Abstract
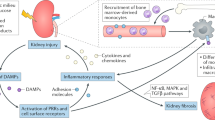
The deposition of immune complexes, activation of complement and infiltration of the kidney by cells of the adaptive and innate immune systems have long been considered responsible for the induction of kidney damage in autoimmune, alloimmune and other inflammatory kidney diseases. However, emerging findings have highlighted the contribution of resident immune cells and of immune molecules expressed by kidney-resident parenchymal cells to disease processes. Several types of kidney parenchymal cells seem to express a variety of immune molecules with a distinct topographic distribution, which may reflect the exposure of these cells to different pathogenic threats or microenvironments. A growing body of literature suggests that these cells can stimulate the infiltration of immune cells that provide protection against infections or contribute to inflammation — a process that is also regulated by draining kidney lymph nodes. Moreover, components of the immune system, such as autoantibodies, cytokines and immune cells, can influence the metabolic profile of kidney parenchymal cells in the kidney, highlighting the importance of crosstalk in pathogenic processes. The development of targeted nanomedicine approaches that modulate the immune response or control inflammation and damage directly within the kidney has the potential to eliminate the need for systemically acting drugs.
Key points
-
Non-myeloid kidney parenchymal cells (KPCs) express molecules that are shared with immune cells; the expression of these molecules may have distinct topological associations and may increase in response to inflammation.
-
Kidney injury can induce the infiltration of immune cells; these cells may have protective or pro-inflammatory features depending on the phase of the disease process. In addition, kidney-resident immune cells may have a surveillance or regulatory role in the kidney but can accumulate in response to injury.
-
Components of the immune system, such as autoantibodies, cytokines and immune cells, can influence the metabolic profile of KPCs in the kidney.
-
Conversely, KPCs can contribute to inflammation by providing metabolites or through the secretion of chemokines and cytokines.
-
The cortex and the medulla of kidney tissue can contain distinct types of tertiary lymphoid organs, which may possess pathogenic or regulatory properties.
-
Targeted delivery of drugs to KPCs holds promise for preventing or reversing the inflammatory response in specific forms of kidney injury and disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
23 November 2023
A Correction to this paper has been published: https://doi.org/10.1038/s41581-023-00793-y
References
Pisetsky, D. S. Pathogenesis of autoimmune disease. Nat. Rev. Nephrol. 19, 509–524 (2023).
Mohan, C., Zhang, T. & Putterman, C. Pathogenic cellular and molecular mediators in lupus nephritis. Nat. Rev. Nephrol. 19, 491–508 (2023).
Maeda, K. et al. CaMK4 compromises podocyte function in autoimmune and nonautoimmune kidney disease. J. Clin. Invest. 128, 3445–3459 (2018).
Li, H. et al. IL-23 reshapes kidney resident cell metabolism and promotes local kidney inflammation. J. Clin. Invest. 131, e142428 (2021).
Tsokos, G. C. Systemic lupus erythematosus. N. Engl. J. Med. 365, 2110–2121 (2011).
Davidson, A. What is damaging the kidney in lupus nephritis? Nat. Rev. Rheumatol. 12, 143–153 (2016).
Tsokos, G. C. Autoimmunity and organ damage in systemic lupus erythematosus. Nat. Immunol. 21, 605–614 (2020).
Urbonaviciute, V. et al. Induction of inflammatory and immune responses by HMGB1-nucleosome complexes: implications for the pathogenesis of SLE. J. Exp. Med. 205, 3007–3018 (2008).
Suurmond, J. & Diamond, B. Autoantibodies in systemic autoimmune diseases: specificity and pathogenicity. J. Clin. Invest. 125, 2194–2202 (2015).
Vlahakos, D. V. et al. Anti-DNA antibodies form immune deposits at distinct glomerular and vascular sites. Kidney Int. 41, 1690–1700 (1992).
Jamaly, S., Rakaee, M., Abdi, R., Tsokos, G. C. & Fenton, K. A. Interplay of immune and kidney resident cells in the formation of tertiary lymphoid structures in lupus nephritis. Autoimmun. Rev. 20, 102980 (2021).
Sato, Y., Silina, K., van den Broek, M., Hirahara, K. & Yanagita, M. The roles of tertiary lymphoid structures in chronic diseases. Nat. Rev. Nephrol. 19, 525–537 (2023).
van Bavel, C. C., Fenton, K. A., Rekvig, O. P., van der Vlag, J. & Berden, J. H. Glomerular targets of nephritogenic autoantibodies in systemic lupus erythematosus. Arthritis Rheum. 58, 1892–1899 (2008).
Ge, Y. et al. Cgnz1 allele confers kidney resistance to damage preventing progression of immune complex-mediated acute lupus glomerulonephritis. J. Exp. Med. 210, 2387–2401 (2013).
Vukelic, M., Li, Y. & Kyttaris, V. C. Novel treatments in lupus. Front. Immunol. 9, 2658 (2018).
Li, H. & Tsokos, G. C. IL-23/IL-17 axis in inflammatory rheumatic diseases. Clin. Rev. Allergy Immunol. 60, 31–45 (2021).
Schwartz, N., Goilav, B. & Putterman, C. The pathogenesis, diagnosis and treatment of lupus nephritis. Curr. Opin. Rheumatol. 26, 502–509 (2014).
Krausgruber, T. et al. Structural cells are key regulators of organ-specific immune responses. Nature 583, 296–302 (2020).
Wen, C. P. et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 371, 2173–2182 (2008).
Akchurin, O. M. & Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 39, 84–92 (2015).
Hsieh, C. et al. Predicting outcomes of lupus nephritis with tubulointerstitial inflammation and scarring. Arthritis Care Res. 63, 865–874 (2011).
Rodriguez-Iturbe, B., Johnson, R. J. & Herrera-Acosta, J. Tubulointerstitial damage and progression of renal failure. Kidney Int. Suppl. 68, S82–S86 (2005).
Rodriguez-Iturbe, B. & Garcia Garcia, G. The role of tubulointerstitial inflammation in the progression of chronic renal failure. Nephron Clin. Pract. 116, c81–88, (2010).
Li, H., Tsokos, M. G. & Tsokos, G. C. Lymphocytes in the neighborhood: good or bad for the kidney? J. Clin. Invest. 132, e160657–, (2022).
Kurts, C., Ginhoux, F. & Panzer, U. Kidney dendritic cells: fundamental biology and functional roles in health and disease. Nat. Rev. Nephrol. 16, 391–407 (2020).
Tang, P. M., Nikolic-Paterson, D. J. & Lan, H. Y. Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 15, 144–158 (2019).
Salei, N. et al. The kidney contains ontogenetically distinct dendritic cell and macrophage subtypes throughout development that differ in their inflammatory properties. J. Am. Soc. Nephrol. 31, 257–278 (2020).
Davidson, A. Renal mononuclear phagocytes in lupus nephritis. ACR Open. Rheumatol. 3, 442–450 (2021).
Watanabe, S., Alexander, M., Misharin, A. V. & Budinger, G. R. S. The role of macrophages in the resolution of inflammation. J. Clin. Invest. 129, 2619–2628 (2019).
Chen, T., Cao, Q., Wang, Y. & Harris, D. C. H. M2 macrophages in kidney disease: biology, therapies, and perspectives. Kidney Int. 95, 760–773 (2019).
Arazi, A. et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 20, 902–914 (2019).
Rao, D. A., Arazi, A., Wofsy, D. & Diamond, B. Design and application of single-cell RNA sequencing to study kidney immune cells in lupus nephritis. Nat. Rev. Nephrol. 16, 238–250 (2020).
Klose, C. S. N. & Artis, D. Innate lymphoid cells control signaling circuits to regulate tissue-specific immunity. Cell Res. 30, 475–491 (2020).
Rabb, H. The T cell as a bridge between innate and adaptive immune systems: implications for the kidney. Kidney Int. 61, 1935–1946 (2002).
Shipman, W. D., Dasoveanu, D. C. & Lu, T. T. Tertiary lymphoid organs in systemic autoimmune diseases: pathogenic or protective? F1000Res 6, 196 (2017).
Chang, A. et al. In situ B cell-mediated immune responses and tubulointerstitial inflammation in human lupus nephritis. J. Immunol. 186, 1849–1860 (2011).
Abraham, R. et al. Specific in situ inflammatory states associate with progression to renal failure in lupus nephritis. J. Clin. Invest. 132, e155350 (2022).
Aloisi, F. & Pujol-Borrell, R. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 6, 205–217 (2006).
Chen, P. M. et al. Kidney tissue hypoxia dictates T cell-mediated injury in murine lupus nephritis. Sci. Transl. Med. 12, eaay1620 (2020).
Li, H. & Tsokos, G. C. Double-negative T cells in autoimmune diseases. Curr. Opin. Rheumatol. 33, 163–172 (2021).
Chen, A. et al. Bowman’s capsule provides a protective niche for podocytes from cytotoxic CD8+ T cells. J. Clin. Invest. 128, 3413–3424 (2018).
do Valle Duraes, F. et al. Immune cell landscaping reveals a protective role for regulatory T cells during kidney injury and fibrosis. JCI Insight 5, e130651 (2020).
Bhargava, R., Li, H. & Tsokos, G. C. Pathogenesis of lupus nephritis: the contribution of immune and kidney resident cells. Curr. Opin. Rheumatol. 35, 107–116 (2023).
Liao, X., Pirapakaran, T. & Luo, X. M. Chemokines and chemokine receptors in the development of lupus nephritis. Mediators Inflamm. 2016, 6012715 (2016).
Worthmann, K. et al. Pathogenetic role of glomerular CXCL13 expression in lupus nephritis. Clin. Exp. Immunol. 178, 20–27 (2014).
Lema et al. Chemokine expression precedes inflammatory cell infiltration and chemokine receptor and cytokine expression during the initiation of murine lupus nephritis. J. Am. Soc. Nephrol. 12, 1369–1382 (2001).
Kolodziejczyk, A. A., Kim, J. K., Svensson, V., Marioni, J. C. & Teichmann, S. A. The technology and biology of single-cell RNA sequencing. Mol. Cell 58, 610–620 (2015).
Park, J. et al. Single-cell transcriptomics of the mouse kidney reveals potential cellular targets of kidney disease. Science 360, 758–763 (2018).
Stewart, B. J. et al. Spatiotemporal immune zonation of the human kidney. Science 365, 1461–1466 (2019).
Zeng, H., Yang, X., Luo, S. & Zhou, Y. The advances of single-cell RNA-seq in kidney immunology. Front. Physiol. 12, 752679 (2021).
Fu, J. et al. Single-cell RNA profiling of glomerular cells shows dynamic changes in experimental diabetic kidney disease. J. Am. Soc. Nephrol. 30, 533–545 (2019).
Wilson, P. C. et al. The single-cell transcriptomic landscape of early human diabetic nephropathy. Proc. Natl Acad. Sci. USA 116, 19619–19625 (2019).
Zheng, Y. et al. Single-cell transcriptomics reveal immune mechanisms of the onset and progression of IgA nephropathy. Cell Rep. 33, 108525 (2020).
Krebs, C. F. et al. Pathogen-induced tissue-resident memory TH17 (TRM17) cells amplify autoimmune kidney disease. Sci. Immunol. 5, eaba4163 (2020).
Der, E. et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat. Immunol. 20, 915–927 (2019).
Sigdel, T. K. et al. Near-single-cell proteomics profiling of the proximal tubular and glomerulus of the normal human kidney. Front. Med. 7, 499 (2020).
Lake, B. B. et al. An atlas of healthy and injured cell states and niches in the human kidney. Nature 619, 585–594 (2023).
Xia, H., Bao, W. & Shi, S. Innate immune activity in glomerular podocytes. Front. Immunol. 8, 122 (2017).
Bhargava, R. & Tsokos, G. C. The immune podocyte. Curr. Opin. Rheumatol. 31, 167–174 (2019).
Hong, S., Healy, H. & Kassianos, A. J. The emerging role of renal tubular epithelial cells in the immunological pathophysiology of lupus nephritis. Front. Immunol. 11, 578952 (2020).
Zhang, C. et al. Activation of Nod-like receptor protein 3 inflammasomes turns on podocyte injury and glomerular sclerosis in hyperhomocysteinemia. Hypertension 60, 154–162 (2012).
Flur, K. et al. Viral RNA induces type I interferon-dependent cytokine release and cell death in mesangial cells via melanoma-differentiation-associated gene-5: implications for viral infection-associated glomerulonephritis. Am. J. Pathol. 175, 2014–2022 (2009).
Patole, P. S. et al. Expression and regulation of Toll-like receptors in lupus-like immune complex glomerulonephritis of MRL-Faslpr mice. Nephrol. Dial. Transpl. 21, 3062–3073 (2006).
Yung, S., Cheung, K. F., Zhang, Q. & Chan, T. M. Anti-dsDNA antibodies bind to mesangial annexin II in lupus nephritis. J. Am. Soc. Nephrol. 21, 1912–1927 (2010).
Yung, S. & Chan, T. M. Anti-dsDNA antibodies and resident renal cells — their putative roles in pathogenesis of renal lesions in lupus nephritis. Clin. Immunol. 185, 40–50 (2017).
Kanapathippillai, P., Hedberg, A., Fenton, C. G. & Fenton, K. A. Nucleosomes contribute to increase mesangial cell chemokine expression during the development of lupus nephritis. Cytokine 62, 244–252 (2013).
Ichinose, K. et al. Cutting edge: calcium/calmodulin-dependent protein kinase type IV is essential for mesangial cell proliferation and lupus nephritis. J. Immunol. 187, 5500–5504 (2011).
Groza, Y., Jemelkova, J., Kafkova, L. R., Maly, P. & Raska, M. IL-6 and its role in IgA nephropathy development. Cytokine Growth Factor. Rev. 66, 1–14 (2022).
Lai, K. N. et al. IgA nephropathy. Nat. Rev. Dis. Prim. 2, 16001 (2016).
Coers, W. et al. Podocyte expression of MHC class I and II and intercellular adhesion molecule-1 (ICAM-1) in experimental pauci-immune crescentic glomerulonephritis. Clin. Exp. Immunol. 98, 279–286 (1994).
Goldwich, A. et al. Podocytes are nonhematopoietic professional antigen-presenting cells. J. Am. Soc. Nephrol. 24, 906–916 (2013).
Pyzik, M., Rath, T., Lencer, W. I., Baker, K. & Blumberg, R. S. FcRn: the architect behind the immune and nonimmune functions of IgG and albumin. J. Immunol. 194, 4595–4603 (2015).
Haddad, G., Dylewski, J., Evans, R., Lewis, L. & Blaine, J. Knockout of the neonatal Fc receptor alters immune complex trafficking and lysosomal function in cultured podocytes. PLoS One 18, e0284636 (2023).
Liu, M. & Zen, K. Toll-like receptors regulate the development and progression of renal diseases. Kidney Dis. 7, 14–23 (2021).
Kuravi, S. J. et al. Podocytes regulate neutrophil recruitment by glomerular endothelial cells via IL-6-mediated crosstalk. J. Immunol. 193, 234–243 (2014).
Ren, J. et al. Twist1 in podocytes ameliorates podocyte injury and proteinuria by limiting CCL2-dependent macrophage infiltration. JCI Insight 6, e148109 (2021).
Bruno, V., Muhlig, A. K., Oh, J. & Licht, C. New insights into the immune functions of podocytes: the role of complement. Mol. Cell Pediatr. 10, 3 (2023).
Li, X., Ding, F., Zhang, X., Li, B. & Ding, J. The expression profile of complement components in podocytes. Int. J. Mol. Sci. 17, 471 (2016).
Karasawa, T. et al. Glomerular endothelial expression of type I IFN-stimulated gene, DExD/H-Box helicase 60 via toll-like receptor 3 signaling: possible involvement in the pathogenesis of lupus nephritis. Ren. Fail. 44, 137–145 (2022).
Hirono, K. et al. Endothelial expression of fractalkine (CX3CL1) is induced by Toll-like receptor 3 signaling in cultured human glomerular endothelial cells. Mod. Rheumatol. 30, 1074–1081 (2020).
Dimou, P. et al. The human glomerular endothelial cells are potent pro-inflammatory contributors in an in vitro model of lupus nephritis. Sci. Rep. 9, 8348 (2019).
Kassianos, A. J. et al. Human proximal tubule epithelial cells modulate autologous dendritic cell function. Nephrol. Dial. Transpl. 28, 303–312 (2013).
Sampangi, S. et al. Human proximal tubule epithelial cells modulate autologous B-cell function. Nephrol. Dial. Transpl. 30, 1674–1683 (2015).
Kassianos, A. J. et al. Fractalkine-CX3CR1-dependent recruitment and retention of human CD1c+ myeloid dendritic cells by in vitro-activated proximal tubular epithelial cells. Kidney Int. 87, 1153–1163 (2015).
Starke, A. et al. Renal tubular PD-L1 (CD274) suppresses alloreactive human T-cell responses. Kidney Int. 78, 38–47 (2010).
Castellano, G. et al. Local synthesis of interferon-alpha in lupus nephritis is associated with type I interferons signature and LMP7 induction in renal tubular epithelial cells. Arthritis Res. Ther. 17, 72 (2015).
Schwarting, A. et al. Renal tubular epithelial cell-derived BAFF expression mediates kidney damage and correlates with activity of proliferative lupus nephritis in mouse and men. Lupus 27, 243–256 (2018).
Mistry, P. & Kaplan, M. J. Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin. Immunol. 185, 59–73 (2017).
Jia, P. et al. Chemokine CCL2 from proximal tubular epithelial cells contributes to sepsis-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 323, F107–F119 (2022).
Ijima, S. et al. Fisetin reduces the senescent tubular epithelial cell burden and also inhibits proliferative fibroblasts in murine lupus nephritis. Front. Immunol. 13, 960601 (2022).
Paragas, N. et al. α-Intercalated cells defend the urinary system from bacterial infection. J. Clin. Invest. 124, 2963–2976 (2014).
Gauer, S. et al. IL-18 is expressed in the intercalated cell of human kidney. Kidney Int. 72, 1081–1087 (2007).
Breton, S. & Battistone, M. A. Unexpected participation of intercalated cells in renal inflammation and acute kidney injury. Nephron 146, 268–273 (2022).
Dorraji, S. E. et al. Mesenchymal stem cells and T cells in the formation of tertiary lymphoid structures in lupus nephritis. Sci. Rep. 8, 7861 (2018).
Huang, N. & Perl, A. Metabolism as a target for modulation in autoimmune diseases. Trends Immunol. 39, 562–576 (2018).
Aufhauser, D. D. Jr et al. Improved renal ischemia tolerance in females influences kidney transplantation outcomes. J. Clin. Invest. 126, 1968–1977 (2016).
McEvoy, C. M. et al. Single-cell profiling of healthy human kidney reveals features of sex-based transcriptional programs and tissue-specific immunity. Nat. Commun. 13, 7634 (2022).
Zhao, J. L., Qiao, X. H., Mao, J. H., Liu, F. & Fu, H. D. The interaction between cellular senescence and chronic kidney disease as a therapeutic opportunity. Front. Pharmacol. 13, 974361 (2022).
O’Sullivan, E. D., Hughes, J. & Ferenbach, D. A. Renal aging: causes and consequences. J. Am. Soc. Nephrol. 28, 407–420 (2017).
Sato, Y. et al. CD153/CD30 signaling promotes age-dependent tertiary lymphoid tissue expansion and kidney injury. J. Clin. Invest. 132, e146071 (2022).
Gottschalk, C. et al. Batf3-dependent dendritic cells in the renal lymph node induce tolerance against circulating antigens. J. Am. Soc. Nephrol. 24, 543–549 (2013).
Dong, X. et al. Antigen presentation by dendritic cells in renal lymph nodes is linked to systemic and local injury to the kidney. Kidney Int. 68, 1096–1108 (2005).
Fletcher, A. L., Acton, S. E. & Knoblich, K. Lymph node fibroblastic reticular cells in health and disease. Nat. Rev. Immunol. 15, 350–361 (2015).
Lutge, M., Pikor, N. B. & Ludewig, B. Differentiation and activation of fibroblastic reticular cells. Immunol. Rev. 302, 32–46 (2021).
Perez-Shibayama, C., Gil-Cruz, C. & Ludewig, B. Fibroblastic reticular cells at the nexus of innate and adaptive immune responses. Immunol. Rev. 289, 31–41 (2019).
Kasinath, V. et al. Activation of fibroblastic reticular cells in kidney lymph node during crescentic glomerulonephritis. Kidney Int. 95, 310–320 (2019).
Li, X. et al. Kidney-draining lymph node fibrosis following unilateral ureteral obstruction. Front. Immunol. 12, 768412 (2021).
Maarouf, O. H. et al. Repetitive ischemic injuries to the kidneys result in lymph node fibrosis and impaired healing. JCI Insight 3, e120546 (2018).
Der, E., Suryawanshi, H., Buyon, J., Tuschl, T. & Putterman, C. Single-cell RNA sequencing for the study of lupus nephritis. Lupus Sci. Med. 6, e000329 (2019).
Disteldorf, E. M. et al. CXCL5 drives neutrophil recruitment in TH17-mediated GN. J. Am. Soc. Nephrol. 26, 55–66 (2015).
Alli, A. A. et al. Kidney tubular epithelial cell ferroptosis links glomerular injury to tubulointerstitial pathology in lupus nephritis. Clin. Immunol. 248, 109213 (2023).
Giuliani, K. T. K. et al. Hypoxic human proximal tubular epithelial cells undergo ferroptosis and elicit an NLRP3 inflammasome response in CD1c+ dendritic cells. Cell Death Dis. 13, 739 (2022).
Li, H., Boulougoura, A., Endo, Y. & Tsokos, G. C. Abnormalities of T cells in systemic lupus erythematosus: new insights in pathogenesis and therapeutic strategies. J. Autoimmun. 132, 102870 (2022).
Krebs, C. F., Turner, J. E., Riedel, J. H. & Panzer, U. Tissue-specific therapy in immune-mediated kidney diseases: new ARGuments for targeting the IL-23/IL-17 axis. J. Clin. Invest. 131, e150588 (2021).
Dai, H., He, F., Tsokos, G. C. & Kyttaris, V. C. IL-23 limits the production of IL-2 and promotes autoimmunity in lupus. J. Immunol. 199, 903–910 (2017).
Li, P. et al. Glutathione peroxidase 4-regulated neutrophil ferroptosis induces systemic autoimmunity. Nat. Immunol. 22, 1107–1117 (2021).
Yu, H. et al. Mesangial cells exhibit features of antigen-presenting cells and activate CD4+ T cell responses. J. Immunol. Res. 2019, 2121849 (2019).
Fu, R. et al. Podocyte activation of NLRP3 inflammasomes contributes to the development of proteinuria in lupus nephritis. Arthritis Rheumatol. 69, 1636–1646 (2017).
Wright, R. D. & Beresford, M. W. Podocytes contribute, and respond, to the inflammatory environment in lupus nephritis. Am. J. Physiol. Renal Physiol. 315, F1683–F1694 (2018).
Kellum, J. A. et al. Acute kidney injury. Nat. Rev. Dis. Prim. 7, 52 (2021).
He, L. et al. AKI on CKD: heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 92, 1071–1083 (2017).
Venkatachalam, M. A., Weinberg, J. M., Kriz, W. & Bidani, A. K. Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J. Am. Soc. Nephrol. 26, 1765–1776 (2015).
Puthumana, J. et al. Biomarkers of inflammation and repair in kidney disease progression. J. Clin. Invest. 131, e139927 (2021).
Xu, L., Guo, J., Moledina, D. G. & Cantley, L. G. Immune-mediated tubule atrophy promotes acute kidney injury to chronic kidney disease transition. Nat. Commun. 13, 4892 (2022).
Noel, S. et al. Immune checkpoint molecule TIGIT regulates kidney T cell functions and contributes to AKI. J. Am. Soc. Nephrol. 34, 755–771 (2023).
Eleftheriadis, T. et al. A role for human renal tubular epithelial cells in direct allo-recognition by CD4+ T-cells and the effect of ischemia-reperfusion. Int. J. Mol. Sci. 22, 1733 (2021).
Breda, P. C. et al. Renal proximal tubular epithelial cells exert immunomodulatory function by driving inflammatory CD4+ T cell responses. Am. J. Physiol. Renal Physiol. 317, F77–F89 (2019).
Metalidis, C. & Kuypers, D. R. Emerging immunosuppressive drugs in kidney transplantation. Curr. Clin. Pharmacol. 6, 130–136 (2011).
Sis, B. et al. Renal medullary changes in renal allograft recipients with raised serum creatinine. J. Clin. Pathol. 59, 377–381 (2006).
Chessa, F. et al. The renal microenvironment modifies dendritic cell phenotype. Kidney Int. 89, 82–94 (2016).
Thaunat, O. et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc. Natl Acad. Sci. USA 102, 14723–14728 (2005).
Thaunat, O. et al. Chronic rejection triggers the development of an aggressive intragraft immune response through recapitulation of lymphoid organogenesis. J. Immunol. 185, 717–728 (2010).
Koenig, A. & Thaunat, O. Lymphoid neogenesis and tertiary lymphoid organs in transplanted organs. Front. Immunol. 7, 646 (2016).
Koga, T., Ichinose, K., Mizui, M., Crispin, J. C. & Tsokos, G. C. Calcium/calmodulin-dependent protein kinase IV suppresses IL-2 production and regulatory T cell activity in lupus. J. Immunol. 189, 3490–3496 (2012).
Ferretti, A. P., Bhargava, R., Dahan, S., Tsokos, M. G. & Tsokos, G. C. Calcium/Calmodulin kinase IV controls the function of both T cells and kidney resident cells. Front. Immunol. 9, 2113 (2018).
Otomo, K. et al. Cutting edge: nanogel-based delivery of an inhibitor of CaMK4 to CD4+ T cells suppresses experimental autoimmune encephalomyelitis and lupus-like disease in mice. J. Immunol. 195, 5533–5537 (2015).
Bhargava, R. et al. Aberrantly glycosylated IgG elicits pathogenic signaling in podocytes and signifies lupus nephritis. JCI Insight 6, e147789 (2021).
Blanchard, L. & Girard, J. P. High endothelial venules (HEVs) in immunity, inflammation and cancer. Angiogenesis 24, 719–753 (2021).
Bahmani, B. et al. Targeted delivery of immune therapeutics to lymph nodes prolongs cardiac allograft survival. J. Clin. Invest. 128, 4770–4786 (2018).
Jiang, L. et al. Simultaneous targeting of primary tumor, draining lymph node, and distant metastases through high endothelial venule-targeted delivery. Nano Today 36, 101045 (2021).
Morel, L. Immunometabolism in systemic lupus erythematosus. Nat. Rev. Rheumatol. 13, 280–290 (2017).
Kleinewietfeld, M. et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496, 518–522 (2013).
Wu, C. et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 496, 513–517 (2013).
Crispin, J. C. et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J. Immunol. 181, 8761–8766 (2008).
Sharabi, A. et al. Regulatory T cells in the treatment of disease. Nat. Rev. Drug. Discov. 17, 823–844 (2018).
Munoz-Rojas, A. R. & Mathis, D. Tissue regulatory T cells: regulatory chameleons. Nat. Rev. Immunol. 21, 597–611 (2021).
Arpaia, N. et al. A distinct function of regulatory T cells in tissue protection. Cell 162, 1078–1089 (2015).
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed substantially to discussion of the content and wrote the article. G.C.T., A.B., Y.E., R.A. and H.L reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Nephrology thanks Benjamin Stewart, Ulf Panzer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tsokos, G.C., Boulougoura, A., Kasinath, V. et al. The immunoregulatory roles of non-haematopoietic cells in the kidney. Nat Rev Nephrol 20, 206–217 (2024). https://doi.org/10.1038/s41581-023-00786-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-023-00786-x