Abstract
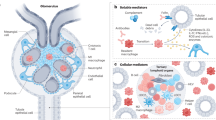
Insights into the relationship between immunometabolism and inflammation have enabled the targeting of several immunity-mediated inflammatory processes that underlie infectious diseases and cancer or drive transplant rejection, but this field remains largely unexplored in kidney diseases. The kidneys comprise heterogeneous cell populations, contain distinct microenvironments such as areas of hypoxia and hypersalinity, and are responsible for a functional triad of filtration, reabsorption and secretion. These distinctive features create myriad potential metabolic therapeutic targets in the kidney. Immune cells have crucial roles in the maintenance of kidney homeostasis and in the response to kidney injury, and their function is intricately connected to their metabolic properties. Changes in nutrient availability and biomolecules, such as cytokines, growth factors and hormones, initiate cellular signalling events that involve energy-sensing molecules and other metabolism-related proteins to coordinate immune cell differentiation, activation and function. Disruption of homeostasis promptly triggers the metabolic reorganization of kidney immune and non-immune cells, which can promote inflammation and tissue damage. The metabolic differences between kidney and immune cells offer an opportunity to specifically target immunometabolism in the kidney.
Key points
-
The unique kidney anatomy and physiology might enable the targeting of immunometabolism as a new therapeutic strategy to treat immunity-driven kidney diseases.
-
Immune and kidney parenchymal cells respond to a variety of stimuli by reprogramming their metabolism, which coordinates their effector functions.
-
Targeting metabolic pathways and energy-sensing molecules has potential to prevent or ameliorate acute kidney injury and chronic kidney disease.
-
Metabolic reprogramming of immune cells towards an anti-inflammatory profile might prevent the establishment of chronic inflammation and contribute to organ preservation after kidney injury.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 395, 709–733 (2020).
Duffield, J. S. Cellular and molecular mechanisms in kidney fibrosis. J. Clin. Invest. 124, 2299–2306 (2014).
Moore, P. K., Hsu, R. K. & Liu, K. D. Management of acute kidney injury: core curriculum 2018. Am. J. Kidney Dis. 72, 136–148 (2018).
Chen, T. K., Knicely, D. H. & Grams, M. E. Chronic kidney disease diagnosis and management: a review. JAMA 322, 1294–1304 (2019).
Perkovic, V. et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 380, 2295–2306 (2019).
Gupta, N. & Wish, J. B. Hypoxia-inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am. J. Kidney Dis. 69, 815–826 (2017).
Marton, A. et al. Organ protection by SGLT2 inhibitors: role of metabolic energy and water conservation. Nat. Rev. Nephrol. 17, 65–77 (2021).
Sato, Y. & Yanagita, M. Immunology of the ageing kidney. Nat. Rev. Nephrol. 15, 625–640 (2019).
Stewart, B. J. et al. Spatiotemporal immune zonation of the human kidney. Science 365, 1461–1466 (2019).
Kurts, C., Panzer, U., Anders, H. J. & Rees, A. J. The immune system and kidney disease: basic concepts and clinical implications. Nat. Rev. Immunol. 13, 738–753 (2013).
Kurts, C., Ginhoux, F. & Panzer, U. Kidney dendritic cells: fundamental biology and functional roles in health and disease. Nat. Rev. Nephrol. 16, 391–407 (2020).
Tang, P. M., Nikolic-Paterson, D. J. & Lan, H. Y. Macrophages: versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 15, 144–158 (2019).
Turner, J. E., Rickassel, C., Healy, H. & Kassianos, A. J. Natural killer cells in kidney health and disease. Front. Immunol. 10, 587 (2019).
Turner, J. E., Becker, M., Mittrucker, H. W. & Panzer, U. Tissue-resident lymphocytes in the kidney. J. Am. Soc. Nephrol. 29, 389–399 (2018).
Oleinika, K., Mauri, C. & Salama, A. D. Effector and regulatory B cells in immune-mediated kidney disease. Nat. Rev. Nephrol. 15, 11–26 (2019).
Brahler, S. et al. Opposing roles of dendritic cell subsets in experimental GN. J. Am. Soc. Nephrol. 29, 138–154 (2018).
Durai, V. & Murphy, K. M. Functions of murine dendritic cells. Immunity 45, 719–736 (2016).
Kohli, K., Janssen, A. & Forster, R. Plasmacytoid dendritic cells induce tolerance predominantly by cargoing antigen to lymph nodes. Eur. J. Immunol. 46, 2659–2668 (2016).
Galicia, G. & Gommerman, J. L. Plasmacytoid dendritic cells and autoimmune inflammation. Biol. Chem. 395, 335–346 (2014).
Larson, S. R. et al. Ly6C+ monocyte efferocytosis and cross-presentation of cell-associated antigens. Cell Death Differ. 23, 997–1003 (2016).
Wang, J. & Kubes, P. A reservoir of mature cavity macrophages that can rapidly invade visceral organs to affect tissue repair. Cell 165, 668–678 (2016).
Breda, C. N. S., Davanzo, G. G., Basso, P. J., Saraiva Camara, N. O. & Moraes-Vieira, P. M. M. Mitochondria as central hub of the immune system. Redox Biol. 26, 101255 (2019).
Nemeth, T., Sperandio, M. & Mocsai, A. Neutrophils as emerging therapeutic targets. Nat. Rev. Drug Discov. 19, 253–275 (2020).
Bogoslowski, A., Butcher, E. C. & Kubes, P. Neutrophils recruited through high endothelial venules of the lymph nodes via PNAd intercept disseminating Staphylococcus aureus. Proc. Natl Acad. Sci. USA 115, 2449–2454 (2018).
Kumar, B. V., Connors, T. J. & Farber, D. L. Human T cell development, localization, and function throughout life. Immunity 48, 202–213 (2018).
Ren, W. et al. Amino-acid transporters in T-cell activation and differentiation. Cell Death Dis. 8, e2655 (2017).
St Paul, M. & Ohashi, P. S. The roles of CD8(+) T cell subsets in antitumor immunity. Trends Cell Biol. 30, 695–704 (2020).
Deseke, M. & Prinz, I. Ligand recognition by the gammadelta TCR and discrimination between homeostasis and stress conditions. Cell Mol. Immunol. 17, 914–924 (2020).
Dellepiane, S., Leventhal, J. S. & Cravedi, P. T cells and acute kidney injury: a two-way relationship. Front. Immunol. 11, 1546 (2020).
Winterberg, P. D. & Ford, M. L. The effect of chronic kidney disease on T cell alloimmunity. Curr. Opin. Organ. Transpl. 22, 22–28 (2017).
Reilly, E. C. et al. TRM integrins CD103 and CD49a differentially support adherence and motility after resolution of influenza virus infection. Proc. Natl Acad. Sci. USA 117, 12306–12314 (2020).
Krebs, C. F. et al. Pathogen-induced tissue-resident memory TH17 (TRM17) cells amplify autoimmune kidney disease. Sci. Immunol. 5, eaba4163 (2020).
Bjorkstrom, N. K., Ljunggren, H. G. & Michaelsson, J. Emerging insights into natural killer cells in human peripheral tissues. Nat. Rev. Immunol. 16, 310–320 (2016).
Carrega, P. et al. CD56brightperforinlow noncytotoxic human NK cells are abundant in both healthy and neoplastic solid tissues and recirculate to secondary lymphoid organs via afferent lymph. J. Immunol. 192, 3805–3815 (2014).
Crosby, C. M. & Kronenberg, M. Tissue-specific functions of invariant natural killer T cells. Nat. Rev. Immunol. 18, 559–574 (2018).
Singh, A. K., Tripathi, P. & Cardell, S. L. Type II NKT cells: an elusive population with immunoregulatory properties. Front. Immunol. 9, 1969 (2018).
Ascon, D. B. et al. Normal mouse kidneys contain activated and CD3+CD4−CD8− double-negative T lymphocytes with a distinct TCR repertoire. J. Leukoc. Biol. 84, 1400–1409 (2008).
Murphy, M. P. & O’Neill, L. A. J. How should we talk about metabolism? Nat. Immunol. 21, 713–715 (2020).
Russell, D. G., Huang, L. & VanderVen, B. C. Immunometabolism at the interface between macrophages and pathogens. Nat. Rev. Immunol. 19, 291–304 (2019).
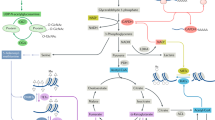
Thorens, B. & Mueckler, M. Glucose transporters in the 21st century. Am. J. Physiol. Endocrinol. Metab. 298, E141–E145 (2010).
Macintyre, A. N. et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 20, 61–72 (2014).
Caro-Maldonado, A. et al. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J. Immunol. 192, 3626–3636 (2014).
Freemerman, A. J. et al. Myeloid Slc2a1-deficient murine model revealed macrophage activation and metabolic phenotype are fueled by GLUT1. J. Immunol. 202, 1265–1286 (2019).
Seki, S. M. & Gaultier, A. Exploring non-metabolic functions of glycolytic enzymes in immunity. Front. Immunol. 8, 1549 (2017).
Alves, R. W., Doretto-Silva, L., da Silva, E. M., Fürstenau, C. R. & Andrade-Oliveira, V. The non-canonical role of metabolic enzymes in immune cells and its impact on diseases. Curr. Tissue Microenviron. Rep. 1, 221–237 (2020).
Wolf, A. J. et al. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell 166, 624–636 (2016).
Damasceno, L. E. A. et al. PKM2 promotes Th17 cell differentiation and autoimmune inflammation by fine-tuning STAT3 activation. J. Exp. Med. 217, e20190613 (2020).
Angiari, S. et al. Pharmacological activation of pyruvate kinase M2 inhibits CD4+ T cell pathogenicity and suppresses autoimmunity. Cell Metab. 31, 391–405.e8 (2020).
Chang, C. H. et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153, 1239–1251 (2013).
Subramanian, A. & Miller, D. M. Structural analysis of alpha-enolase. Mapping the functional domains involved in down-regulation of the c-myc protooncogene. J. Biol. Chem. 275, 5958–5965 (2000).
Wang, R. et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011).
De Rosa, V. et al. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat. Immunol. 16, 1174–1184 (2015).
Patra, K. C. & Hay, N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 39, 347–354 (2014).
Haschemi, A. et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell Metab. 15, 813–826 (2012).
Clarke, A. J., Riffelmacher, T., Braas, D., Cornall, R. J. & Simon, A. K. B1a B cells require autophagy for metabolic homeostasis and self-renewal. J. Exp. Med. 215, 399–413 (2018).
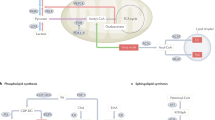
Martinez-Reyes, I. & Chandel, N. S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 11, 102 (2020).
Howie, D. et al. Foxp3 drives oxidative phosphorylation and protection from lipotoxicity. JCI Insight 2, e89160 (2017).
Namgaladze, D. & Brune, B. Macrophage fatty acid oxidation and its roles in macrophage polarization and fatty acid-induced inflammation. Biochim. Biophys. Acta 1861, 1796–1807 (2016).
Qian, X., Yang, Z., Mao, E. & Chen, E. Regulation of fatty acid synthesis in immune cells. Scand. J. Immunol. 88, e12713 (2018).
Kemp, R. G. & Foe, L. G. Allosteric regulatory properties of muscle phosphofructokinase. Mol. Cell Biochem. 57, 147–154 (1983).
Iacobazzi, V. & Infantino, V. Citrate–new functions for an old metabolite. Biol. Chem. 395, 387–399 (2014).
Altman, B. J., Stine, Z. E. & Dang, C. V. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat. Rev. Cancer 16, 619–634 (2016).
Klysz, D. et al. Glutamine-dependent alpha-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci. Signal. 8, ra97 (2015).
Liu, P. S. et al. α-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 18, 985–994 (2017).
Basit, F., Mathan, T., Sancho, D. & de Vries, I. J. M. Human dendritic cell subsets undergo distinct metabolic reprogramming for immune response. Front. Immunol. 9, 2489 (2018).
Lam, W. Y. et al. Mitochondrial pyruvate import promotes long-term survival of antibody-secreting plasma cells. Immunity 45, 60–73 (2016).
Michalek, R. D. et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 (2011).
van der Windt, G. J. et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc. Natl Acad. Sci. USA 110, 14336–14341 (2013).
van der Windt, G. J. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
Wang, Y. P. & Lei, Q. Y. Metabolite sensing and signaling in cell metabolism. Signal. Transduct. Target. Ther. 3, 30 (2018).
Liu, G. Y. & Sabatini, D. M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203 (2020).
Tang, Y. et al. Adipose tissue mTORC2 regulates ChREBP-driven de novo lipogenesis and hepatic glucose metabolism. Nat. Commun. 7, 11365 (2016).
Moloughney, J. G. et al. mTORC2 responds to glutamine catabolite levels to modulate the hexosamine biosynthesis enzyme GFAT1. Mol. Cell 63, 811–826 (2016).
Lee, J. W., Ko, J., Ju, C. & Eltzschig, H. K. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 51, 1–13 (2019).
Waickman, A. T. & Powell, J. D. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunol. Rev. 249, 43–58 (2012).
Powell, J. D., Pollizzi, K. N., Heikamp, E. B. & Horton, M. R. Regulation of immune responses by mTOR. Annu. Rev. Immunol. 30, 39–68 (2012).
Jeon, S. M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 48, e245 (2016).
Galic, S. et al. Hematopoietic AMPK beta1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J. Clin. Invest. 121, 4903–4915 (2011).
Sag, D., Carling, D., Stout, R. D. & Suttles, J. Adenosine 5’-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J. Immunol. 181, 8633–8641 (2008).
Blagih, J. et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity 42, 41–54 (2015).
Rao, E. et al. Deficiency of AMPK in CD8+ T cells suppresses their anti-tumor function by inducing protein phosphatase-mediated cell death. Oncotarget 6, 7944–7958 (2015).
Foretz, M., Guigas, B. & Viollet, B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 15, 569–589 (2019).
Ma, Q. et al. PlGF signaling and macrophage repolarization contribute to the anti-neoplastic effect of metformin. Eur. J. Pharmacol. 863, 172696 (2019).
Cavaglieri, R. C., Day, R. T., Feliers, D. & Abboud, H. E. Metformin prevents renal interstitial fibrosis in mice with unilateral ureteral obstruction. Mol. Cell Endocrinol. 412, 116–122 (2015).
Zhang, D. et al. Metabolic regulation of gene expression by histone lactylation. Nature 574, 575–580 (2019).
Certo, M., Tsai, C. H., Pucino, V., Ho, P. C. & Mauro, C. Lactate modulation of immune responses in inflammatory versus tumour microenvironments. Nat. Rev. Immunol. 21, 151–161 (2021).
Kane, D. A. Lactate oxidation at the mitochondria: a lactate-malate-aspartate shuttle at work. Front. Neurosci. 8, 366 (2014).
Tavakoli, S. et al. Characterization of macrophage polarization states using combined measurement of 2-deoxyglucose and glutamine accumulation: implications for imaging of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 37, 1840–1848 (2017).
Scialo, F., Fernandez-Ayala, D. J. & Sanz, A. Role of mitochondrial reverse electron transport in ROS signaling: potential roles in health and disease. Front. Physiol. 8, 428 (2017).
Garaude, J. et al. Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense. Nat. Immunol. 17, 1037–1045 (2016).
den Besten, G. et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340 (2013).
Andrade-Oliveira, V. et al. Gut bacteria products prevent AKI induced by ischemia-reperfusion. J. Am. Soc. Nephrol. 26, 1877–1888 (2015).
Smith, P. M. et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573 (2013).
Park, J., Goergen, C. J., HogenEsch, H. & Kim, C. H. Chronically elevated levels of short-chain fatty acids induce T cell-mediated ureteritis and hydronephrosis. J. Immunol. 196, 2388–2400 (2016).
Bader, J. E., Voss, K. & Rathmell, J. C. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol. Cell 78, 1019–1033 (2020).
Bettencourt, I. A. & Powell, J. D. Targeting metabolism as a novel therapeutic approach to autoimmunity, inflammation, and transplantation. J. Immunol. 198, 999–1005 (2017).
Lee, J. B., Vance, V. K. & Cahill, G. F. Jr. Metabolism of C14-labeled substrates by rabbit kidney cortex and medulla. Am. J. Physiol. 203, 27–36 (1962).
Chan, D. A. et al. Targeting GLUT1 and the Warburg effect in renal cell carcinoma by chemical synthetic lethality. Sci. Transl. Med. 3, 94ra70 (2011).
Di Dedda, C., Vignali, D., Piemonti, L. & Monti, P. Pharmacological targeting of GLUT1 to control autoreactive T cell responses. Int. J. Mol. Sci. 20, 4962 (2019).
Ma, R. et al. A Pck1-directed glycogen metabolic program regulates formation and maintenance of memory CD8+ T cells. Nat. Cell Biol. 20, 21–27 (2018).
Masopust, D., Vezys, V., Marzo, A. L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Aichler, M. & Walch, A. MALDI imaging mass spectrometry: current frontiers and perspectives in pathology research and practice. Lab. Invest. 95, 422–431 (2015).
Zhou, T. T. et al. Small molecule IVQ, as a prodrug of gluconeogenesis inhibitor QVO, efficiently ameliorates glucose homeostasis in type 2 diabetic mice. Acta Pharmacol. Sin. 40, 1193–1204 (2019).
Andrade-Oliveira, V., Foresto-Neto, O., Watanabe, I. K. M., Zatz, R. & Camara, N. O. S. Inflammation in renal diseases: new and old players. Front. Pharmacol. 10, 1192 (2019).
Perico, N., Cattaneo, D., Sayegh, M. H. & Remuzzi, G. Delayed graft function in kidney transplantation. Lancet 364, 1814–1827 (2004).
Kako, K., Kato, M., Matsuoka, T. & Mustapha, A. Depression of membrane-bound Na+-K+-ATPase activity induced by free radicals and by ischemia of kidney. Am. J. Physiol. 254, C330–C337 (1988).
Inoki, K. et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J. Clin. Invest. 121, 2181–2196 (2011).
Godel, M. et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Invest. 121, 2197–2209 (2011).
Grahammer, F. et al. mTOR regulates endocytosis and nutrient transport in proximal tubular cells. J. Am. Soc. Nephrol. 28, 230–241 (2017).
Lee, H. et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 22, 225–234 (2020).
Lieberthal, W., Tang, M., Lusco, M., Abate, M. & Levine, J. S. Preconditioning mice with activators of AMPK ameliorates ischemic acute kidney injury in vivo. Am. J. Physiol. Renal Physiol. 311, F731–F739 (2016).
Efeyan, A. et al. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature 493, 679–683 (2013).
Jones, R. G. & Pearce, E. J. MenTORing immunity: mTOR signaling in the development and function of tissue-resident immune cells. Immunity 46, 730–742 (2017).
Chen, G. et al. mTOR signaling regulates protective activity of transferred CD4+Foxp3+ T cells in repair of acute kidney injury. J. Immunol. 197, 3917–3926 (2016).
Zeng, H. et al. mTORC1 couples immune signals and metabolic programming to establish Treg-cell function. Nature 499, 485–490 (2013).
Meng, X. et al. Hypoxia-inducible factor-1alpha is a critical transcription factor for IL-10-producing B cells in autoimmune disease. Nat. Commun. 9, 251 (2018).
Schreiber, K. H. et al. A novel rapamycin analog is highly selective for mTORC1 in vivo. Nat. Commun. 10, 3194 (2019).
Cameron, A. M. et al. Inflammatory macrophage dependence on NAD+ salvage is a consequence of reactive oxygen species-mediated DNA damage. Nat. Immunol. 20, 420–432 (2019).
Fortner, K. A. et al. Targeting mitochondrial oxidative stress with MitoQ reduces NET formation and kidney disease in lupus-prone MRL-lpr mice. Lupus Sci. Med. 7, e000387 (2020).
Xiao, L. et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 11, 297–311 (2017).
Qiu, J. et al. Acetate promotes T cell effector function during glucose restriction. Cell Rep. 27, 2063–2074.e5 (2019).
Meyer, F. et al. Propionate supplementation promotes the expansion of peripheral regulatory T-cells in patients with end-stage renal disease. J. Nephrol. 33, 817–827 (2020).
Zheng, Z. et al. Enhanced glycolytic metabolism contributes to cardiac dysfunction in polymicrobial sepsis. J. Infect. Dis. 215, 1396–1406 (2017).
McCall, C. E. et al. Pyruvate dehydrogenase complex stimulation promotes immunometabolic homeostasis and sepsis survival. JCI Insight 3, e99292 (2018).
Giustina, A. D. et al. Dimethyl fumarate modulates oxidative stress and inflammation in organs after sepsis in rats. Inflammation 41, 315–327 (2018).
Liao, S. T. et al. 4-Octyl itaconate inhibits aerobic glycolysis by targeting GAPDH to exert anti-inflammatory effects. Nat. Commun. 10, 5091 (2019).
Nikolic-Paterson, D. J., Wang, S. & Lan, H. Y. Macrophages promote renal fibrosis through direct and indirect mechanisms. Kidney Int. Suppl. 4, 34–38 (2014).
Ding, H. et al. Inhibiting aerobic glycolysis suppresses renal interstitial fibroblast activation and renal fibrosis. Am. J. Physiol. Renal Physiol. 313, F561–F575 (2017).
Wei, Q. et al. Glycolysis inhibitors suppress renal interstitial fibrosis via divergent effects on fibroblasts and tubular cells. Am. J. Physiol. Renal Physiol. 316, F1162–F1172 (2019).
Oh, C. J. et al. Dimethylfumarate attenuates renal fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-beta/Smad signaling. PLoS ONE 7, e45870 (2012).
Smith, J. A., Stallons, L. J. & Schnellmann, R. G. Renal cortical hexokinase and pentose phosphate pathway activation through the EGFR/Akt signaling pathway in endotoxin-induced acute kidney injury. Am. J. Physiol. Renal Physiol. 307, F435–F444 (2014).
Grayson, P. C. et al. Metabolic pathways and immunometabolism in rare kidney diseases. Ann. Rheum. Dis. 77, 1226–1233 (2018).
Ghergurovich, J. M. et al. A small molecule G6PD inhibitor reveals immune dependence on pentose phosphate pathway. Nat. Chem. Biol. 16, 731–739 (2020).
Allison, A. C. & Eugui, E. M. The design and development of an immunosuppressive drug, mycophenolate mofetil. Springer Semin. Immunopathol. 14, 353–380 (1993).
Allison, A. C. & Eugui, E. M. Purine metabolism and immunosuppressive effects of mycophenolate mofetil (MMF). Clin. Transpl. 10, 77–84 (1996).
Feldkamp, T. et al. Evidence for involvement of nonesterified fatty acid-induced protonophoric uncoupling during mitochondrial dysfunction caused by hypoxia and reoxygenation. Nephrol. Dial. Transpl. 24, 43–51 (2009).
Bienholz, A. et al. Adverse effects of alpha-ketoglutarate/malate in a rat model of acute kidney injury. Am. J. Physiol. Renal Physiol. 303, F56–F63 (2012).
Hou, E. et al. Malate and aspartate increase L-arginine and nitric oxide and attenuate hypertension. Cell Rep. 19, 1631–1639 (2017).
Le, A. et al. Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab. 15, 110–121 (2012).
Xu, X. et al. Overview of the development of glutaminase inhibitors: achievements and future directions. J. Med. Chem. 62, 1096–1115 (2019).
Flowers, E. M. et al. Lkb1 deficiency confers glutamine dependency in polycystic kidney disease. Nat. Commun. 9, 814 (2018).
Nguyen, H. D. et al. Metabolic reprogramming of alloantigen-activated T cells after hematopoietic cell transplantation. J. Clin. Invest. 126, 1337–1352 (2016).
Gatza, E. et al. Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease. Sci. Transl. Med. 3, 67ra68 (2011).
Gerner, R. R. et al. Targeting NAD immunometabolism limits severe graft-versus-host disease and has potent antileukemic activity. Leukemia 34, 1885–1897 (2020).
Lee, C. F. et al. Preventing allograft rejection by targeting immune metabolism. Cell Rep. 13, 760–770 (2015).
Markey, K. A. et al. Microbe-derived short chain fatty acids butyrate and propionate are associated with protection from chronic GVHD. Blood 136, 130–136 (2020).
Wu, H. et al. Gut microbial metabolites induce donor-specific tolerance of kidney allografts through induction of T regulatory cells by short-chain fatty acids. J. Am. Soc. Nephrol. 31, 1445–1461 (2020).
Mishra, M. K. et al. Laquinimod reduces neuroaxonal injury through inhibiting microglial activation. Ann. Clin. Transl. Neurol. 1, 409–422 (2014).
Slattery, K. & Gardiner, C. M. NK cell metabolism and TGFbeta – implications for immunotherapy. Front. Immunol. 10, 2915 (2019).
Fernandez, H. R. et al. The mitochondrial citrate carrier, SLC25A1, drives stemness and therapy resistance in non-small cell lung cancer. Cell Death Differ. 25, 1239–1258 (2018).
Chang, C. H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015).
Gemta, L. F. et al. Impaired enolase 1 glycolytic activity restrains effector functions of tumor-infiltrating CD8+ T cells. Sci. Immunol. 4, eaap9520 (2019).
Labani-Motlagh, A., Ashja-Mahdavi, M. & Loskog, A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front. Immunol. 11, 940 (2020).
Sukumar, M. et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Invest. 123, 4479–4488 (2013).
Leone, R. D. et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science 366, 1013–1021 (2019).
Schulte, M. L. et al. Pharmacological blockade of ASCT2-dependent glutamine transport leads to antitumor efficacy in preclinical models. Nat. Med. 24, 194–202 (2018).
Oh, M. H. et al. Targeting glutamine metabolism enhances tumor specific immunity by modulating suppressive myeloid cells. J. Clin. Invest. 130, 3865–3884 (2020).
Rini, B. I., Campbell, S. C. & Escudier, B. Renal cell carcinoma. Lancet 373, 1119–1132 (2009).
Wettersten, H. I. Reprogramming of metabolism in kidney cancer. Semin. Nephrol. 40, 2–13 (2020).
Simon, A. G. et al. Targeting glycolysis with 2-deoxy-D-glucose sensitizes primary cell cultures of renal cell carcinoma to tyrosine kinase inhibitors. J. Cancer Res. Clin. Oncol. 146, 2255–2265 (2020).
Kuang, H. et al. Therapeutic effect of sodium glucose Co-transporter 2 inhibitor dapagliflozin on renal cell carcinoma. Med. Sci. Monit. 23, 3737–3745 (2017).
Zhang, Q. et al. Overexpression of G6PD represents a potential prognostic factor in clear cell renal cell carcinoma. J. Cancer 8, 665–673 (2017).
Jiang, P., Du, W. & Wu, M. Regulation of the pentose phosphate pathway in cancer. Protein Cell 5, 592–602 (2014).
Mele, L. et al. A new inhibitor of glucose-6-phosphate dehydrogenase blocks pentose phosphate pathway and suppresses malignant proliferation and metastasis in vivo. Cell Death Dis. 9, 572 (2018).
Matsumoto, K., Fujiwara, Y., Nagai, R., Yoshida, M. & Ueda, S. Expression of two isozymes of acyl-coenzyme A: cholesterol acyltransferase-1 and -2 in clear cell type renal cell carcinoma. Int. J. Urol. 15, 166–170 (2008).
Yang, W. et al. Potentiating the antitumour response of CD8+ T cells by modulating cholesterol metabolism. Nature 531, 651–655 (2016).
Pearce, E. L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
Bruce, J. Y. et al. A phase II study of 2-methoxyestradiol nanocrystal colloidal dispersion alone and in combination with sunitinib malate in patients with metastatic renal cell carcinoma progressing on sunitinib malate. Invest. New Drugs 30, 794–802 (2012).
Ronnen, E. A. et al. A phase II trial of 17-(Allylamino)-17-demethoxygeldanamycin in patients with papillary and clear cell renal cell carcinoma. Invest. New Drugs 24, 543–546 (2006).
Jeong, W. et al. Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1α), in patients with refractory solid tumors. Cancer Chemother. Pharmacol. 73, 343–348 (2014).
Felizardo, R. J. F. et al. Gut microbial metabolite butyrate protects against proteinuric kidney disease through epigenetic- and GPR109a-mediated mechanisms. FASEB J. 33, 11894–11908 (2019).
Hoste, E. A. et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 41, 1411–1423 (2015).
Leemans, J. C., Kors, L., Anders, H. J. & Florquin, S. Pattern recognition receptors and the inflammasome in kidney disease. Nat. Rev. Nephrol. 10, 398–414 (2014).
Zindel, J. & Kubes, P. DAMPs, PAMPs, and LAMPs in immunity and sterile inflammation. Annu. Rev. Pathol. 15, 493–518 (2020).
Chawla, L. S. & Kimmel, P. L. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 82, 516–524 (2012).
Wang, Z. et al. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am. J. Clin. Nutr. 92, 1369–1377 (2010).
O’Connor, P. M. Renal oxygen delivery: matching delivery to metabolic demand. Clin. Exp. Pharmacol. Physiol. 33, 961–967 (2006).
Mather, A. & Pollock, C. Glucose handling by the kidney. Kidney Int. 79 (Suppl. 120), S1–S6 (2011).
Acknowledgements
The authors are extremely thankful to A. Stacy at the National Institute of Allergy and Infectious Diseases, National Institute of Health (NAID/NIH) for carefully reading the manuscript before submission. This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2015/26682-6; 2017/02564-7; 2019/14755-0), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), financial code 001, CAPES COFECUB and The PEW Latin American fellowship from the Pew Charitable Trusts (VA-O). The authors apologize to all colleagues whose work could not be cited owing to space restrictions.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, made substantial contributions to discussions of the content, wrote the manuscript, and reviewed or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Nephrology thanks T. Huber, C. Mauro and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Immunosenescence
-
Ageing-associated changes in the immune system.
- Contraction phase
-
A phase of inflammatory response whereby homeostasis is restored, and effector immune cells die or become memory cells.
- Anaplerotic reactions
-
Metabolic reactions aimed at replenishing tricarboxylic acid cycle intermediates.
- Lactylation
-
Post-translational modification characterized by the addition of a lactyl group to a histone.
- Ferroptotic cell death
-
An iron-dependent form of cell death.
Rights and permissions
About this article
Cite this article
Basso, P.J., Andrade-Oliveira, V. & Câmara, N.O.S. Targeting immune cell metabolism in kidney diseases. Nat Rev Nephrol 17, 465–480 (2021). https://doi.org/10.1038/s41581-021-00413-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41581-021-00413-7
This article is cited by
-
Drp1: Focus on Diseases Triggered by the Mitochondrial Pathway
Cell Biochemistry and Biophysics (2024)
-
Label-Free and Real-Time Electrical Impedance Monitoring of Macrophage Polarization of THP-1 Monocytes on Indium Tin Oxide Electrode
BioChip Journal (2024)
-
Mitochondrial control of inflammation
Nature Reviews Immunology (2023)
-
Chemiluminescence Immunoassay Method of Urinary Liver Fatty-acid-binding Protein as a Promising Candidate for Kidney Disease
Journal of Fluorescence (2023)
-
Immune cell metabolism and metabolic reprogramming
Molecular Biology Reports (2022)