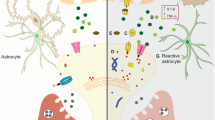
Abstract
A growing body of evidence has demonstrated a link between Alzheimer disease (AD) and epilepsy. Late-onset epilepsy and epileptiform activity can precede cognitive deterioration in AD by years, and its presence has been shown to predict a faster disease course. In animal models of AD, amyloid and tau pathology are linked to cortical network hyperexcitability that precedes the first signs of memory decline. Thus, detection of epileptiform activity in AD has substantial clinical importance as a potential novel modifiable risk factor for dementia. In this Review, we summarize the epidemiological evidence for the complex bidirectional relationship between AD and epilepsy, examine the effect of epileptiform activity and seizures on cognition in people with AD, and discuss the precision medicine treatment strategies based on the latest research in human and animal models. Finally, we outline some of the unresolved questions of the field that should be addressed by rigorous research, including whether particular clinicopathological subtypes of AD have a stronger association with epilepsy, and the sequence of events between epileptiform activity and amyloid and tau pathology.
Key points
-
Pathological neuronal hyperexcitability in individuals with Alzheimer disease is two to three times higher than in healthy individuals and is associated with accelerated cognitive deterioration.
-
Late-onset epilepsy might be a non-cognitive prodromal sign of Alzheimer disease and a novel modifiable risk factor.
-
Neuropsychological testing in late-onset epilepsy as well as long-term EEG in early Alzheimer disease is necessary to ensure timely antidementia and/or antiseizure interventions.
-
Studies in transgenic Alzheimer disease mouse models have revealed cellular and molecular mechanisms linking neuronal hyperexcitability to amyloid and tau pathology, and have demonstrated that hyperexcitability can accelerate disease progression.
-
Epilepsy in Alzheimer disease is a hard-to-treat condition. Experimental and clinical studies have found levetiracetam to be the most promising antiseizure medication among currently available compounds.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Jack, C. R. Jr et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 14, 535–562 (2018).
Jellinger, K. A. Recent update on the heterogeneity of the Alzheimer’s disease spectrum. J. Neural Transm. 129, 1–24 (2022). A detailed review on the four different histopathological subtypes of Alzheimer disease, which also discusses the main clinical characteristics, the specific dementia phenotypes and the impact of co-pathologies.
Leng, F. & Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: where do we go from here? Nat. Rev. Neurol. 17, 157–172 (2021).
Dubois, B. et al. Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 12, 292–323 (2016).
Horvath, A., Szűcs, A., Barcs, G., Noebels, J. L. & Kamondi, A. Epileptic seizures in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 30, 186–192 (2016).
Vossel, K. A., Tartaglia, M. C., Nygaard, H. B., Zeman, A. Z. & Miller, B. L. Epileptic activity in Alzheimer’s disease: causes and clinical relevance. Lancet Neurol. 16, 311–322 (2017).
Palop, J. J. & Mucke, L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 17, 777–792 (2016).
Romoli, M., Sen, A., Parnetti, L., Calabresi, P. & Costa, C. Amyloid-β, a potential link between epilepsy and cognitive decline. Nat. Rev. Neurol. 17, 469–485 (2021). This paper presents the most recent findings on the role of amyloid-β in the development of both cognitive decline and epilepsy in Alzheimer disease through shared pathomechanistic pathways.
Sen, A., Jette, N., Husain, M. & Sander, J. W. Epilepsy in older people. Lancet 395, 735–748 (2020).
Lee, S. K. Epilepsy in the elderly: treatment and consideration of comorbid diseases. J. Epilepsy Res. 9, 27–35 (2019).
Johnson, E. L. et al. Dementia in late-onset epilepsy: the Atherosclerosis Risk in Communities study. Neurology 95, e3248–e3256 (2020).
Tang, T., Zhang, R. & Pan, X. Meta-analysis of the risk of dementia in elderly patients with late-onset epilepsy. Clin. Neurol. Neurosurg. 223, 107499 (2022).
Huang, L., Fu, C., Li, J. & Peng, S. Late-onset epilepsy and the risk of dementia: a systematic review and meta-analysis. Aging Clin. Exp. Res. 34, 1771–1779 (2022).
Cvetkovska, E. et al. Prevalence of various risk factors associated with new-onset epilepsy after the age of 50: a retrospective population-based study. Epileptic Disord. 24, 95–101 (2022).
Süße, M., Zank, M., von Podewils, V. & von Podewils, F. Autoimmune encephalitis in late-onset seizures: when to suspect and how to treat. Front. Neurol. 2, 633999 (2021).
Kawakami, O. et al. Incidence of dementia in patients with adult-onset epilepsy of unknown causes. J. Neurol. Sci. 395, 71–76 (2018).
Costa, C. et al. Alzheimer’s disease and late-onset epilepsy of unknown origin: two faces of beta amyloid pathology. Neurobiol. Aging 73, 61–67 (2019).
Keret, O., Hoang, T. D., Xia, F., Rosen, H. J. & Yaffe, K. Association of late-onset unprovoked seizures of unknown etiology with the risk of developing dementia in older veterans. JAMA Neurol. 77, 710–715 (2020).
Nardi Cesarini, E. et al. Late-onset epilepsy with unknown etiology: a pilot study on neuropsychological profile, cerebrospinal fluid biomarkers, and quantitative EEG characteristics. Front. Neurol. 11, 199 (2020).
Ophir, K., Ran, B., Felix, B. & Amir, G. Ten year cumulative incidence of dementia after late onset epilepsy of unknown etiology. J. Clin. Neurosci. 86, 247–251 (2021).
Reyes, A. et al. Diagnosing cognitive disorders in older adults with epilepsy. Epilepsia 62, 460–471 (2021).
Costa, C. et al. Cognitive decline risk stratification in people with late-onset epilepsy of unknown etiology: an electroencephalographic connectivity and graph theory pilot study. J. Alzheimers Dis. 88, 893–901 (2022).
Fernandes, M. et al. Cognitive functioning, cerebrospinal fluid Alzheimer’s disease biomarkers and cerebral glucose metabolism in late-onset epilepsy of unknown aetiology: a prospective study. Eur. J. Neurosci. 56, 5384–5396 (2022).
Hansson, O. Biomarkers for neurodegenerative diseases. Nat. Med. 27, 954–963 (2021).
Podcasy, J. L. & Epperson, C. N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 18, 437–446 (2016).
Scheyer, O. et al. Female sex and Alzheimer’s risk: the menopause connection. J. Prev. Alzheimers Dis. 5, 225–230 (2018).
Zetterberg, H. & Blennow, K. Moving fluid biomarkers for Alzheimer’s disease from research tools to routine clinical diagnostics. Mol. Neurodegener. 16, 10 (2021).
Banote, R. K., Håkansson, S., Zetterberg, H. & Zelano, J. CSF biomarkers in patients with epilepsy in Alzheimer’s disease: a nation-wide study. Brain Commun. 4, fcac210 (2022).
Johnson, E. L. et al. Association between midlife risk factors and late-onset epilepsy: results from the Atherosclerosis Risk in Communities study. JAMA Neurol. 75, 1375–1382 (2018).
Stefanidou, M. et al. Bi-directional association between epilepsy and dementia: the Framingham Heart Study. Neurology 95, e3241–e3247 (2020).
Zhao, B. et al. Risk of seizures and subclinical epileptiform activity in patients with dementia: a systematic review and meta-analysis. Ageing Res. Rev. 72, 101478 (2021).
Dun, C. et al. Bi-directional associations of epilepsy with dementia and Alzheimer’s disease: a systematic review and meta-analysis of longitudinal studies. Age Ageing 51, afac010 (2022).
Beagle, A. J. et al. Relative incidence of seizures and myoclonus in Alzheimer’s disease, dementia with Lewy bodies, and frontotemporal dementia. J. Alzheimers Dis. 60, 211–223 (2017).
DiFrancesco, J. C. et al. Adult-onset epilepsy in presymptomatic Alzheimer’s disease: a retrospective study. J. Alzheimers Dis. 60, 1267–1274 (2017).
Horvath, A. et al. Prevalence, semiology, and risk factors of epilepsy in Alzheimer’s disease: an ambulatory EEG study. J. Alzheimers Dis. 63, 1045–1054 (2018).
Lyou, H. J., Seo, K. D., Lee, J. E., Pak, H. Y. & Lee, J. H. Association of Alzheimer’s disease with the risk of developing epilepsy: a 10-year nationwide cohort study. Dement. Neurocogn. Disord. 17, 156–162 (2018).
Rauramaa, T. et al. Epilepsy in neuropathologically verified Alzheimer’s disease. Seizure 58, 9–12 (2018).
Baker, J., Libretto, T., Henley, W. & Zeman, A. The prevalence and clinical features of epileptic seizures in a memory clinic population. Seizure 71, 83–92 (2019).
Tabuas-Pereira, M. et al. Increased CSF tau is associated with a higher risk of seizures in patients with Alzheimer’s disease. Epilepsy Behav. 98, 207–209 (2019).
Arnaldi, D. et al. Epilepsy in neurodegenerative dementias: a clinical, epidemiological, and EEG study. J. Alzheimers Dis. 74, 865–874 (2020).
Vöglein, J. et al. Seizures in Alzheimer’s disease are highly recurrent and associated with a poor disease course. J. Neurol. 267, 2941–2948 (2020). This large retrospective study found a 70.4% risk of seizure recurrence within 7.46 months in people with AD, a finding that highlights the importance of early introduction of antiseizure medication.
Zelano, J., Brigo, F. & Garcia-Patek, S. Increased risk of epilepsy in patients registered in the Swedish Dementia Registry. Eur. J. Neurol. 27, 129–135 (2020).
Blank, L. J., Acton, E. K., Thibault, D. & Willis, A. W. Neurodegenerative disease is associated with increased incidence of epilepsy: a population based study of older adults. Age Ageing 50, 205–212 (2021).
Wang, X. et al. Predictors of new-onset epilepsy in people with younger-onset neurocognitive disorders. Front. Aging Neurosci. 13, 637260 (2021).
Vöglein, J. et al. Seizure prevalence in neurodegenerative diseases – a study of autopsy proven cases. Eur. J. Neurol. 29, 12–18 (2022).
Cretin, B. et al. CSF in epileptic prodromal Alzheimer’s disease: no diagnostic contribution but a pathophysiological one. Front. Neurol. 12, 623777 (2021).
García-Cabrero, A. M. et al. Hyperexcitability and epileptic seizures in a model of frontotemporal dementia. Neurobiol. Dis. 58, 200–208 (2013).
Gomez-Murcia, V. et al. Hyperexcitability and seizures in the THY-Tau22 mouse model of tauopathy. Neurobiol. Aging 94, 265–270 (2020).
Przybyla, M. et al. Onset of hippocampal network aberration and memory deficits in P301S tau mice are associated with an early gene signature. Brain 143, 1889–1904 (2020).
Volicer, L., Smith, S. & Volicer, B. J. Effect of seizures on progression of dementia of the Alzheimer type. Dementia 6, 258–263 (1995).
Baker, J., Libretto, T., Henley, W. & Zeman, A. A longitudinal study of epileptic seizures in Alzheimer’s disease. Front. Neurol. 10, 1266 (2019).
Fisher, R. S. et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 55, 475–482 (2014).
Horvath, A., Szucs, A., Barcs, G. & Kamondi, A. Sleep EEG detects epileptiform activity in Alzheimer’s disease with high sensitivity. J. Alzheimers Dis. 56, 1175–1183 (2017). This study shows that in people with AD, 1-h sleep EEG is enough to detect epileptiform activity with high sensitivity owing to the fact that over 80% of epileptiform discharges occur during non-REM sleep.
Vossel, K. A. et al. Incidence and impact of subclinical epileptiform activity in Alzheimer’s disease. Ann. Neurol. 80, 858–870 (2016). This paper describes significantly faster cognitive decline in people with AD who had SEA on their EEG than in those without epileptiform activity.
Horvath, A. A. et al. Subclinical epileptiform activity accelerates the progression of Alzheimer’s disease: a long-term EEG study. Clin. Neurophysiol. 132, 1982–1989 (2021). This study shows that a higher number of subclinical epileptiform discharges on the EEG positively correlates with more severe cognitive decline in people with AD.
Musaeus, C. S. et al. Detection of subclinical epileptiform discharges in Alzheimer’s disease using long-term outpatient EEG monitoring. Neurobiol. Dis. 183, 106149 (2023).
Chatrian, G. E. et al. A glossary of terms most commonly used by clinical electroencephalographers. Electroencephalogr. Clin. Neurophysiol. 37, 538–548 (1974).
Noachtar, S. et al. A glossary of terms most commonly used by clinical electroencephalographers and proposal for the report form for the EEG findings. The International Federation of Clinical Neurophysiology. Electroencephalogr. Clin. Neurophysiol. 52, 21–41 (1999).
Brunetti, V. et al. Subclinical epileptiform activity during sleep in Alzheimer’s disease and mild cognitive impairment. Clin. Neurophysiol. 131, 1011–1018 (2020).
Kane, N. et al. A revised glossary of terms most commonly used by clinical electroencephalographers and updated proposal for the report format of the EEG findings. Revision 2017. Clin. Neurophysiol. Pract. 2, 170–185 (2017).
Minkeviciene, R. et al. Amyloid β-induced neuronal hyperexcitability triggers progressive epilepsy. J. Neurosci. 29, 3453–3462 (2009). This is a study on the prevalence of epileptic seizures in APP transgenic mice, which also demonstrates that amyloid-β protofibrils directly elicit hyperexcitability of excitatory neurons.
Hall, A. M. et al. Tau-dependent Kv4.2 depletion and dendritic hyperexcitability in a mouse model of Alzheimer’s disease. J. Neurosci. 35, 6221–6230 (2015).
Lam, A. D. et al. Association of epileptiform abnormalities and seizures in Alzheimer disease. Neurology 95, e2259–e2270 (2020). This study shows that frequent small sharp spikes, currently considered benign EEG graphoelements, are strongly related with pathological cortical hyperexcitability in people with AD.
Yeh, W. C. et al. Association between subclinical epileptiform discharge and the severity of cognitive decline in Alzheimer’s disease: a longitudinal cohort study. J. Alzheimers Dis. 90, 305–312 (2022).
Vossel, K. A. et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 70, 1158–1166 (2013).
Issa, N. P. et al. Small sharp spikes as EEG markers of mesiotemporal lobe epilepsy. Clin. Neurophysiol. 129, 1796–1803 (2018).
Abou Jaoude, M. et al. Noninvasive detection of hippocampal epileptiform activity on scalp electroencephalogram. JAMA Neurol. 79, 614–622 (2022).
Stam, C. J., van Nifterick, A. M., de Haan, W. & Gouw, A. A. Network hyperexcitability in early Alzheimer’s disease: is functional connectivity a potential biomarker? Brain Topogr. 36, 595–612 (2023).
Ranasinghe, K. G. et al. Neuronal synchrony abnormalities associated with subclinical epileptiform activity in early-onset Alzheimer’s disease. Brain 145, 744–753 (2022).
Horvath, A. A., Csernus, E. A., Lality, S., Kaminski, R. M. & Kamondi, A. Inhibiting epileptiform activity in cognitive disorders: possibilities for a novel therapeutic approach. Front. Neurosci. 14, 557416 (2020).
Murray, M. E. et al. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet Neurol. 10, 785–796 (2011).
Uretsky, M. et al. Longitudinal cognitive performance of Alzheimer’s disease neuropathological subtypes. Alzheimers Dement. 7, e12201 (2021).
Horvath, A., Kiss, M., Szucs, A. & Kamondi, A. Precuneus-dominant degeneration of parietal lobe is at risk of epilepsy in mild Alzheimer’s disease. Front. Neurol. 10, 878 (2019).
Cretin, B. et al. Epileptic prodromal Alzheimer’s disease, a retrospective study of 13 new cases: expanding the spectrum of Alzheimer’s disease to an epileptic variant? J. Alzheimers Dis. 52, 1125–1133 (2016). This paper describes the clinical characteristics of the epileptic variant of AD.
Palop, J. J. et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 55, 697–711 (2007). This paper reveals the presence of seizures and neuronal hyperactivity in amyloid plaque-forming transgenic mice, and raised the interest of the AD research field to study the link between AD and epilepsy.
Chan, J., Jones, N. C., Bush, A. I., O’Brien, T. J. & Kwan, P. A mouse model of Alzheimer’s disease displays increased susceptibility to kindling and seizure-associated death. Epilepsia 56, e73–e77 (2015).
Liu, S. et al. Accelerated kindling epileptogenesis in Tg4510 tau transgenic mice, but not in tau knockout mice. Epilepsia 58, e136–e141 (2017).
Gureviciene, I. et al. Characterization of epileptic spiking associated with brain amyloidosis in APP/PS1 mice. Front. Neurol. 10, 1151 (2019).
Jin, N., Ziyatdinova, S., Gurevicience, I. & Tanila, H. Response of spike-wave discharges in aged APP/PS1 Alzheimer model mice to antiepileptic, metabolic and cholinergic drugs. Sci. Rep. 10, 11851 (2020).
Kam, K., Duffy, Á. M., Moretto, J., LaFrancois, J. J. & Scharfman, H. E. Interictal spikes during sleep are an early defect in the Tg2576 mouse model of β-amyloid neuropathology. Sci. Rep. 6, 20119 (2016).
Lam, A. D. & Noebels, J. Night watch on the titanic: detecting early signs of epileptogenesis in Alzheimer disease. Epilepsy Curr. 20, 369–374 (2020).
Szabo, A. et al. Neuronal hyperexcitability in the Tg2576 mouse model of Alzheimer’s disease – the influence of sleep and noradrenergic transmission. Neurobiol. Aging 123, 35–48 (2023).
Lisgaras, C. P. & Scharfman, H. E. High-frequency oscillations (250-500 Hz) in animal models of Alzheimer’s disease and two animal models of epilepsy. Epilepsia 64, 231–246 (2023).
Busche, M. A. et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science 321, 1686–1689 (2008).
Gschwind, T. et al. Contribution of early Alzheimer’s disease-related pathophysiology to the development of acquired epilepsy. Eur. J. Neurosci. 47, 1534–1562 (2018).
Born, H. A. et al. Genetic suppression of transgenic APP rescues hypersynchronous network activity in a mouse model of Alzeimer’s disease. J. Neurosci. 11, 3826–3840 (2014).
Johnson, E. C. B. et al. Behavioral and neural network abnormalities in human APP transgenic mice resemble those of App knock-in mice and are modulated by familial Alzheimer’s disease mutations but not by inhibition of BACE1. Mol. Neurodegener. 15, 53 (2020). This paper is a systematic comparison between APP overexpression, APP mutations, amyloid plaque load and the level of amyloid-β oligomers as potential causes of neuronal hyperactivity in genetically modified AD mouse models.
Hascup, K. N. & Hascup, E. R. Soluble amyloid-β42 stimulates glutamate release through activation of the α7 nicotinic acetylcholine receptor. J. Alzheimers Dis. 53, 337–347 (2016).
Zott, B. et al. A vicious cycle of β amyloid-dependent neuronal hyperactivation. Science 365, 559–565 (2019).
Alfaro-Ruiz, R. et al. The expression and localisation of G-protein-coupled inwardly rectifying potassium (GIRK) channels is differentially altered in the hippocampus of two mouse models of Alzheimer’s disease. Int. J. Mol. Sci. 22, 11106 (2021).
Ciccone, R. et al. Amyloid β-induced upregulation of Nav1.6 underlies neuronal hyperactivity in Tg2576 Alzheimer’s disease mouse model. Sci. Rep. 9, 13592 (2019).
Hatch, R. J., Wei, Y., Xia, D. & Götz, J. Hyperphosphorylated tau causes reduced hippocampal CA1 excitability by relocating the axon initial segment. Acta Neuropathol. 133, 717–730 (2017).
Cloyd, R. A., Koren, J. III, Abisambra, J. F. & Smith, B. N. Effects of altered tau expression on dentate granule cell excitability in mice. Exp. Neurol. 343, 113766 (2021).
Busche, M. et al. Tau impairs neural circuits, dominating amyloid-β effects, in Alzheimer models in vivo. Nat. Neurosci. 22, 57–64 (2019).
Kudo, T. et al. Selective dysfunction of fast-spiking inhibitory interneurons and disruption of perineuronal nets in a tauopathy mouse model. iScience 26, 106342 (2023).
Morey, N. et al. Treatment of epilepsy using a targeted p38γ kinase gene therapy. Sci. Adv. 8, eadd2577 (2022).
Brown, J. et al. Tau in cerebrospinal fluid induces neuronal hyperexcitability and alters hippocampal theta oscillations. Acta Neuropathol. Comm. 11, 67 (2023).
Roberson, E. D. et al. Reducing endogenous tau ameliorates amyloid β-induced deficits in an Alzheimer’s disease mouse model. Science 316, 750–754 (2007).
DeVos, S. L. et al. Antisense reduction of tau in adult mice protects against seizures. J. Neurosci. 33, 12887–12897 (2013).
Holth, J. K. et al. Tau loss attenuates neuronal network hyperexcitability in mouse and Drosophila genetic models of epilepsy. J. Neurosci. 33, 1651–1659 (2013).
Wang, C. & Holtzman, D. M. Bidirectional relationship between sleep and Alzheimer’s disease: role of amyloid, tau, and other factors. Neuropsychopharmacology 45, 104–120 (2020).
Roberson, E. D. et al. Amyloid-β/Fyn-induced synaptic, network, and conitive impairments depend on tau levels in multiple mouse models of Alzheimer’s disease. J. Neurosci. 31, 700–711 (2011). This is a thorough investigation of the mechanisms showing that depletion of endogenous tau protein confers protection against amyloid-β-induced neuronal hyperexcitability and seizures.
Ittner, L. M. et al. Dendritic function of tau mediates amyloid-β toxicity in Alzheimer’s disease mouse models. Cell 142, 387–397 (2010).
Peters, F. et al. Tau deletion reduces plaque-associated BACE1 accumulation and decelerates plaque formation in a mouse model of Alzheimer’s disease. EMBO J. 38, e102345 (2019).
Chang, C. W., Evans, M. D., Yu, X., Yu, G. Q. & Mucke, L. Tau reduction affects excitatory and inhibitory neurons differently, reduces excitation/inhibition ratios, and counteracts network hypersynchrony. Cell Rep. 37, 109855 (2021).
DeVos, S. L. et al. Tau reduction prevents neuronal loss and reverses pathological tau deposition and seeding in mice with tauopathy. Sci. Transl. Med 9, eaag0481 (2017).
Hwang, K., Vaknalli, R. N., Addo-Osafo, K., Vicente, M. & Vossel, K. Tauopathy and epilepsy comorbidities and underlying mechanisms. Front. Aging Neurosci. 14, 903973 (2022).
Martinez-Losa, M. et al. Nav1.1-overexpressing interneuron transplants restore brain rhythms and cognition in a mouse model of Alzheimer’s disease. Neuron 98, 75–89 (2018).
Garcia-Marin, V. et al. Diminished perisomatic GABAergic terminals on cortical neurons adjacent to amyloid plaques. Front. Neuroanat. 3, 28 (2009).
Takahashi, H. et al. Hippocampal interneuron loss in an APP/PS1 double mutant mouse and in Alzheimer’s disease. Brain Struct. Funct. 214, 145–160 (2010).
Cattaud, V. et al. Early disruption of parvalbumin expression and perineuronal nets in the hippocampus of the Tg2576 mouse model of Alzheimer’s disease can be rescued by enriched environment. Neurobiol. Aging 72, 147–158 (2018).
Ali, F., Baringer, S. L., Neal, A., Choi, E. Y. & Kwan, A. C. Parvalbumin-positive neuron loss and amyloid-β deposits in the frontal cortex of Alzheimer’s disease-related mice. J. Alzheimers Dis. 72, 1323–1339 (2019).
Petrache, A. L. et al. Aberrant excitatory-inhibitory synaptic mechanisms in entorhinal cortex microcircuits during the pathogenesis of Alzheimer’s disease. Cereb. Cortex 29, 1834–1850 (2019).
Caccavano, A. et al. Inhibitory parvalbumin basket cell activity is selectively reduced during hippocampal sharp wave ripples in a mouse model of familial Alzheimer’s disease. J. Neurosci. 40, 5116–5136 (2020).
Giesers, N. K. & Wirths, O. Loss of hippocampal calretinin and parvalbumin interneurons in the 5XFAD mouse model of Alzheimer’s disease. ASN Neuro 12, 1759091420925356 (2020).
Algamal, M. et al. Reduced excitatory neuron activity and interneuron-type-specific deficits in a mouse model of Alzheimer’s disease. Commun. Biol. 5, 1323 (2022).
Deng, Y. et al. Loss of LAMP5 interneurons drives neuronal network dysfunction in Alzheimer’s disease. Acta Neuropathol. 144, 637–650 (2022).
Sos, K. E. et al. Amyloid β induces interneuron-specific changes in the hippocampus of APPNL-F mice. PLoS ONE 15, e0233700 (2020).
Verdaguer, E. et al. Vulnerability of calbindin, calretinin and parvalbumin in a transgenic/knock-in APPswe/PS1dE9 mouse model of Alzheimer disease together with disruption of hippocampal neurogenesis. Exp. Gerontol. 69, 176–188 (2015).
Hijazi, S. et al. Early restoration of parvalbumin interneuron activity prevents memory loss and network hyperexcitability in a mouse model of Alzheimer’s disease. Mol. Psychiatry 25, 3380–3398 (2020).
Gazestani, V. et al. Early Alzheimer’s disease pathology in human cortex involves transient cell states. Cell 186, 4438–4453 (2023).
Verret, L. et al. Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell 149, 708–721 (2012). This study demonstrates reduced functional voltage-gated sodium channels in the most important type of inhibitory interneurons in APP transgenic mice and the brain of people with AD, and presents a potential mechanism of decreased inhibition without neuronal loss.
Jin, N. et al. Preclinical evaluation of drug treatment options for sleep-related epileptiform spiking in Alzheimer’s disease. Alzheimers Dement. 8, e12291 (2022).
Olah, V. J. et al. Biophysical Kv3 channel alterations dampen excitability of cortical PV interneurons and contribute to network hyperexcitability in early Alzheimer’s. Elife 11, 75316 (2022).
Martín-Belmonte, A. et al. Reduction in the neuronal surface of post and presynaptic GABAB receptors in the hippocampus in a mouse model of Alzheimer’s disease. Brain Pathol. 30, 554–575 (2020).
Devinsky, O., Vezzani, A., Najjar, S., De Lanerolle, N. C. & Rogawski, M. A. Glia and epilepsy: excitability and inflammation. Trends Neurosci. 36, 174–184 (2013).
Talantova, M. et al. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc. Natl Acad. Sci. USA 110, 13691 (2013).
Ren, S. et al. TNF-α-mediated reduction in inhibitory neurotransmission precedes sporadic Alzheimer’s disease pathology in young Trem2R47H rats. J. Biol. Chem. 296, 100089 (2021).
Das, M. et al. Interdependence of neural network dysfunction and microglial alterations in Alzheimer’s disease-related models. iScience 24, 103245 (2021).
Stoiljkovic, M., Gutierrez, K. O., Kelley, C., Horvath, T. L. & Hajós, M. TREM2 deficiency disrupts network oscillations leading to epileptic activity and aggravates amyloid-β-related hippocampal pathophysiology in mice. J. Alzheimers Dis. 88, 837–847 (2022).
Rueda-Carrasco, J. et al. Microglia-synapse engulfment via PtdSer-TREM2 ameliorates neuronal hyperactivity in Alzheimer’s disease models. EMBO J. 42, e113246 (2023).
Das, M. et al. Alzheimer risk-increasing TREM2 variant causes aberrant cortical synapse density and promotes network hyperexcitability in mouse models. Neurobiol. Dis. 186, 106263 (2023).
Hunter, J. M. et al. Emergence of a seizure phenotype in aged apolipoprotein epsilon 4 targeted replacement mice. Brain Res. 1467, 120–132 (2012).
Lamoureux, L., Marottoli, F. M., Tseng, K. Y. & Tai, L. M. APOE4 promotes tonic-clonic seizures, an effect modified by familial Alzheimer’s disease mutations. Front. Cell Dev. Biol. 9, 656521 (2021).
Klein, R. C., Acheson, S. K., Mace, B. E., Sullivan, P. M. & Moore, S. D. Altered neurotransmission in the lateral amygdala in aged human apoE4 targeted replacement mice. Neurobiol. Aging 35, 2046–2052 (2014).
Har-Paz, I., Arieli, E. & Moran, A. ApoE4 attenuates cortical neuronal activity in young behaving apoE4 rats. Neurobiol. Dis. 155, 105373 (2021).
Feyissa, A. M., Hasan, T. F. & Meschia, J. F. Stroke-related epilepsy. Eur. J. Neurol. 26, 18–e3 (2019).
Brigo, F., Tezzon, F. & Nardone, R. Late-onset seizures and risk of subsequent stroke: a systematic review. Epilepsy Behav. 31, 9–12 (2014).
Dziadkowiak, E., Guziński, M., Chojdak-Łukasiewicz, J., Wieczorek, M. & Paradowski, B. Predictive factors in post-stroke epilepsy: retrospective analysis. Adv. Clin. Exp. Med. 30, 29–34 (2021).
Wall, J., Knight, J. & Emsley, H. C. A. Late-onset epilepsy predicts stroke: systematic review and meta-analysis. Epilepsy Behav. 115, 107634 (2021).
Phan, J., Ramos, M., Soares, T. & Parmar, M. S. Poststroke seizure and epilepsy: a review of incidence, risk factors, diagnosis, pathophysiology, and pharmacological therapies. Oxid. Med. Cell. Longev. 2022, 7692215 (2022).
Lee, S. H., Aw, K. L., Banik, S. & Myint, P. K. Post-stroke seizure risk prediction models: a systematic review and meta-analysis. Epileptic Disord. 24, 302–314 (2022).
Galovic, M. et al. Prediction of late seizures after ischaemic stroke with a novel prognostic model (the SeLECT score): a multivariable prediction model development and validation study. Lancet Neurol. 17, 143–152 (2018).
Lekoubou, A. et al. Poststroke seizures and the risk of dementia among young stroke survivors. Neurology 99, e385–e392 (2022).
Gibson, L. M., Allan, S. M., Parkes, L. M. & Emsley, H. C. Occult cerebrovascular disease and late-onset epilepsy: could loss of neurovascular unit integrity be a viable model? Cardiovasc. Psychiatry Neurol. 2011, 130406 (2011).
Friedman, D., Honig, L. S. & Scarmeas, N. Seizures and epilepsy in Alzheimer’s disease. CNS Neurosci. Ther. 18, 285–294 (2012).
Löscher, W. & Klein, P. New approaches for developing multi-targeted drug combinations for disease modification of complex brain disorders. Does epilepsy prevention become a realistic goal? Pharmacol. Ther. 229, 107934 (2022).
van Vliet, E. A. & Marchi, N. Neurovascular unit dysfunction as a mechanism of seizures and epilepsy during aging. Epilepsia 63, 1297–1313 (2022).
Reiss, Y. et al. The neurovasculature as a target in temporal lobe epilepsy. Brain Pathol. 33, e13147 (2023).
Löscher, W. & Friedman, A. Structural, molecular, and functional alterations of the blood-brain barrier during epileptogenesis and epilepsy: a cause, consequence, or both? Int. J. Mol. Sci. 21, 591 (2020).
Apátiga-Pérez, R. et al. Neurovascular dysfunction and vascular amyloid accumulation as early events in Alzheimer’s disease. Metab. Brain Dis. 37, 39–50 (2022).
Hussain, B., Fang, C. & Chang, J. Blood-brain barrier breakdown: an emerging biomarker of cognitive impairment in normal aging and dementia. Front. Neurosci. 15, 688090 (2021).
Chauhan, P. S., Mishra, M., Koul, B., Sharma, M. & Yadav, D. Modifiable risk factors associated with Alzheimer’s disease with special reference to sleep disturbance. CNS Neurol. Disord. Drug Targets 20, 594–601 (2021).
Sabia, S. et al. Association of sleep duration in middle and old age with incidence of dementia. Nat. Commun. 12, 2289 (2021).
Holth, J. K. et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363, 880–884 (2019).
Shokri-Kojori, E. et al. β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc. Natl Acad. Sci. USA 115, 4483–4488 (2018).
Das, R. & Luczak, A. Epileptic seizures and link to memory processes. AIMS Neurosci. 9, 114–127 (2022).
Puentes-Mestril, C., Roach, J., Niethard, N., Zochowski, M. & Aton, S. J. How rhythms of the sleeping brain tune memory and synaptic plasticity. Sleep 42, zsz095 (2019).
Irwin, M. R. & Vitiello, M. V. Implications of sleep disturbance and inflammation for Alzheimer’s disease dementia. Lancet Neurol. 18, 296–306 (2019).
Winer, J. R. et al. Sleep as a potential biomarker of tau and β-amyloid burden in the human brain. J. Neurosci. 39, 6315–6324 (2019).
Frederiksen, K. S. et al. European Academy of Neurology guideline on medical management issues in dementia. Eur. J. Neurol. 27, 1805–1820 (2020).
Liu, J. & Wang, L. N. Treatment of epilepsy for people with Alzheimer’s disease. Cochrane Database Syst. Rev. 5, CD011922 (2021).
Taipale, H. et al. Use of antiepileptic drugs and dementia risk – an analysis of Finnish health register and German health insurance data. J. Am. Geriatr. Soc. 66, 1123–1129 (2018).
Beghi, E. & Beghi, M. Epilepsy, antiepileptic drugs and dementia. Curr. Opin. Neurol. 33, 191–197 (2020).
Sarycheva, T. et al. Antiepileptic drug use and mortality among community-dwelling persons with Alzheimer disease. Neurology 94, e2099–e2108 (2020).
Brodie, M. J. et al. Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia 54, 11–27 (2013).
Sills, G. J. & Rogawski, M. A. Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 168, 107966 (2020).
Löscher, W., Gillard, M., Sands, Z. A., Kaminski, R. M. & Klitgaard, H. Synaptic vesicle glycoprotein 2A ligands in the treatment of epilepsy and beyond. CNS Drugs 30, 1055–1077 (2016).
Stout, K. A., Dunn, A. R., Hoffman, C. & Miller, G. W. The synaptic vesicle glycoprotein 2: structure, function, and disease relevance. ACS Chem. Neurosci. 10, 3927–3938 (2019).
Kong, Y. et al. The synaptic vesicle protein 2A interacts with key pathogenic factors in Alzheimer’s disease: implications for treatment. Front. Cell Dev. Biol. 9, 609908 (2021).
Rossi, R., Arjmand, S., Bærentzen, S. L., Gjedde, A. & Landau, A. M. Synaptic vesicle glycoprotein 2A: features and functions. Front. Neurosci. 16, 864514 (2022).
Sanz-Blasco, S., Piña-Crespo, J. C., Zhang, X., McKercher, S. R. & Lipton, S. A. Levetiracetam inhibits oligomeric Aβ-induced glutamate release from human astrocytes. Neuroreport 27, 705–709 (2016).
Sanchez, P. E. et al. Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proc. Natl Acad. Sci. USA 109, E2895–E2903 (2012). This landmark study in human-APP mice shows that among multiple antiseizure drugs, only levetiracetam effectively suppresses abnormal spiking activity and reverses behavioural abnormalities, cognitive impairment and remodelling of hippocampal circuits.
Fu, C. H. et al. Early seizure activity accelerates depletion of hippocampal neural stem cells and impairs spatial discrimination in an Alzheimer’s disease model. Cell Rep. 27, 3741–3751 (2019).
Rao, N. R. & Savas, J. N. Levetiracetam treatment normalizes levels of presynaptic endocytosis machinery and restores nonamyloidogenic APP processing in App knock-in mice. J. Proteome Res. 20, 3580–3589 (2021).
Alavi, M. S., Fanoudi, S., Hosseini, M. & Sadeghnia, H. R. Beneficial effects of levetiracetam in streptozotocin-induced rat model of Alzheimer’s disease. Metab. Brain Dis. 37, 689–700 (2022).
Nandini, H. S., Krishna, K. L. & Apattira, C. Combination of Ocimum sanctum extract and levetiracetam ameliorates cognitive dysfunction and hippocampal architecture in rat model of Alzheimer’s disease. J. Chem. Neuroanat. 120, 102069 (2022).
Nygaard, H. B. et al. Brivaracetam, but not ethosuximide, reverses memory impairments in an Alzheimer’s disease mouse model. Alzheimers Res. Ther. 7, 25 (2015).
Silva, J. C., Shen, Y., Chan, J., Kwan, P. & Jones, N. C. Anti-epileptogenic effects of synaptic vesicle protein 2A modulation in a mouse model of Alzheimer’s disease. Epilepsy Res. 186, 106994 (2022).
Cai, Z., Li, S., Matuskey, D., Nabulsi, N. & Huang, Y. PET imaging of synaptic density: a new tool for investigation of neuropsychiatric diseases. Neurosci. Lett. 691, 44–50 (2019).
Mecca, A. P. et al. In vivo measurement of widespread synaptic loss in Alzheimer’s disease with SV2A PET. Alzheimers Dement. 16, 974–982 (2020). This study shows that brain PET with the SV2A ligand [11C]UCB-JPE is a suitable in vivo method to determine the regional extent of synaptic loss in AD.
Vossel, K. et al. Effect of levetiracetam on cognition in patients with Alzheimer disease with and without epileptiform activity: a randomized clinical trial. JAMA Neurol. 78, 1345–1354 (2021). This study suggests that stratification of patient populations based on EEG results might be an important new aspect when designing drug trials to find the most effective medication for AD-related epilepsy.
Bakker, A. et al. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron 74, 467–474 (2012). This clinical study shows that levetiracetam normalizes hippocampal hyperexcitability and improves memory performance in mildly impaired individuals with AD.
Bakker, A., Albert, M. S., Krauss, G., Speck, C. L. & Gallagher, M. Response of the medial temporal lobe network in amnestic mild cognitive impairment to therapeutic intervention assessed by fMRI and memory task performance. Neuroimage Clin. 7, 688–698 (2015).
Musaeus, C. S. et al. Levetiracetam increases hippocampal blood flow in Alzheimer’s disease as measured by arterial spin labelling MRI. J. Alzheimers Dis. 93, 939–948 (2023).
US National Library of Medicine. ClinicalTrials.govclinicaltrials.gov/study/NCT03875638 (2023).
US National Library of Medicine. ClinicalTrials.govclinicaltrials.gov/study/NCT04004702 (2019).
Celdran de Castro, A. et al. Levetiracetam, from broad-spectrum use to precision prescription: a narrative review and expert opinion. Seizure 107, 121–131 (2023).
Rizzello, E. et al. Lamotrigine rescues neuronal alterations and prevents seizure-induced memory decline in an Alzheimer’s disease mouse model. Neurobiol. Dis. 181, 106106 (2023).
de Paula Faria, D. et al. Cannabidiol treatment improves glucose metabolism and memory in streptozotocin-induced Alzheimer’s disease rat model: a proof-of-concept study. Int. J. Mol. Sci. 23, 1076 (2022).
Xiong, Y. & Lim, C. S. Understanding the modulatory effects of cannabidiol on Alzheimer’s disease. Brain Sci. 11, 1211 (2021).
Chen, S. J. et al. Allopregnanolone promotes neuronal and oligodendrocyte differentiation in vitro and in vivo: therapeutic implication for Alzheimer’s disease. Neurotherapeutics 17, 1813–1824 (2020).
Cretin, B. Pharmacotherapeutic strategies for treating epilepsy in patients with Alzheimer’s disease. Expert. Opin. Pharmacother. 19, 1201–1209 (2018).
Löscher, W., Potschka, H., Sisodiya, S. M. & Vezzani, A. Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol. Rev. 72, 606–638 (2020).
Kalilani, L., Sun, X., Pelgrims, B., Noack-Rink, M. & Villanueva, V. The epidemiology of drug-resistant epilepsy: a systematic review and meta-analysis. Epilepsia 59, 2179–2193 (2018).
Cumbo, E. & Ligori, L. D. Levetiracetam, lamotrigine, and phenobarbital in patients with epileptic seizures and Alzheimer’s disease. Epilepsy Behav. 17, 461–466 (2010). This is a prospective randomized clinical trial on the efficacy of antiseizure medications in people with AD and focal epilepsy, showing high treatment-resistance of seizures in these individuals.
Vande Vyver, M. et al. Higher susceptibility to 6 Hz corneal kindling and lower responsiveness to antiseizure drugs in mouse models of Alzheimer’s disease. Epilepsia 63, 2703–2715 (2022).
Mahamud, Z., Mononen, C. P., Brigo, F., Garcia-Ptacek, S. & Zelano, J. Risk of epilepsy diagnosis after a first unprovoked seizure in dementia. Seizure 82, 118–124 (2020).
Smith, P. E. M. Initial management of seizure in adults. N. Engl. J. Med 385, 251–263 (2021).
Larner, A. J. & Marson, A. G. Epileptic seizures in Alzheimer’s disease: what are the implications of SANAD II? J. Alzheimers Dis. 85, 527–529 (2022).
Bruzzone, M. J. et al. Hippocampal spikes have heterogeneous scalp EEG correlates important for defining IEDs. Epilepsy Res. 182, 106914 (2022).
Lam, A. D. et al. Silent hippocampal seizures and spikes identified by foramen ovale electrodes in Alzheimer’s disease. Nat. Med. 23, 678–680 (2017).
Horváth, A. et al. Interictal epileptiform activity in the foramen ovale electrodes of a frontotemporal dementia patient. J. Alzheimers Dis. Rep. 1, 89–96 (2017).
Vos, S. J. B. et al. Preclinical Alzheimer’s disease and its outcome, a longitudinal cohort study. Lancet Neurol. 12, 957–965 (2013).
Frisoni, G. et al. The probabilistic model of Alzheimer disease: the amyloid hypothesis revised. Nat. Rev. Neurosci. 23, 53–66 (2022).
Jack, C. R. et al. The bivariate distribution of amyloid-β and tau: relationship with established neurocognitive clinical syndromes. Brain 142, 3230–3242 (2019).
Witt, J. A. & Helmstaedter, C. Should cognition be screened in new-onset epilepsies? A study in 247 untreated patients. J. Neurol. 259, 1727–1731 (2012).
Gourmaud, S. et al. Alzheimer-like amyloid and tau alterations associated with cognitive deficit in temporal lobe epilepsy. Brain 143, 191–209 (2020).
Vico Varela, E., Etter, G. & Williams, S. Excitatory-inhibitory imbalance in Alzheimer’s disease and therapeutic significance. Neurobiol. Dis. 127, 605–615 (2019).
Kazim, S. F. et al. Early-onset network hyperexcitability in presymptomatic Alzheimer’s disease transgenic mice is suppressed by passive immunization with anti-human APP/Aβ antibody and by mGluR5 blockade. Front. Aging Neurosci. 9, 71 (2017).
Ossenkoppele, R. et al. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat. Med. 28, 2381–2387 (2022).
Thal, D. R., Rüb, U., Orantes, M. & Braak, H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58, 1791–1800 (2002).
Braak, H. & Braak, E. Evolution of the neuropathology of Alzheimer’s disease. Acta Neurol. Scand. 165, 3–12 (1996).
Jack, C. R. et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 9, 119–128 (2010).
Vogel, J. W. et al. Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat. Med. 27, 871–881 (2021).
Harris, S. S., Wolf, F., De Strooper, B. & Busche, M. A. Tipping the scales: peptide-dependent dysregulation of neural circuit dynamics in Alzheimer’s disease. Neuron 107, 417–435 (2020).
Jin, N., Lipponen, A., Koivisto, H., Gurevicius, K. & Tanila, H. Increased cortical beta power and spike-wave discharges in middle-aged APP/PS1 mice. Neurobiol. Aging 71, 127–141 (2018).
Santiago, J. A. & Potashkin, J. A. The impact of disease comorbidities in Alzheimer’s disease. Front. Aging Neurosci. 12, 631770 (2021).
Pascual-Leone, A. & Bartres-Faz, D. Human brain resilience: a call to action. Ann. Neurol. 90, 336–349 (2021).
Bocancea, D. I. et al. Determinants of cognitive and brain resilience to tau pathology: a longitudinal analysis. Brain 146, 3719–3734 (2023).
van Loenhoud, A. C. et al. Cognitive reserve and clinical progression in Alzheimer disease. A paradoxical relationship. Neurology 93, e334–e346 (2019).
Petersen, R. C. et al. Current concepts in mild cognitive impairment. Arch. Neurol. 58, 1985–1992 (2001).
Kramer, M., Sartorius, N., Jablensky, A. & Gulbinat, W. The ICD-9 classification of mental disorders: a review of its development and contents. Acta Psychiatr. Scand. 59, 241–262 (1979).
Dubois, B. et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS–ADRDA criteria. Lancet Neurol. 6, 734–746 (2007).
Centers for Disease Control and Prevention. International classification of diseases, tenth revision (ICD-10). CDC www.cdc.gov/nchs/icd/icd10.htm (2021).
Wang, Y. & Mattson, M. P. L-type Ca2+ currents at CA1 synapses, but not CA3 or dentate granule neuron synapses, are increased in 3xTgAD mice in an age-dependent manner. Neurobiol. Aging 35, 88–95 (2014).
Acknowledgements
The authors are very grateful to G. Buzsáki and L. Mucke for their comments and constructive criticisms on an earlier draft of the manuscript. The authors are thankful to J. Cole and S. J. Damberger for their intellectual contribution to the article.
Author information
Authors and Affiliations
Contributions
A.K. developed the primary content and structure of the article. All authors selected the references for the article. A.K. and A.A.H. wrote the first draft of the manuscript. AK., A.A.H., W.L. and H.T. prepared the figures and tables for the manuscript. All authors reviewed and/or edited the final version of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
A.K. received grant from the Hungarian Academy of Sciences, National Brain Research Program III (NAP2022-I-9/2022). M.G.-D. receives book royalties from Oxford University Press, and article royalties from Up-to-Date; and is Associate Editor of the Journal of Clinical Neurophysiology and Journal of Clinical Sleep Medicine. She received honoraria for presentations at the annual meeting of the American Academy of Sleep Medicine, and for teaching presentations at the annual meeting and fall course of the American Clinical Neurophysiology Society. W.L. received grants from the German Research Foundation (LO 274/15-1; 274/16-2); consultancy fees from AC Immune, Angelini Pharma, Clexio Biosciences, Lundbeck and Selene Therapeutics. He is co-founder and Chief Scientific Officer of PrevEp Inc. (Bethesda, MD). PrevEp is focusing on preventing epilepsy after traumatic brain injury, stroke and encephalitis. The company is not developing any current therapies to prevent epilepsy in Alzheimer disease, and none of its professional activities has any relationship to Alzheimer disease. H.T. received grants from the Academy of Finland (310059 and 340377), Jane and Aatos Erkko Foundation (Finland), and Olav Thon Foundation (Norway). A.A.H. received grants from the Hungarian Academy of Sciences, National Brain Research Program III (NAP2022-I-9/2022) and Momentum Research Grant (Lendület-2023_94); the Hungarian Scientific Research Fund 2019 of the National Research, Development and Innovation Office (PD-132652); a János Bolyai Research Scholarship of the Hungarian Academy of Sciences (bo-78-20-2020); the EU Joint Program Neurodegenerative Disease Research (JPND) project (via the National Research, Development and Innovation Office, 2019-2-1-7-ERA-NET-2020-00006).
Peer review
Peer review information
Nature Reviews Neurology thanks Benjamin Cretin, Jin-Tai Yu and Cinzia Costa for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Review criteria Authors reviewed the scientific literature from 1 January 2017 to 15 October 2023, published in English, by searching PubMed, Scopus, Web of Science, Cochrane Library, EMBASE, CINAHL Plus, Science Direct, DOAJ and Google Scholar databases. Search terms included: Alzheimer’s disease; epilepsy; spike; seizures; epileptiform (activity); anticonvulsants; antiseizure drugs; antiepileptic drugs; (network) hyperexcitability; tau; amyloid; Alzheimer’s disease and epilepsy; and sleep. Although epilepsy occurs frequently in genetically determined early-onset Alzheimer disease this entity is not covered in this Review; therefore related papers were excluded. Seminal references published before 2017 were also included. Information about clinical trials were obtained from ClinicalTrials.gov.
Related links
Alzheimer’s Disease Neuroimaging Initiative database: https://adni.loni.usc.edu/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kamondi, A., Grigg-Damberger, M., Löscher, W. et al. Epilepsy and epileptiform activity in late-onset Alzheimer disease: clinical and pathophysiological advances, gaps and conundrums. Nat Rev Neurol 20, 162–182 (2024). https://doi.org/10.1038/s41582-024-00932-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-024-00932-4