ning, Z., Li, G., Melvin, D.L.R. et al. Dendrite initiation and propagation in lithium metal solid-state batteries. Nature 618, 287–293 (2023). https://doi.org/10.1038/s41586-023-05970-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-05970-4
This article is cited by
-
External-pressure–electrochemistry coupling in solid-state lithium metal batteries
Nature Reviews Materials (2024)
-
A Review on Engineering Design for Enhancing Interfacial Contact in Solid-State Lithium–Sulfur Batteries
Nano-Micro Letters (2024)
-
Interface design for all-solid-state lithium batteries
Nature (2023)
-
Lithium filaments wedge open cracks in solid-state batteries
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.