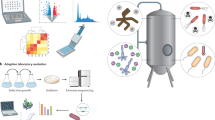
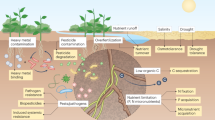
Abstract
Microorganisms are a promising means to address many societal sustainability challenges owing to their ability to thrive in diverse environments and interface with the microscale chemical world via diverse metabolic capacities. Synthetic biology can engineer microorganisms by rewiring their regulatory networks or introducing new functionalities, enhancing their utility for target applications. In this Review, we provide a broad, high-level overview of various research efforts addressing sustainability challenges through synthetic biology, emphasizing foundational microbiological research questions that can accelerate the development of these efforts. We introduce an organizational framework that categorizes these efforts along three domains — factory, farm and field — that are defined by the extent to which the engineered microorganisms interface with the natural external environment. Different application areas within the same domain share many fundamental challenges, highlighting productive opportunities for cross-disciplinary collaborations between researchers working in historically disparate fields.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
IPCC. Climate Change and Land: an IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems (eds Shukla, P .R. et al.) (IPCC, 2019).
IPCC. Climate Change 2022: Impacts, Adaptation and Vulnerability (eds Pörtner, H.-O. et al.) (IPCC, 2022).
Jansson, C. G. & Northen, T. Calcifying cyanobacteria — the potential of biomineralization for carbon capture and storage. Curr. Opin. Biotechnol. 21, 365–371 (2010).
van Aswegen, P. C., van Niekerk, J. & Olivier, W. in Biomining (eds Rawlings, D. E. & Johnson, D. B.) 1–33 (Springer, 2007).
Li, J., Yang, H., Tong, L. & Sand, W. Some aspects of industrial heap bioleaching technology: from basics to practice. Miner. Process. Extr. Metall. Rev. 43, 510–528 (2022).
Marcellin, E. et al. Low carbon fuels and commodity chemicals from waste gases — systematic approach to understand energy metabolism in a model acetogen. Green Chem. 18, 3020–3028 (2016).
Hwang, I. Y. et al. Biological conversion of methane to chemicals and fuels: technical challenges and issues. Appl. Microbiol. Biotechnol. 102, 3071–3080 (2018).
Torella, J. P. et al. Efficient solar-to-fuels production from a hybrid microbial–water-splitting catalyst system. Proc. Natl Acad. Sci. USA 112, 2337–2342 (2015). This article introduces a novel hybrid organic–inorganic system that converts solar energy directly into biomass.
Liew, F. E. et al. Carbon-negative production of acetone and isopropanol by gas fermentation at industrial pilot scale. Nat. Biotechnol. 40, 335–344 (2022). This article demonstrates the potential for commodity chemical production at industrial scale using acetogen-based gas fermentation.
Klemenčič, M. et al. in Microalgae-Based Biofuels and Bioproducts (eds Gonzalez-Fernandez, C. & Muñoz, R.) 305–325 (Woodhead, 2017).
Liu, C., Colón, B. C., Ziesack, M., Silver, P. A. & Nocera, D. G. Water splitting–biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 352, 1210–1213 (2016). This article showcases the potential of genetic engineering by improving a hybrid inorganic–organic solar-to-biomass system.
Nangle, S. N. et al. Valorization of CO2 through lithoautotrophic production of sustainable chemicals in Cupriavidus necator. Metab. Eng. 62, 207–220 (2020).
Chen, J. S. et al. Production of fatty acids in Ralstonia eutropha H16 by engineering β-oxidation and carbon storage. PeerJ 3, e1468 (2015).
Rodríguez, Y., Firmino, P. I. M., Pérez, V., Lebrero, R. & Muñoz, R. Biogas valorization via continuous polyhydroxybutyrate production by Methylocystis hirsuta in a bubble column bioreactor. Waste Manag. 113, 395–403 (2020).
Raberg, M., Volodina, E., Lin, K. & Steinbüchel, A. Ralstonia eutropha H16 in progress: applications beside PHAs and establishment as production platform by advanced genetic tools. Crit. Rev. Biotechnol. 38, 494–510 (2018).
Xiong, B. et al. Genome editing of Ralstonia eutropha using an electroporation-based CRISPR-Cas9 technique. Biotechnol. Biofuels 11, 172 (2018).
Averesch, N. J. et al. High-performance polyesters from carbon dioxide — novel polyhydroxyarylates from engineered Cupriavidus necator. Preprint at bioRxiv https://doi.org/10.1101/2021.12.12.472320 (2023).
Cantera, S. et al. A systematic comparison of ectoine production from upgraded biogas using Methylomicrobium alcaliphilum and a mixed haloalkaliphilic consortium. Waste Manag. 102, 773–781 (2020).
Sherbo, R. S., Silver, P. A. & Nocera, D. G. Riboflavin synthesis from gaseous nitrogen and carbon dioxide by a hybrid inorganic-biological system. Proc. Natl Acad. Sci. USA 119, e2210538119 (2022).
Khoshnevisan, B., Tsapekos, P., Zhang, Y., Valverde-Pérez, B. & Angelidaki, I. Urban biowaste valorization by coupling anaerobic digestion and single cell protein production. Bioresour. Technol. 290, 121743 (2019).
Zha, X. et al. Bioconversion of wastewater to single cell protein by methanotrophic bacteria. Bioresour. Technol. 320, 124351 (2021).
Graham, A. E. & Ledesma-Amaro, R. The microbial food revolution. Nat. Commun. 14, 2231 (2023).
Clomburg, J. M., Crumbley, A. M. & Gonzalez, R. Industrial biomanufacturing: the future of chemical production. Science 355, aag0804 (2017).
Cho, J. S., Kim, G. B., Eun, H., Moon, C. W. & Lee, S. Y. Designing microbial cell factories for the production of chemicals. JACS Au 2, 1781–1799 (2022).
Crater, J. S. & Lievense, J. C. Scale-up of industrial microbial processes. FEMS Microbiol. Lett. 365, fny138 (2018).
Lee, S. Y. et al. A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2, 18–33 (2019).
Wehrs, M. et al. Engineering robust production microbes for large-scale cultivation. Trends Microbiol. 27, 524–537 (2019).
Nikel, P. I. & de Lorenzo, V. Pseudomonas putida as a functional chassis for industrial biocatalysis: from native biochemistry to trans-metabolism. Metab. Eng. 50, 142–155 (2018).
Cordell, W. T., Avolio, G., Takors, R. & Pfleger, B. F. Milligrams to kilograms: making microbes work at scale. Trends Biotechnol. 41, 1442–1457 (2023).
Fabris, M. et al. Emerging technologies in algal biotechnology: toward the establishment of a sustainable, algae-based bioeconomy. Front. Plant Sci. 11, 279 (2020).
Tanner, R. S., Miller, L. M. & Yang, D. Clostridium ljungdahlii sp. nov., an acetogenic species in clostridial rRNA homology group I. Int. J. Syst. Bacteriol. 43, 232–236 (1993).
Takors, R. et al. Using gas mixtures of CO, CO2 and H2 as microbial substrates: the do’s and don’ts of successful technology transfer from laboratory to production scale. Microb. Biotechnol. 11, 606–625 (2018).
LanzaTech. New waste-to-ethanol facility in Japan turns municipal solid waste into products. https://lanzatech.com/new-waste-to-ethanol-facility-in-japan-turns-municipal-solid-waste-into-products/ (2022).
Bioenergy International. LanzaTech commission world’s first commercial waste gas to ethanol plant. https://bioenergyinternational.com/lanzatech-commission-worlds-first-commercial-waste-gas-ethanol-plant-china/ (2018).
Sahoo, K. K., Goswami, G. & Das, D. Biotransformation of methane and carbon dioxide into high-value products by methanotrophs: current state of art and future prospects. Front. Microbiol. 12, 636486 (2021).
Pieja, A. J., Morse, M. C. & Cal, A. J. Methane to bioproducts: the future of the bioeconomy? Curr. Opin. Chem. Biol. 41, 123–131 (2017).
Nguyen, A. D. & Lee, E. Y. Engineered methanotrophy: a sustainable solution for methane-based industrial biomanufacturing. Trends Biotechnol. 39, 381–396 (2021).
Cantera, S. et al. Bio-conversion of methane into high profit margin compounds: an innovative, environmentally friendly and cost-effective platform for methane abatement. World J. Microbiol. Biotechnol. 35, 16 (2019).
Cantera, S. et al. Technologies for the bioconversion of methane into more valuable products. Curr. Opin. Biotechnol. 50, 128–135 (2018).
Claassens, N. J. et al. Replacing the Calvin cycle with the reductive glycine pathway in Cupriavidus necator. Metab. Eng. 62, 30–41 (2020).
Alagesan, S. et al. Functional genetic elements for controlling gene expression in Cupriavidus necator H16. Appl. Environ. Microbiol. 84, e00878-18 (2018).
Sydow, A. et al. Expanding the genetic tool box for Cupriavidus necator by a stabilized L-rhamnose inducible plasmid system. J. Biotechnol. 263, 1–10 (2017).
Gleizer, S. et al. Conversion of Escherichia coli to generate all biomass carbon from CO2. Cell 179, 1255–1263.e12 (2019). This article demonstrates the ability of synthetic microbiologists to use continuous evolution to rewire a heterotrophic organism to autotrophy.
Kim, S. et al. Growth of E. coli on formate and methanol via the reductive glycine pathway. Nat. Chem. Biol. 16, 538–545 (2020).
Chen, F. Y.-H., Jung, H.-W., Tsuei, C.-Y. & Liao, J. C. Converting Escherichia coli to a synthetic methylotroph growing solely on methanol. Cell 182, 933–946.e14 (2020).
Keller, P. et al. Generation of an Escherichia coli strain growing on methanol via the ribulose monophosphate cycle. Nat. Commun. 13, 5243 (2022).
Weijma, J., Wolthoorn, A. & Huisman, J. Solutions in practice for removal of selenium and molybdenum. Proc. Eur. Metall. Conf. EMC 2007, 519–527 (2007).
Hageman, S. P. W., Weijden, R. D., van der, Stams, A. J. M., van Cappellen, P. & Buisman, C. J. N. Microbial selenium sulfide reduction for selenium recovery from wastewater. J. Hazard. Mater. 329, 110–119 (2017).
Jeswiet, J. & Szekeres, A. Energy consumption in mining comminution. Procedia CIRP 48, 140–145 (2016).
Allen, M. A high-level study into mining energy use for the key mineral commodities of the future. CEEC International https://www.ceecthefuture.org/resources/mining-energy-consumption-2021 (2021).
Johnson, D. B. Biomining goes underground. Nat. Geosci. 8, 165–166 (2015).
Pakostova, E., Grail, B. M. & Johnson, D. B. Indirect oxidative bioleaching of a polymetallic black schist sulfide ore. Miner. Eng. 106, 102–107 (2017).
Bomberg, M., Miettinen, H. & Kinnunen, P. The diverse indigenous bacterial community in the Rudna mine does not cause dissolution of copper from Kupferschiefer in oxic conditions. Minerals 12, 366 (2022).
Vera, M., Schippers, A. & Sand, W. Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation — part A. Appl. Microbiol. Biotechnol. 97, 7529–7541 (2013).
Riekkola-Vanhanen, M. & Palmu, L. in Ni-Co 2013 (eds Battle, T. et al.) 269–278 (Springer, 2016).
Olson, G. J. & Clark, T. R. Bioleaching of molybdenite. Hydrometallurgy 93, 10–15 (2008).
Vahidi, E. & Zhao, F. in REWAS 2016: Towards Materials Resource Sustainability (eds Kirchain, R. E. et al.) 113–120 (Springer, 2016).
Arshi, P. S., Vahidi, E. & Zhao, F. Behind the scenes of clean energy: the environmental footprint of rare earth products. ACS Sustain. Chem. Eng. 6, 3311–3320 (2018).
Cheisson, T. & Schelter, E. J. Rare earth elements: Mendeleev’s bane, modern marvels. Science 363, 489–493 (2019).
Voncken, J. H. L. The Rare Earth Elements (Springer, 2016).
Shin, D., Kim, J., Kim, B., Jeong, J. & Lee, J. Use of phosphate solubilizing bacteria to leach rare earth elements from monazite-bearing ore. Minerals 5, 189–202 (2015).
Corbett, M. K., Eksteen, J. J., Niu, X.-Z., Croue, J.-P. & Watkin, E. L. J. Interactions of phosphate solubilising microorganisms with natural rare-earth phosphate minerals: a study utilizing Western Australian monazite. Bioprocess. Biosyst. Eng. 40, 929–942 (2017).
Zeng, Q., Wu, X., Wang, J. & Ding, X. Phosphate solubilization and gene expression of phosphate-solubilizing bacterium Burkholderia multivorans WS-FJ9 under different levels of soluble phosphate. J. Microbiol. Biotechnol. 27, 844–855 (2017).
Fathollahzadeh, H., Becker, T., Eksteen, J. J., Kaksonen, A. H. & Watkin, E. L. J. Microbial contact enhances bioleaching of rare earth elements. Bioresour. Technol. Rep. 3, 102–108 (2018).
Zhang, L. et al. Bioleaching of rare earth elements from bastnaesite-bearing rock by actinobacteria. Chem. Geol. 483, 544–557 (2018).
Cockell, C. S. et al. Space station biomining experiment demonstrates rare earth element extraction in microgravity and Mars gravity. Nat. Commun. 11, 5523 (2020).
Schmitz, A. M. et al. Generation of a Gluconobacter oxydans knockout collection for improved extraction of rare earth elements. Nat. Commun. 12, 6693 (2021).
Schmitz, A. M. et al. High efficiency rare earth element biomining with systems biology guided engineering of Gluconobacter oxydans. Preprint at bioRxiv https://doi.org/10.1101/2023.02.09.527855 (2023).
Mattocks, J. A. et al. Enhanced rare-earth separation with a metal-sensitive lanmodulin dimer. Nature 618, 87–93 (2023). This article reports the discovery and structure of a novel dimeric lanmodulin and demonstrates its utility through the creation of a single-stage purification system capable of separating heavy and light rare earth elements to >98% purity.
Cook, E. C., Featherston, E. R., Showalter, S. A. & Cotruvo, J. A. Structural basis for rare earth element recognition by Methylobacterium extorquens lanmodulin. Biochemistry 58, 120–125 (2019).
Deblonde, G. J.-P. et al. Selective and efficient biomacromolecular extraction of rare-earth elements using lanmodulin. Inorg. Chem. 59, 11855–11867 (2020).
Featherston, E. R. & Cotruvo, J. A. The biochemistry of lanthanide acquisition, trafficking, and utilization. Biochim. Biophys. Acta Mol. Cell Res. 1868, 118864 (2021).
Zytnick, A. M. et al. Discovery and characterization of the first known biological lanthanide chelator. Preprint at bioRxiv https://doi.org/10.1101/2022.01.19.476857 (2023).
Wegner, C.-E. et al. Extracellular and intracellular lanthanide accumulation in the methylotrophic Beijerinckiaceae bacterium RH AL1. Appl. Environ. Microbiol. 87, e03144–20 (2021).
Medin, S. et al. Genomic characterization of rare earth binding by Shewanella oneidensis. Sci. Rep. 13, 15975 (2023).
Dong, Z. et al. Bridging hydrometallurgy and biochemistry: a protein-based process for recovery and separation of rare earth elements. ACS Cent. Sci. 7, 1798–1808 (2021).
Carrasco-López, C., García-Echauri, S. A., Kichuk, T. & Avalos, J. L. Optogenetics and biosensors set the stage for metabolic cybergenetics. Curr. Opin. Biotechnol. 65, 296–309 (2020).
Nasu, Y., Shen, Y., Kramer, L. & Campbell, R. E. Structure- and mechanism-guided design of single fluorescent protein-based biosensors. Nat. Chem. Biol. 17, 509–518 (2021).
Nadler, D. C., Morgan, S.-A., Flamholz, A., Kortright, K. E. & Savage, D. F. Rapid construction of metabolite biosensors using domain-insertion profiling. Nat. Commun. 7, 12266 (2016).
Yaginuma, H. et al. Diversity in ATP concentrations in a single bacterial cell population revealed by quantitative single-cell imaging. Sci. Rep. 4, 6522 (2014).
Greenwald, E. C., Mehta, S. & Zhang, J. Genetically encoded fluorescent biosensors illuminate the spatiotemporal regulation of signaling networks. Chem. Rev. 118, 11707–11794 (2018).
Hackley, C. R., Mazzoni, E. O. & Blau, J. cAMPr: a single-wavelength fluorescent sensor for cyclic AMP. Sci. Signal. 11, eaah3738 (2018).
Hu, H. et al. Glucose monitoring in living cells with single fluorescent protein-based sensors. RSC Adv. 8, 2485–2489 (2018).
Kostyuk, A. I., Demidovich, A. D., Kotova, D. A., Belousov, V. V. & Bilan, D. S. Circularly permuted fluorescent protein-based indicators: history, principles, and classification. Int. J. Mol. Sci. 20, 4200 (2019).
Baumschlager, A. & Khammash, M. Synthetic biological approaches for optogenetics and tools for transcriptional light-control in bacteria. Adv. Biol. 5, 2000256 (2021).
Baumschlager, A., Aoki, S. K. & Khammash, M. Dynamic blue light-inducible T7 RNA polymerases (opto-t7rnaps) for precise spatiotemporal gene expression control. ACS Synth. Biol. 6, 2157–2167 (2017).
Romano, E. et al. Engineering AraC to make it responsive to light instead of arabinose. Nat. Chem. Biol. 17, 817–827 (2021).
Fernandez-Rodriguez, J., Moser, F., Song, M. & Voigt, C. A. Engineering RGB color vision into Escherichia coli. Nat. Chem. Biol. 13, 706–708 (2017).
Weber, A. M. et al. A blue light receptor that mediates RNA binding and translational regulation. Nat. Chem. Biol. 15, 1085–1092 (2019).
Chen, X. et al. An extraordinary stringent and sensitive light-switchable gene expression system for bacterial cells. Cell Res. 26, 854–857 (2016).
Li, X. et al. A single-component light sensor system allows highly tunable and direct activation of gene expression in bacterial cells. Nucleic Acids Res. 48, e33 (2020).
Pu, J., Zinkus-Boltz, J. & Dickinson, B. C. Evolution of a split RNA polymerase as a versatile biosensor platform. Nat. Chem. Biol. 13, 432–438 (2017).
Multamäki, E. et al. Optogenetic control of bacterial expression by red light. ACS Synth. Biol. 11, 3354–3367 (2022).
Piraner, D. I., Wu, Y. & Shapiro, M. G. Modular thermal control of protein dimerization. ACS Synth. Biol. 8, 2256–2262 (2019).
Xiong, L. L., Garrett, M. A., Buss, M. T., Kornfield, J. A. & Shapiro, M. G. Tunable temperature-sensitive transcriptional activation based on lambda repressor. ACS Synth. Biol. 11, 2518–2522 (2022).
Chee, W. K. D., Yeoh, J. W., Dao, V. L. & Poh, C. L. Highly reversible tunable thermal-repressible split-T7 RNA polymerases (Thermal-T7RNAPs) for dynamic gene regulation. ACS Synth. Biol. 11, 921–937 (2022).
Bhokisham, N. et al. A redox-based electrogenetic CRISPR system to connect with and control biological information networks. Nat. Commun. 11, 2427 (2020).
Tschirhart, T. et al. Electronic control of gene expression and cell behaviour in Escherichia coli through redox signalling. Nat. Commun. 8, 14030 (2017).
Lawrence, J. M. et al. Synthetic biology and bioelectrochemical tools for electrogenetic system engineering. Sci. Adv. 8, eabm5091 (2022).
Terrell, J. L. et al. Bioelectronic control of a microbial community using surface-assembled electrogenetic cells to route signals. Nat. Nanotechnol. 16, 688–697 (2021).
Wu, D. et al. Biomolecular actuators for genetically selective acoustic manipulation of cells. Sci. Adv. 9, eadd9186 (2023).
Halleran, A. D. & Murray, R. M. Cell-free and in vivo characterization of Lux, Las, and Rpa quorum activation systems in E. coli. ACS Synth. Biol. 7, 752–755 (2018).
Schuster, L. A. & Reisch, C. R. A plasmid toolbox for controlled gene expression across the Proteobacteria. Nucleic Acids Res. 49, 7189–7202 (2021).
Meyer, A. J., Segall-Shapiro, T. H., Glassey, E., Zhang, J. & Voigt, C. A. Escherichia coli ‘Marionette’ strains with 12 highly optimized small-molecule sensors. Nat. Chem. Biol. 15, 196–204 (2019).
Klewer, L. & Wu, Y. Light‐induced dimerization approaches to control cellular processes. Chem. Weinh. Bergstr. Ger. 25, 12452–12463 (2019).
Haskett, T. L., Tkacz, A. & Poole, P. S. Engineering rhizobacteria for sustainable agriculture. ISME J. 15, 949–964 (2021). This comprehensive review covers the various challenges and potentials of engineering plant growth promoting traits into rhizobacteria.
Marsh, J. W. & Ley, R. E. Microbiome engineering: taming the untractable. Cell 185, 416–418 (2022).
Dundas, C. M. & Dinneny, J. R. Genetic circuit design in rhizobacteria. BioDesign Res. 2022, 9858049 (2022).
Pirttilä, A. M., Mohammad Parast Tabas, H., Baruah, N. & Koskimäki, J. J. Biofertilizers and biocontrol agents for agriculture: how to identify and develop new potent microbial strains and traits. Microorganisms 9, 817 (2021).
Pankievicz, V. C. S., Irving, T. B., Maia, L. G. S. & Ané, J.-M. Are we there yet? The long walk towards the development of efficient symbiotic associations between nitrogen-fixing bacteria and non-leguminous crops. BMC Biol. 17, 99 (2019).
Ke, J., Wang, B. & Yoshikuni, Y. Microbiome engineering: synthetic biology of plant-associated microbiomes in sustainable agriculture. Trends Biotechnol. 39, 244–261 (2021).
Han, S.-W. & Yoshikuni, Y. Microbiome engineering for sustainable agriculture: using synthetic biology to enhance nitrogen metabolism in plant-associated microbes. Curr. Opin. Microbiol. 68, 102172 (2022).
M, B. B. & R, D. Manipulating nitrogen regulation in diazotrophic bacteria for agronomic benefit. Biochem. Soc. Trans. 47, 603–614 (2019).
Menegat, S., Ledo, A. & Tirado, R. Greenhouse gas emissions from global production and use of nitrogen synthetic fertilisers in agriculture. Sci. Rep. 12, 14490 (2022).
Henryson, K., Kätterer, T., Tidåker, P. & Sundberg, C. Soil N2O emissions, N leaching and marine eutrophication in life cycle assessment — a comparison of modelling approaches. Sci. Total Environ. 725, 138332 (2020).
Ryu, M.-H. et al. Control of nitrogen fixation in bacteria that associate with cereals. Nat. Microbiol. 5, 314–330 (2020). This impressive study illustrates the promises of synthetic biology approaches for implementing nitrogen fixation in alternative bacterial strains.
Espah Borujeni, A., Zhang, J., Doosthosseini, H., Nielsen, A. A. K. & Voigt, C. A. Genetic circuit characterization by inferring RNA polymerase movement and ribosome usage. Nat. Commun. 11, 5001 (2020).
Haskett, T. L. et al. Engineered plant control of associative nitrogen fixation. Proc. Natl Acad. Sci. USA 119, e2117465119 (2022).
Bergkessel, M., Basta, D. W. & Newman, D. K. The physiology of growth arrest: uniting molecular and environmental microbiology. Nat. Rev. Microbiol. 14, 549–562 (2016).
Bergkessel, M. Regulation of protein biosynthetic activity during growth arrest. Curr. Opin. Microbiol. 57, 62–69 (2020).
Bergkessel, M. & Delavaine, L. Diversity in starvation survival strategies and outcomes among heterotrophic Proteobacteria. Microb. Physiol. 31, 146–162 (2021).
Nguyen, P. Q., Courchesne, N.-M. D., Duraj-Thatte, A., Praveschotinunt, P. & Joshi, N. S. Engineered living materials: prospects and challenges for using biological systems to direct the assembly of smart materials. Adv. Mater. 30, 1704847 (2018).
Gilbert, C. & Ellis, T. Biological engineered living materials: growing functional materials with genetically programmable properties. ACS Synth. Biol. 8, 1–15 (2019).
Rodrigo-Navarro, A., Sankaran, S., Dalby, M. J., del Campo, A. & Salmeron-Sanchez, M. Engineered living biomaterials. Nat. Rev. Mater. 6, 1175–1190 (2021).
Molinari, S., Tesoriero, R. F. & Ajo-Franklin, C. M. Bottom-up approaches to engineered living materials: challenges and future directions. Matter 4, 3095–3120 (2021).
Tang, T.-C. et al. Materials design by synthetic biology. Nat. Rev. Mater. 6, 332–350 (2021).
An, B. et al. Engineered living materials for sustainability. Chem. Rev. 123, 2349–2419 (2022).
Chen, B. et al. Programmable living assembly of materials by bacterial adhesion. Nat. Chem. Biol. 18, 289–294 (2022).
Molinari, S. et al. A de novo matrix for macroscopic living materials from bacteria. Nat. Commun. 13, 5544 (2022).
Huang, J. et al. Programmable and printable Bacillus subtilis biofilms as engineered living materials. Nat. Chem. Biol. 15, 34–41 (2019).
Gilbert, C. et al. Living materials with programmable functionalities grown from engineered microbial co-cultures. Nat. Mater. 20, 691–700 (2021).
McBee, R. M. et al. Engineering living and regenerative fungal–bacterial biocomposite structures. Nat. Mater. 21, 471–478 (2022). This paper presents a promising approach towards functionalized macroscale engineered living materials that embeds engineered microorganisms into blocks made from fungal mycelia.
Jo, H. & Sim, S. Programmable living materials constructed with the dynamic covalent interface between synthetic polymers and engineered B. subtilis. ACS Appl. Mater. Interfaces 14, 20729–20738 (2022).
Meyer, V. Connecting materials sciences with fungal biology: a sea of possibilities. Fungal Biol. Biotechnol. 9, 5 (2022).
Delvendahl, N. et al. Narratives of fungal-based materials for a new bioeconomy era. Innov. Eur. J. Soc. Sci. Res. 36, 96–106 (2023).
Bagga, M. et al. Advancements in bacteria based self-healing concrete and the promise of modelling. Constr. Build. Mater. 358, 129412 (2022).
Jonkers, H. M., Thijssen, A., Muyzer, G., Copuroglu, O. & Schlangen, E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 36, 230–235 (2010).
Vijay, K., Murmu, M. & Deo, S. V. Bacteria based self healing concrete — a review. Constr. Build. Mater. 152, 1008–1014 (2017).
Castro-Alonso, M. J. et al. Microbially induced calcium carbonate precipitation (MICP) and its potential in bioconcrete: microbiological and molecular concepts. Front. Mater. https://doi.org/10.3389/fmats.2019.00126 (2019).
Biswas, M. et al. Bioremediase a unique protein from a novel bacterium BKH1, ushering a new hope in concrete technology. Enzym. Microb. Technol. 46, 581–587 (2010).
Sarkar, M., Adak, D., Tamang, A., Chattopadhyay, B. & Mandal, S. Genetically-enriched microbe-facilitated self-healing concrete — a sustainable material for a new generation of construction technology. RSC Adv. 5, 105363–105371 (2015).
Grand View Research Inc. Self-healing concrete market size, share & trends analysis report by form (intrinsic, capsule based, vascular), by application (residential, industrial, commercial, infrastructure), by region, and segment forecasts, 2020–2027 https://www.grandviewresearch.com/industry-analysis/self-healing-concrete-market (2020).
Silva, F. B., da, Boon, N., Belie, N. D. & Verstraete, W. Industrial application of biological self-healing concrete: challenges and economical feasibility. J. Commer. Biotechnol. 21, 31–38 (2015).
National Institute of Health. NIH guidelines for research involving recombinant or synthetic nucleic acid molecules (NIH Guidelines) — April 2019. NIH https://osp.od.nih.gov/wp-content/uploads/NIH_Guidelines.pdf (2019).
Stirling, F. & Silver, P. A. Controlling the implementation of transgenic microbes: are we ready for what synthetic biology has to offer? Mol. Cell 78, 614–623 (2020). This paper provides a comprehensive review on the challenges associated with biocontainment of engineered organisms, with a particular emphasis on the evolutionary stability of these systems.
Lee, J. W., Chan, C. T. Y., Slomovic, S. & Collins, J. J. Next-generation biocontainment systems for engineered organisms. Nat. Chem. Biol. 14, 530–537 (2018).
Diwo, C. & Budisa, N. Alternative biochemistries for alien life: basic concepts and requirements for the design of a robust biocontainment system in genetic isolation. Genes 10, 17 (2019).
Mandell, D. J. et al. Biocontainment of genetically modified organisms by synthetic protein design. Nature 518, 55–60 (2015).
Robertson, W. E. et al. Sense codon reassignment enables viral resistance and encoded polymer synthesis. Science 372, 1057–1062 (2021).
Nyerges, A. et al. A swapped genetic code prevents viral infections and gene transfer. Nature 615, 720–727 (2023).
Zürcher, J. F. et al. Refactored genetic codes enable bidirectional genetic isolation. Science 378, 516–523 (2022).
Guindani, C., da Silva, L. C., Cao, S., Ivanov, T. & Landfester, K. Synthetic cells: from simple bio-inspired modules to sophisticated integrated systems. Angew. Chem. Int. Ed. Engl. 61, e202110855 (2022).
Gaut, N. J. & Adamala, K. P. Reconstituting natural cell elements in synthetic cells. Adv. Biol. 5, 2000188 (2021).
Tang, T.-C. et al. Hydrogel-based biocontainment of bacteria for continuous sensing and computation. Nat. Chem. Biol. 17, 724–731 (2021).
French, K. E., Zhou, Z. & Terry, N. Horizontal ‘gene drives’ harness indigenous bacteria for bioremediation. Sci. Rep. 10, 15091 (2020).
Valderrama, J. A., Kulkarni, S. S., Nizet, V. & Bier, E. A bacterial gene-drive system efficiently edits and inactivates a high copy number antibiotic resistance locus. Nat. Commun. 10, 5726 (2019).
Coban, O., De Deyn, G. B. & van der Ploeg, M. Soil microbiota as game-changers in restoration of degraded lands. Science 375, abe0725 (2022).
Downie, H. et al. Transparent soil for imaging the rhizosphere. PLoS ONE 7, e44276 (2012).
Downie, H. F., Valentine, T. A., Otten, W., Spiers, A. J. & Dupuy, L. X. Transparent soil microcosms allow 3D spatial quantification of soil microbiological processes in vivo. Plant Signal. Behav. 9, e970421 (2014).
Ma, L. et al. Hydrogel-based transparent soils for root phenotyping in vivo. Proc. Natl Acad. Sci. USA 116, 11063–11068 (2019).
Sharma, K., Palatinszky, M., Nikolov, G., Berry, D. & Shank, E. A. Transparent soil microcosms for live-cell imaging and non-destructive stable isotope probing of soil microorganisms. eLife 9, e56275 (2020).
Tecon, R., Ebrahimi, A., Kleyer, H., Erev Levi, S. & Or, D. Cell-to-cell bacterial interactions promoted by drier conditions on soil surfaces. Proc. Natl Acad. Sci. USA 115, 9791–9796 (2018). This paper is an elegant study linking experimental soil microcosms with mathematical modelling to reveal the central role of cell density in driving plasmid conjugation rates in soil.
Van Elsas, J. D., Turner, S. & Bailey, M. J. Horizontal gene transfer in the phytosphere. N. Phytol. 157, 525–537 (2003).
Fernandez-Lopez, R. et al. Unsaturated fatty acids are inhibitors of bacterial conjugation. Microbiology 151, 3517–3526 (2005).
Lima, T., Domingues, S. & Da Silva, G. J. Manure as a potential hotspot for antibiotic resistance dissemination by horizontal gene transfer events. Vet. Sci. 7, 110 (2020).
Berthold, T. et al. Mycelia as a focal point for horizontal gene transfer among soil bacteria. Sci. Rep. 6, 36390 (2016).
Greenlon, A. et al. Global-level population genomics reveals differential effects of geography and phylogeny on horizontal gene transfer in soil bacteria. Proc. Natl Acad. Sci. USA 116, 15200–15209 (2019).
Del Valle, I., Gao, X., Ghezzehei, T. A., Silberg, J. J. & Masiello, C. A. Artificial soils reveal individual factor controls on microbial processes. mSystems 7, e00301–e00322 (2022).
Wang, Y.-J. & Leadbetter, J. R. Rapid acyl-homoserine lactone quorum signal biodegradation in diverse soils. Appl. Environ. Microbiol. 71, 1291–1299 (2005).
Piraner, D. I., Abedi, M. H., Moser, B. A., Lee-Gosselin, A. & Shapiro, M. G. Tunable thermal bioswitches for in vivo control of microbial therapeutics. Nat. Chem. Biol. 13, 75–80 (2017).
Herron, P. M., Gage, D. J. & Cardon, Z. G. Micro-scale water potential gradients visualized in soil around plant root tips using microbiosensors. Plant Cell Env. 33, 199–210 (2010).
Stirling, F. et al. Synthetic cassettes for pH-mediated sensing, counting, and containment. Cell Rep. 30, 3139–3148.e4 (2020).
Del Valle, I. et al. Translating new synthetic biology advances for biosensing into the earth and environmental sciences. Front. Microbiol. 11, 618373 (2021). This excellent review covers the challenges and potentials of engineering and deploying microbial biosensors into environments.
Dieterle, P. B., Min, J., Irimia, D. & Amir, A. Dynamics of diffusive cell signaling relays. eLife 9, e61771 (2020).
Larkin, J. W. et al. Signal percolation within a bacterial community. Cell Syst. 7, 137–145.e3 (2018).
Raynaud, X. & Nunan, N. Spatial ecology of bacteria at the microscale in soil. PLoS ONE 9, e87217 (2014).
Gantner, S. et al. In situ quantitation of the spatial scale of calling distances and population density-independent N-acylhomoserine lactone-mediated communication by rhizobacteria colonized on plant roots. FEMS Microbiol. Ecol. 56, 188–194 (2006).
Wilpiszeski, R. L. et al. Soil aggregate microbial communities: towards understanding microbiome interactions at biologically relevant scales. Appl. Environ. Microbiol. 85, e00324-19 (2019).
Or, D., Smets, B. F., Wraith, J. M., Dechesne, A. & Friedman, S. P. Physical constraints affecting bacterial habitats and activity in unsaturated porous media — a review. Adv. Water Resour. 30, 1505–1527 (2007).
Schmieder, S. S. et al. Bidirectional propagation of signals and nutrients in fungal networks via specialized hyphae. Curr. Biol. 29, 217–228.e4 (2019).
Cheng, H.-Y., Masiello, C. A., Bennett, G. N. & Silberg, J. J. Volatile gas production by methyl halide transferase: an in situ reporter of microbial gene expression in soil. Environ. Sci. Technol. 50, 8750–8759 (2016).
Fulk, E. M. et al. A split methyl halide transferase AND gate that reports by synthesizing an indicator gas. ACS Synth. Biol. 9, 3104–3113 (2020).
Fulk, E. M. et al. Nondestructive chemical sensing within bulk soil using 1000 biosensors per gram of matrix. ACS Synth. Biol. 11, 2372–2383 (2022).
Chemla, Y. et al. Parallel engineering of environmental bacteria and performance over years under jungle-simulated conditions. PLoS ONE 17, e0278471 (2022).
Koerner, E. Evolution, Function and Manipulation of Methyl Halide Production in Plants. PhD thesis, University of East Anglia (2012).
Fisher, J. B. et al. Tree-mycorrhizal associations detected remotely from canopy spectral properties. Glob. Change Biol. 22, 2596–2607 (2016).
Barbour, K. M., Barrón‐Sandoval, A., Walters, K. E. & Martiny, J. B. H. Towards quantifying microbial dispersal in the environment. Environ. Microbiol. 25, 137–142 (2023).
Choudoir, M. J. & DeAngelis, K. M. A framework for integrating microbial dispersal modes into soil ecosystem ecology. iScience 25, 103887 (2022).
Custer, G. F., Bresciani, L. & Dini-Andreote, F. Ecological and evolutionary implications of microbial dispersal. Front. Microbiol. 13, 855859 (2022). Along with Barbour et al. (ref. 187) and Choudoir and DeAngelis (ref. 188), these reviews and perspectives are an excellent introduction to the emerging research area of microbial dispersal in natural environments.
Carini, P. et al. Relic DNA is abundant in soil and obscures estimates of soil microbial diversity. Nat. Microbiol. 2, 16242 (2016).
Harrison, J. B., Sunday, J. M. & Rogers, S. M. Predicting the fate of eDNA in the environment and implications for studying biodiversity. Proc. R. Soc. B Biol. Sci. 286, 20191409 (2019).
Kittredge, H. A., Dougherty, K. M. & Evans, S. E. Dead but not forgotten: how extracellular DNA, moisture, and space modulate the horizontal transfer of extracellular antibiotic resistance genes in soil. Appl. Environ. Microbiol. 88, e02280-21 (2022).
Qian, J. et al. Barcoded microbial system for high-resolution object provenance. Science 368, 1135–1140 (2020).
Brito, I. L. Examining horizontal gene transfer in microbial communities. Nat. Rev. Microbiol. 19, 442–453 (2021).
Acknowledgements
The authors thank Q. Justman for the insightful comments and revisions of the manuscript.
Author information
Authors and Affiliations
Contributions
E.M.J. and J.P.M. conceptualized the narrative framework, researched the literature and wrote the article. All authors contributed to discussions of the content and reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
Pamela Silver is a founder of KulaBio and Circe Bioscience. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Nico J. Claassens and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jones, E.M., Marken, J.P. & Silver, P.A. Synthetic microbiology in sustainability applications. Nat Rev Microbiol (2024). https://doi.org/10.1038/s41579-023-01007-9
Accepted:
Published:
DOI: https://doi.org/10.1038/s41579-023-01007-9