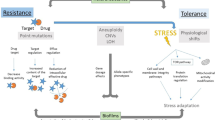
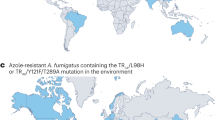
Abstract
Infections caused by non-tuberculous mycobacteria (NTM) are increasing globally and are notoriously difficult to treat due to intrinsic resistance of these bacteria to many common antibiotics. NTM are diverse and ubiquitous in the environment, with only a few species causing serious and often opportunistic infections in humans, including Mycobacterium abscessus. This rapidly growing mycobacterium is one of the most commonly identified NTM species responsible for severe respiratory, skin and mucosal infections in humans. It is often regarded as one of the most antibiotic-resistant mycobacteria, leaving us with few therapeutic options. In this Review, we cover the proposed infection process of M. abscessus, its virulence factors and host interactions and highlight the commonalities and differences of M. abscessus with other NTM species. Finally, we discuss drug resistance mechanisms and future therapeutic options. Taken together, this knowledge is essential to further our understanding of this overlooked and neglected global threat.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tortoli, E. Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin. Microbiol. Rev. 27, 727–752 (2014).
Tortoli, E. et al. The new phylogeny of the genus Mycobacterium: the old and the news. Infect. Genet. Evol. 56, 19–25 (2017).
Turenne, C. Y. Nontuberculous mycobacteria: Insights on taxonomy and evolution. Infect. Genet. Evol. 72, 159–168 (2019).
Runyon, E. H. Anonymous mycobacteria in pulmonary disease. Med. Clin. North Am. 43, 273–290 (1959).
Wolinsky, E. Mycobacterial diseases other than tuberculosis. Clin. Infect. Dis. 15, 1–10 (1992).
Cole, S. T. et al. Massive gene decay in the leprosy bacillus. Nature 409, 1007–1011 (2001).
Brown-Elliott, B. A. & Philley, J. V. Rapidly growing mycobacteria. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.TNMI7-0027-2016 (2017).
Falkinham, J. O. Environmental sources of nontuberculous mycobacteria. Clin. Chest Med. 36, 35–41 (2015).
Roux, A.-L. et al. Multicenter study of prevalence of nontuberculous mycobacteria in patients with cystic fibrosis in France. J. Clin. Microbiol. 47, 4124–4128 (2009).
Olivier, K. N. et al. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am. J. Respir. Crit. Care Med. 167, 828–834 (2003).
Collins, F. M. AIDS-related mycobacterial disease. Springer Semin. Immunopathol. 10, 375–391 (1988).
Collins, F. M. Mycobacterial disease, immunosuppression, and acquired immunodeficiency syndrome. Clin. Microbiol. Rev. 2, 360–377 (1989).
Catherinot, E. et al. Mycobacterium avium and Mycobacterium abscessus complex target distinct cystic fibrosis patient subpopulations. J. Cyst. Fibros. 12, 74–80 (2013).
Baldwin, S. L., Larsen, S. E., Ordway, D., Cassell, G. & Coler, R. N. The complexities and challenges of preventing and treating nontuberculous mycobacterial diseases. PLoS Negl. Trop. Dis. 13, e0007083 (2019).
Wu, M.-L., Aziz, D. B., Dartois, V. & Dick, T. NTM drug discovery: status, gaps and the way forward. Drug Discov. Today 23, 1502–1519 (2018). This article provides a good overview of the current status of NTM drug discovery.
Adjemian, J., Olivier, K. N., Seitz, A. E., Holland, S. M. & Prevots, D. R. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am. J. Respir. Crit. Care Med. 185, 881–886 (2012).
Swenson, C., Zerbe, C. S. & Fennelly, K. Host variability in NTM disease: implications for research needs. Front. Microbiol. 9, 2901 (2018).
Falkinham, J. O. The changing pattern of nontuberculous mycobacterial disease. Can. J. Infect. Dis. 14, 281–286 (2003).
Brown-Elliott, B. A. & Wallace, R. J. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin. Microbiol. Rev. 15, 716–746 (2002).
Mei, Y. et al. Cutaneous tuberculosis and nontuberculous mycobacterial infections at a national specialized hospital in China. Acta Derm. Venereol. https://doi.org/10.2340/00015555-3283 (2019).
Misch, E. A., Saddler, C. & Davis, J. M. Skin and soft tissue infections due to nontuberculous mycobacteria. Curr. Infect. Dis. Rep. 20, 6 (2018).
Griffith, D. E. et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175, 367–416 (2007).
Floto, R. A. et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis: executive summary. Thorax 71, 88–90 (2016).
Koh, W.-J. et al. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M. avium complex lung disease. Chest 142, 1482–1488 (2012).
Kim, B.-J. et al. A description of Mycobacterium chelonae subsp. gwanakae subsp. nov., a rapidly growing mycobacterium with a smooth colony phenotype due to glycopeptidolipids. Int. J. Syst. Evol. Microbiol. 68, 3772–3780 (2018).
Jankovic, M. et al. Microbiological criteria in non-tuberculous mycobacteria pulmonary disease: a tool for diagnosis and epidemiology. Int. J. Tuberc. Lung Dis. 20, 934–940 (2016).
Choo, S. W. et al. Genomic reconnaissance of clinical isolates of emerging human pathogen Mycobacterium abscessus reveals high evolutionary potential. Sci. Rep. 4, 4061 (2014).
Sapriel, G. et al. Genome-wide mosaicism within Mycobacterium abscessus: evolutionary and epidemiological implications. BMC Genomics 17, 118 (2016).
Ringuet, H. et al. hsp65 sequencing for identification of rapidly growing mycobacteria. J. Clin. Microbiol. 37, 852–857 (1999).
Adékambi, T. & Drancourt, M. Dissection of phylogenetic relationships among 19 rapidly growing Mycobacterium species by 16S rRNA, hsp65, sodA, recA and rpoB gene sequencing. Int. J. Syst. Evol. Microbiol. 54, 2095–2105 (2004).
Adékambi, T., Berger, P., Raoult, D. & Drancourt, M. rpoB gene sequence-based characterization of emerging non-tuberculous mycobacteria with descriptions of Mycobacterium bolletii sp. nov., Mycobacterium phocaicum sp. nov. and Mycobacterium aubagnense sp. nov. Int. J. Syst. Evol. Microbiol. 56, 133–143 (2006).
Tortoli, E. et al. Emended description of Mycobacterium abscessus, Mycobacterium abscessus subsp. abscessus and Mycobacterium abscessus subsp. bolletii and designation of Mycobacterium abscessus subsp. massiliense comb. nov. Int. J. Syst. Evol. Microbiol. 66, 4471–4479 (2016).
Ryan, K. & Byrd, T. F. Mycobacterium abscessus: shapeshifter of the mycobacterial world. Front. Microbiol. 9, 2642 (2018).
Gutiérrez, A. V., Viljoen, A., Ghigo, E., Herrmann, J.-L. & Kremer, L. Glycopeptidolipids, a double-edged sword of the Mycobacterium abscessus complex. Front. Microbiol. 9, 1145 (2018).
Rottman, M. et al. Importance of T cells, gamma interferon, and tumor necrosis factor in immune control of the rapid grower Mycobacterium abscessus in C57BL/6 mice. Infect. Immun. 75, 5898–5907 (2007).
Bernut, A. et al. Mycobacterium abscessus-induced granuloma formation is strictly dependent on TNF signaling and neutrophil trafficking. PLoS Pathog. 12, e1005986 (2016).
Dorhoi, A., Reece, S. T. & Kaufmann, S. H. E. For better or for worse: the immune response against Mycobacterium tuberculosis balances pathology and protection: immunology and pathology in tuberculosis. Immunol. Rev. 240, 235–251 (2011).
Bernut, A. et al. Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc. Natl Acad. Sci. USA 111, E943–E952 (2014). This article describes the use of zebrafish to study pathogenicity of M. abscessus with special emphasis on cording in escaping innate immunity.
Rosain, J. et al. Mendelian susceptibility to mycobacterial disease: 2014-2018 update. Immunol. Cell Biol. 97, 360–367 (2019).
Casanova, J.-L. & Abel, L. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 20, 581–620 (2002).
Mufti, A. H., Toye, B. W., Mckendry, R. R. J. & Angel, J. B. Mycobacterium abscessus infection after use of tumor necrosis factor alpha inhibitor therapy: case report and review of infectious complications associated with tumor necrosis factor alpha inhibitor use. Diagn. Microbiol. Infect. Dis. 53, 233–238 (2005).
Sfeir, M. et al. Mycobacterium abscessus complex infections: a retrospective cohort study. Open. Forum Infect. Dis. 5, ofy022 (2018).
Esther, C. R., Esserman, D. A., Gilligan, P., Kerr, A. & Noone, P. G. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J. Cyst. Fibros. 9, 117–123 (2010).
Qvist, T. et al. Epidemiology of nontuberculous mycobacteria among patients with cystic fibrosis in Scandinavia. J. Cyst. Fibros. 14, 46–52 (2015).
Park, I. K. & Olivier, K. N. Nontuberculous mycobacteria in cystic fibrosis and non-cystic fibrosis bronchiectasis. Semin. Respir. Crit. Care Med. 36, 217–224 (2015).
Kwak, N. et al. Mycobacterium abscessus pulmonary disease: individual patient data meta-analysis. Eur. Respir. J. 54, 1801991 (2019).
Choi, H. et al. Treatment outcomes of macrolide-susceptible Mycobacterium abscessus lung disease. Diagn. Microbiol. Infect. Dis. 90, 293–295 (2018).
Koh, W.-J. et al. Mycobacterial characteristics and treatment outcomes in Mycobacterium abscessus lung disease. Clin. Infect. Dis. 64, 309–316 (2017).
Pierre-Audigier, C. et al. Age-related prevalence and distribution of nontuberculous mycobacterial species among patients with cystic fibrosis. J. Clin. Microbiol. 43, 3467–3470 (2005).
Cullen, A. R., Cannon, C. L., Mark, E. J. & Colin, A. A. Mycobacterium abscessus infection in cystic fibrosis. Colonization or infection? Am. J. Respir. Crit. Care Med. 161, 641–645 (2000).
Jönsson, B. E. et al. Molecular epidemiology of Mycobacterium abscessus, with focus on cystic fibrosis. J. Clin. Microbiol. 45, 1497–1504 (2007).
Catherinot, E. et al. Acute respiratory failure involving an R variant of Mycobacterium abscessus. J. Clin. Microbiol. 47, 271–274 (2009).
Tomashefski, J. F., Stern, R. C., Demko, C. A. & Doershuk, C. F. Nontuberculous mycobacteria in cystic fibrosis. An autopsy study. Am. J. Respir. Crit. Care Med. 154, 523–528 (1996).
Drancourt, M. Looking in amoebae as a source of mycobacteria. Microb. Pathog. 77, 119–124 (2014).
Adékambi, T. et al. Amoebal coculture of ‘Mycobacterium massiliense’ sp. nov. from the sputum of a patient with hemoptoic pneumonia. J. Clin. Microbiol. 42, 5493–5501 (2004).
Bakala N’Goma, J. C. et al. Mycobacterium abscessus phospholipase C expression is induced during coculture within amoebae and enhances M. abscessus virulence in mice. Infect. Immun. 83, 780–791 (2015).
Le Moigne, V. et al. MgtC as a host-induced factor and vaccine candidate against Mycobacterium abscessus infection. Infect. Immun. 84, 2895–2903 (2016).
Ovrutsky, A. R. et al. Cooccurrence of free-living amoebae and nontuberculous mycobacteria in hospital water networks, and preferential growth of Mycobacterium avium in Acanthamoeba lenticulata. Appl. Environ. Microbiol. 79, 3185–3192 (2013).
Dubois, V. et al. Mycobacterium abscessus virulence traits unraveled by transcriptomic profiling in amoeba and macrophages. PLoS Pathog. 15, e1008069 (2019).
Burgess, W., Margolis, A., Gibbs, S., Duarte, R. S. & Jackson, M. Disinfectant susceptibility profiling of glutaraldehyde-resistant nontuberculous mycobacteria. Infect. Control. Hosp. Epidemiol. 38, 784–791 (2017).
Thomson, R. et al. Isolation of nontuberculous mycobacteria (NTM) from household water and shower aerosols in patients with pulmonary disease caused by NTM. J. Clin. Microbiol. 51, 3006–3011 (2013).
Feazel, L. M. et al. Opportunistic pathogens enriched in showerhead biofilms. Proc. Natl Acad. Sci. USA 106, 16393–16399 (2009).
Thomson, R., Tolson, C., Sidjabat, H., Huygens, F. & Hargreaves, M. Mycobacterium abscessus isolated from municipal water - a potential source of human infection. BMC Infect. Dis. 13, 241 (2013).
September, S. M., Brözel, V. S. & Venter, S. N. Diversity of nontuberculoid Mycobacterium species in biofilms of urban and semiurban drinking water distribution systems. Appl. Environ. Microbiol. 70, 7571–7573 (2004).
Huang, W.-C., Chiou, C.-S., Chen, J.-H. & Shen, G.-H. Molecular epidemiology of Mycobacterium abscessus infections in a subtropical chronic ventilatory setting. J. Med. Microbiol. 59, 1203–1211 (2010).
Falkinham, J. O., Norton, C. D. & Le Chevallier, M. W. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other mycobacteria in drinking water distribution systems. Appl. Environ. Microbiol. 67, 1225–1231 (2001).
Dubrou, S. et al. Diversity, community composition, and dynamics of nonpigmented and late-pigmenting rapidly growing mycobacteria in an urban tap water production and distribution system. Appl. Environ. Microbiol. 79, 5498–5508 (2013).
van Ingen, J., Blaak, H., de Beer, J., de Roda Husman, A. M. & van Soolingen, D. Rapidly growing nontuberculous mycobacteria cultured from home tap and shower water. Appl. Environ. Microbiol. 76, 6017–6019 (2010).
Bryant, J. M. et al. Whole-genome sequencing to identify transmission of Mycobacterium abscessus between patients with cystic fibrosis: a retrospective cohort study. Lancet 381, 1551–1560 (2013).
Bryant, J. M. et al. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 354, 751–757 (2016). This article proposes that M. abscessus infections are acquired through transmission, presumably via fomites and aerosols.
Malcolm, K. C. et al. Mycobacterium abscessus displays fitness for fomite transmission. Appl. Environ. Microbiol. https://doi.org/10.1128/AEM.00562-17 (2017).
Bernut, A. et al. Insights into the smooth-to-rough transitioning in Mycobacterium bolletii unravels a functional Tyr residue conserved in all mycobacterial MmpL family members. Mol. Microbiol. 99, 866–883 (2016).
Belisle, J. T. & Brennan, P. J. Chemical basis of rough and smooth variation in mycobacteria. J. Bacteriol. 171, 3465–3470 (1989).
Agustí, G., Astola, O., Rodríguez-Güell, E., Julián, E. & Luquin, M. Surface spreading motility shown by a group of phylogenetically related, rapidly growing pigmented mycobacteria suggests that motility is a common property of mycobacterial species but is restricted to smooth colonies. J. Bacteriol. 190, 6894–6902 (2008).
Prinzis, S., Rivoire, B. & Brennan, P. J. Search for the molecular basis of morphological variation in Mycobacterium avium. Infect. Immun. 62, 1946–1951 (1994).
Schaefer, W. B., Davis, C. L. & Cohn, M. L. Pathogenicity of transparent, opaque, and rough variants of Mycobacterium avium in chickens and mice. Am. Rev. Respir. Dis. 102, 499–506 (1970).
Howard, S. T. et al. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiol. Read. Engl. 152, 1581–1590 (2006).
Pawlik, A. et al. Identification and characterization of the genetic changes responsible for the characteristic smooth-to-rough morphotype alterations of clinically persistent Mycobacterium abscessus. Mol. Microbiol. 90, 612–629 (2013). This report describes the genetic changes that are associated with the smooth-to-rough transition.
Ripoll, F. et al. Genomics of glycopeptidolipid biosynthesis in Mycobacterium abscessus and M. chelonae. BMC Genomics 8, 114 (2007).
Schorey, J. S. & Sweet, L. The mycobacterial glycopeptidolipids: structure, function, and their role in pathogenesis. Glycobiology 18, 832–841 (2008).
Belisle, J. T., Klaczkiewicz, K., Brennan, P. J., Jacobs, W. R. & Inamine, J. M. Rough morphological variants of Mycobacterium avium. Characterization of genomic deletions resulting in the loss of glycopeptidolipid expression. J. Biol. Chem. 268, 10517–10523 (1993).
Brennan, P. J. & Nikaido, H. The envelope of mycobacteria. Annu. Rev. Biochem. 64, 29–63 (1995).
Billman-Jacobe, H., McConville, M. J., Haites, R. E., Kovacevic, S. & Coppel, R. L. Identification of a peptide synthetase involved in the biosynthesis of glycopeptidolipids of Mycobacterium smegmatis. Mol. Microbiol. 33, 1244–1253 (1999).
Medjahed, H. & Reyrat, J.-M. Construction of Mycobacterium abscessus defined glycopeptidolipid mutants: comparison of genetic tools. Appl. Environ. Microbiol. 75, 1331–1338 (2009). This is the first description of specific gene disruption in M. abscessus.
Deshayes, C. et al. MmpS4 promotes glycopeptidolipids biosynthesis and export in Mycobacterium smegmatis. Mol. Microbiol. 78, 989–1003 (2010).
Viljoen, A. et al. The diverse family of MmpL transporters in mycobacteria: from regulation to antimicrobial developments. Mol. Microbiol. 104, 889–904 (2017).
Sondén, B. et al. Gap, a mycobacterial specific integral membrane protein, is required for glycolipid transport to the cell surface. Mol. Microbiol. 58, 426–440 (2005).
Park, I. K. et al. Clonal diversification and changes in lipid traits and colony morphology in Mycobacterium abscessus clinical isolates. J. Clin. Microbiol. 53, 3438–3447 (2015).
Zhang, B. et al. Crystal structures of membrane transporter MmpL3, an anti-TB drug target. Cell 176, 636–648.e13 (2019). This article provides the first high-resolution crystal structure of MmpL3 in mycobacteria.
Kocíncová, D. et al. Spontaneous transposition of IS1096 or ISMsm3 leads to glycopeptidolipid overproduction and affects surface properties in Mycobacterium smegmatis. Tuberculosis 88, 390–398 (2008).
Le Moigne, V. et al. Lsr2 Is an important determinant of intracellular growth and virulence in Mycobacterium abscessus. Front. Microbiol. 10, 905 (2019).
Jankute, M. et al. The role of hydrophobicity in tuberculosis evolution and pathogenicity. Sci. Rep. 7, 1315 (2017).
Viljoen, A. et al. A simple and rapid gene disruption strategy in Mycobacterium abscessus: on the design and application of glycopeptidolipid mutants. Front. Cell. Infect. Microbiol. 8, 69 (2018).
Krasowska, A. & Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 4, 112 (2014).
Brambilla, C. et al. Mycobacteria clumping increase their capacity to damage macrophages. Front. Microbiol. 7, 1562 (2016).
Clary, G. et al. Mycobacterium abscessus smooth and rough morphotypes form antimicrobial-tolerant biofilm phenotypes but are killed by acetic acid. Antimicrob. Agents Chemother. 62, e01782-17 (2018).
Kansal, R. G., Gomez-Flores, R. & Mehta, R. T. Change in colony morphology influences the virulence as well as the biochemical properties of the Mycobacterium avium complex. Microb. Pathog. 25, 203–214 (1998).
Catherinot, E. et al. Hypervirulence of a rough variant of the Mycobacterium abscessus type strain. Infect. Immun. 75, 1055–1058 (2007).
Laencina, L. et al. Identification of genes required for Mycobacterium abscessus growth in vivo with a prominent role of the ESX-4 locus. Proc. Natl Acad. Sci. USA 115, E1002–E1011 (2018). This article describes the importance of ESX-4 in M. abscessus virulence.
Richard, M. et al. Mutations in the MAB_2299c TetR regulator confer cross-resistance to clofazimine and bedaquiline in Mycobacterium abscessus. Antimicrob. Agents Chemother. 63, e01316–e01318 (2019).
Ripoll, F. et al. Non mycobacterial virulence genes in the genome of the emerging pathogen Mycobacterium abscessus. PLoS One 4, e5660 (2009). This is the first report of the complete genome of M. abscessus.
Maloney, K. E. & Valvano, M. A. The mgtC gene of Burkholderia cenocepacia is required for growth under magnesium limitation conditions and intracellular survival in macrophages. Infect. Immun. 74, 5477–5486 (2006).
Mathee, K. et al. Dynamics of Pseudomonas aeruginosa genome evolution. Proc. Natl Acad. Sci. USA 105, 3100–3105 (2008).
Blanc-Potard, A.-B. & Lafay, B. MgtC as a horizontally-acquired virulence factor of intracellular bacterial pathogens: evidence from molecular phylogeny and comparative genomics. J. Mol. Evol. 57, 479–486 (2003).
Belon, C., Gannoun-Zaki, L., Lutfalla, G., Kremer, L. & Blanc-Potard, A.-B. Mycobacterium marinum MgtC plays a role in phagocytosis but is dispensable for intracellular multiplication. PLoS One 9, e116052 (2014).
Titball, R. W. Bacterial phospholipases C. Microbiol. Rev. 57, 347–366 (1993).
Le Chevalier, F. et al. Revisiting the role of phospholipases C in virulence and the lifecycle of Mycobacterium tuberculosis. Sci. Rep. 5, 16918 (2015).
Cirillo, J. D., Falkow, S., Tompkins, L. S. & Bermudez, L. E. Interaction of Mycobacterium avium with environmental amoebae enhances virulence. Infect. Immun. 65, 3759–3767 (1997).
Astarie-Dequeker, C. et al. Phthiocerol dimycocerosates of M. tuberculosis participate in macrophage invasion by inducing changes in the organization of plasma membrane lipids. PLoS Pathog. 5, e1000289 (2009).
Cambier, C. J., O’Leary, S. M., O’Sullivan, M. P., Keane, J. & Ramakrishnan, L. Phenolic glycolipid facilitates mycobacterial escape from microbicidal tissue-resident macrophages. Immunity 47, 552–565.e4 (2017).
Cao, Z., Casabona, M. G., Kneuper, H., Chalmers, J. D. & Palmer, T. The type VII secretion system of Staphylococcus aureus secretes a nuclease toxin that targets competitor bacteria. Nat. Microbiol. 2, 16183 (2016).
Vaziri, F. & Brosch, R. ESX/Type VII secretion systems — An important way out for mycobacterial proteins. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.PSIB-0029-2019 (2019).
Abdallah, A. M. et al. Type VII secretion–mycobacteria show the way. Nat. Rev. Microbiol. 5, 883–891 (2007).
Gray, T. A. et al. Intercellular communication and conjugation are mediated by ESX secretion systems in mycobacteria. Science 354, 347–350 (2016).
Dumas, E. et al. Mycobacterial pan-genome analysis suggests important role of plasmids in the radiation of type VII secretion systems. Genome Biol. Evol. 8, 387–402 (2016).
Simeone, R. et al. Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog. 8, e1002507 (2012).
Guinn, K. M. et al. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 51, 359–370 (2004).
Lienard, J. et al. The Mycobacterium marinum ESX-1 system mediates phagosomal permeabilization and type I interferon production via separable mechanisms. Proc. Natl Acad. Sci. USA 117, 1160–1166 (2019).
Coros, A., Callahan, B., Battaglioli, E. & Derbyshire, K. M. The specialized secretory apparatus ESX-1 is essential for DNA transfer in Mycobacterium smegmatis. Mol. Microbiol. 69, 794–808 (2008).
Clark, R. R. et al. Direct cell-cell contact activates SigM to express the ESX-4 secretion system in Mycobacterium smegmatis. Proc. Natl Acad. Sci. USA 115, E6595–E6603 (2018).
McNamara, M., Danelishvili, L. & Bermudez, L. E. The Mycobacterium avium ESX-5 PPE protein, PPE25-MAV, interacts with an ESAT-6 family protein, MAV_2921, and localizes to the bacterial surface. Microb. Pathog. 52, 227–238 (2012).
Li, Y., Miltner, E., Wu, M., Petrofsky, M. & Bermudez, L. E. A Mycobacterium avium PPE gene is associated with the ability of the bacterium to grow in macrophages and virulence in mice. Cell. Microbiol. 7, 539–548 (2005). This is the first report of a PPE protein in M. avium virulence.
Abdallah, A. M. et al. The ESX-5 secretion system of Mycobacterium marinum modulates the macrophage response. J. Immunol. 181, 7166–7175 (2008).
Mackenzie, N., Alexander, D. C., Turenne, C. Y., Behr, M. A. & De Buck, J. M. Genomic comparison of PE and PPE genes in the Mycobacterium avium complex. J. Clin. Microbiol. 47, 1002–1011 (2009).
Soler-Arnedo, P., Sala, C., Zhang, M., Cole, S. T. & Piton, J. Polarly localized EccE 1 is required for ESX-1 function and stabilization of ESX-1 membrane proteins in Mycobacterium tuberculosis. J. Bacteriol. 202, e00662-19 (2019).
Trias, J., Jarlier, V. & Benz, R. Porins in the cell wall of mycobacteria. Science 258, 1479–1481 (1992).
Luthra, S., Rominski, A. & Sander, P. The role of antibiotic-target-modifying and antibiotic-modifying enzymes in Mycobacterium abscessus drug resistance. Front. Microbiol. 9, 219 (2018). This is a compelling review describing the drug resistance mechanisms involving modification of either drug targets or drug-modifying enzymes in M. abscessus.
Nessar, R., Cambau, E., Reyrat, J. M., Murray, A. & Gicquel, B. Mycobacterium abscessus: a new antibiotic nightmare. J. Antimicrob. Chemother. 67, 810–818 (2012).
Trias, J. & Benz, R. Permeability of the cell wall of Mycobacterium smegmatis. Mol. Microbiol. 14, 283–290 (1994).
Jarlier, V. & Nikaido, H. Mycobacterial cell wall: structure and role in natural resistance to antibiotics. FEMS Microbiol. Lett. 123, 11–18 (1994).
Lambert, P. A. Cellular impermeability and uptake of biocides and antibiotics in Gram-positive bacteria and mycobacteria. J. Appl. Microbiol. 92, 46S–54S (2002).
Alcaide, F., Pfyffer, G. E. & Telenti, A. Role of embB in natural and acquired resistance to ethambutol in mycobacteria. Antimicrob. Agents Chemother. 41, 2270–2273 (1997).
Guillemin, I., Jarlier, V. & Cambau, E. Correlation between quinolone susceptibility patterns and sequences in the A and B subunits of DNA gyrase in mycobacteria. Antimicrob. Agents Chemother. 42, 2084–2088 (1998).
Rominski, A., Roditscheff, A., Selchow, P., Böttger, E. C. & Sander, P. Intrinsic rifamycin resistance of Mycobacterium abscessus is mediated by ADP-ribosyltransferase MAB_0591. J. Antimicrob. Chemother. 72, 376–384 (2017). This study demonstrates that ADP-ribosylation inactivates rifamycins in M. abscessus.
Obata, S. et al. Association of rpoB mutations with rifampicin resistance in Mycobacterium avium. Int. J. Antimicrob. Agents 27, 32–39 (2006).
Soroka, D. et al. Characterization of broad-spectrum Mycobacterium abscessus class A β-lactamase. J. Antimicrob. Chemother. 69, 691–696 (2014).
Dubée, V. et al. β-Lactamase inhibition by avibactam in Mycobacterium abscessus. J. Antimicrob. Chemother. 70, 1051–1058 (2015).
Lefebvre, A.-L. et al. Inhibition of the β-lactamase BlaMab by avibactam improves the in vitro and in vivo efficacy of imipenem against Mycobacterium abscessus. Antimicrob. Agents Chemother. 61, e02440-16 (2017).
Rominski, A. et al. Elucidation of Mycobacterium abscessus aminoglycoside and capreomycin resistance by targeted deletion of three putative resistance genes. J. Antimicrob. Chemother. 72, 2191–2200 (2017).
Ung, K. L., Alsarraf, H. M. A. B., Olieric, V., Kremer, L. & Blaise, M. Crystal structure of the aminoglycosides N-acetyltransferase Eis2 from Mycobacterium abscessus. FEBS J. 286, 4342–4355 (2019).
Dal Molin, M. et al. Molecular mechanisms of intrinsic streptomycin resistance in Mycobacterium abscessus. Antimicrob. Agents Chemother. 62, e01427–17 (2018).
Halloum, I. et al. Resistance to thiacetazone derivatives active against Mycobacterium abscessus involves mutations in the MmpL5 transcriptional repressor MAB_4384. Antimicrob. Agents Chemother. 61, 02509–02516 (2017).
Richard, M. et al. Mechanistic and structural insights into the unique TetR-dependent regulation of a drug efflux pump in Mycobacterium abscessus. Front. Microbiol. 9, 649 (2018).
Gutiérrez, A. V., Richard, M., Roquet-Banères, F., Viljoen, A. & Kremer, L. The TetR-family transcription factor MAB_2299c regulates the expression of two distinct MmpS-MmpL efflux pumps involved in cross-resistance to clofazimine and bedaquiline in Mycobacterium abscessus. Antimicrob. Agents Chemother. 63, e01000-19 (2019).
Rodrigues, L. et al. The role of efflux pumps in macrolide resistance in Mycobacterium avium complex. Int. J. Antimicrob. Agents 34, 529–533 (2009).
Alexander, D. C. et al. Emergence of mmpT5 variants during bedaquiline treatment of Mycobacterium intracellulare lung disease. J. Clin. Microbiol. 55, 574–584 (2017).
Nash, K. A., Brown-Elliott, B. A. & Wallace, R. J. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob. Agents Chemother. 53, 1367–1376 (2009). This is the first description of erm41 in macrolide-inducible resistance in M. abscessus.
Kim, H.-Y. et al. Mycobacterium massiliense is differentiated from Mycobacterium abscessus and Mycobacterium bolletii by erythromycin ribosome methyltransferase gene (erm) and clarithromycin susceptibility patterns. Microbiol. Immunol. 54, 347–353 (2010).
Richard, M., Gutiérrez, A. V. & Kremer, L. Dissecting erm(41)-mediated macrolide inducible resistance in Mycobacterium abscessus. Antimicrob. Agents Chemother. 64, e01879-19 (2019).
Hurst-Hess, K., Rudra, P. & Ghosh, P. Mycobacterium abscessus WhiB7 regulates a species-specific repertoire of genes to confer extreme antibiotic resistance. Antimicrob. Agents Chemother. 61, e01347-17 (2017). This study highlights the importance of WhiB7 regulating expression of various genes conferring resistance to antibiotics in M. abscessus.
Pryjma, M., Burian, J., Kuchinski, K. & Thompson, C. J. Antagonism between front-line antibiotics clarithromycin and amikacin in the treatment of Mycobacterium abscessus infections is mediated by the whiB7 gene. Antimicrob. Agents Chemother. 61, 01353-17 (2017).
Bastian, S. et al. Assessment of clarithromycin susceptibility in strains belonging to the Mycobacterium abscessus group by erm(41) and rrl sequencing. Antimicrob. Agents Chemother. 55, 775–781 (2011).
Maurer, F. P., Rüegger, V., Ritter, C., Bloemberg, G. V. & Böttger, E. C. Acquisition of clarithromycin resistance mutations in the 23S rRNA gene of Mycobacterium abscessus in the presence of inducible erm(41). J. Antimicrob. Chemother. 67, 2606–2611 (2012).
Prammananan, T. et al. A single 16S ribosomal RNA substitution is responsible for resistance to amikacin and other 2-deoxystreptamine aminoglycosides in Mycobacterium abscessus and Mycobacterium chelonae. J. Infect. Dis. 177, 1573–1581 (1998).
Moon, S. M. et al. Clinical characteristics, treatment outcomes, and resistance mutations associated with macrolide-resistant Mycobacterium avium complex lung disease. Antimicrob. Agents Chemother. 60, 6758–6765 (2016).
Brown-Elliott, B. A. et al. In vitro activity of amikacin against isolates of Mycobacterium avium complex with proposed MIC breakpoints and finding of a 16S rRNA gene mutation in treated isolates. J. Clin. Microbiol. 51, 3389–3394 (2013).
Lefebvre, A.-L. et al. Bactericidal and intracellular activity of β-lactams against Mycobacterium abscessus. J. Antimicrob. Chemother. 71, 1556–1563 (2016).
Kaushik, A. et al. In vitro activity of the New β-lactamase inhibitors relebactam and vaborbactam in combination with β-lactams against Mycobacterium abscessus complex clinical isolates. Antimicrob. Agents Chemother. 63, e02623-18 (2019).
Pandey, R. et al. Dual β-lactam combinations highly active against Mycobacterium abscessus complex in vitro. mBio 10, e02895-18 (2019).
Ganapathy, U. S., Dartois, V. & Dick, T. Repositioning rifamycins for Mycobacterium abscessus lung disease. Expert Opin. Drug Discov. 14, 867–878 (2019).
Pryjma, M., Burian, J. & Thompson, C. J. Rifabutin acts in synergy and is bactericidal with frontline Mycobacterium abscessus antibiotics clarithromycin and tigecycline, suggesting a potent treatment combination. Antimicrob. Agents Chemother. 62, e00283-18 (2018).
Rudra, P., Hurst-Hess, K., Lappierre, P. & Ghosh, P. High levels of intrinsic tetracycline resistance in Mycobacterium abscessus are conferred by a tetracycline-modifying monooxygenase. Antimicrob. Agents Chemother. 62, e00119-18 (2018).
Wallace, R. J. et al. Clinical experience in 52 patients with tigecycline-containing regimens for salvage treatment of Mycobacterium abscessus and Mycobacterium chelonae infections. J. Antimicrob. Chemother. 69, 1945–1953 (2014).
Yang, B. et al. Clofazimine-containing regimen for the treatment of Mycobacterium abscessus lung disease. Antimicrob. Agents Chemother. 61, e02052-16 (2017).
Dupont, C. et al. Bedaquiline inhibits the ATP synthase in Mycobacterium abscessus and is effective in infected zebrafish. Antimicrob. Agents Chemother. 61, e01225-17 (2017).
Ruth, M. M. et al. A bedaquiline/clofazimine combination regimen might add activity to the treatment of clinically relevant non-tuberculous mycobacteria. J. Antimicrob. Chemother. 74, 935–943 (2019).
Viljoen, A. et al. Verapamil improves the activity of bedaquiline against Mycobacterium abscessus in vitro and in macrophages. Antimicrob. Agents Chemother. 63, e00705-19 (2019).
Griffith, D. E. et al. Amikacin liposome inhalation suspension for treatment-refractory lung disease caused by Mycobacterium avium complex (CONVERT). A prospective, open-label, randomized study. Am. J. Respir. Crit. Care Med. 198, 1559–1569 (2018).
Dupont, C. et al. A new piperidinol derivative targeting mycolic acid transport in Mycobacterium abscessus. Mol. Microbiol. 101, 515–529 (2016).
Kozikowski, A. P. et al. Targeting mycolic acid transport by indole-2-carboxamides for the treatment of Mycobacterium abscessus infections. J. Med. Chem. 60, 5876–5888 (2017).
Franz, N. D. et al. Design, synthesis and evaluation of indole-2-carboxamides with pan anti-mycobacterial activity. Bioorg. Med. Chem. 25, 3746–3755 (2017).
Pandya, A. N. et al. Indole-2-carboxamides are active against Mycobacterium abscessus in a mouse model of acute infection. Antimicrob. Agents Chemother. 63, e02245-18 (2019).
Raynaud, C. et al. Active benzimidazole derivatives targeting the MmpL3 transporter in Mycobacterium abscessus. ACS Infect. Dis. https://doi.org/10.1021/acsinfecdis.9b00389 (2019).
Locher, C. P. et al. A novel inhibitor of gyrase B is a potent drug candidate for treatment of tuberculosis and nontuberculosis mycobacterial infections. Antimicrob. Agents Chemother. 59, 1455–1465 (2015).
Brown-Elliott, B. A., Rubio, A. & Wallace, R. J. In vitro susceptibility testing of a novel benzimidazole, SPR719, against nontuberculous mycobacteria. Antimicrob. Agents Chemother. 62, e01503-18 (2018).
Madani, A. et al. Cyclipostins and cyclophostin analogues as multitarget inhibitors that impair growth of Mycobacterium abscessus. ACS Infect. Dis. 5, 1597–1608 (2019).
Dedrick, R. M. et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 25, 730–733 (2019). This study describes the first administration of genetically engineered phages in a patient with cystic fibrosis chronically infected with a drug-resistant M. massiliense strain.
Bernut, A., Herrmann, J.-L., Ordway, D. & Kremer, L. The diverse cellular and animal models to decipher the physiopathological traits of Mycobacterium abscessus infection. Front. Cell. Infect. Microbiol. 7, 100 (2017).
Bernut, A. et al. In vivo assessment of drug efficacy against Mycobacterium abscessus using the embryonic zebrafish test system. Antimicrob. Agents Chemother. 58, 4054–4063 (2014).
Lerat, I. et al. In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J. Infect. Dis. 209, 905–912 (2014).
De Groote, M. A. et al. GM-CSF knockout mice for preclinical testing of agents with antimicrobial activity against Mycobacterium abscessus. J. Antimicrob. Chemother. 69, 1057–1064 (2014).
Obregón-Henao, A. et al. Susceptibility of Mycobacterium abscessus to antimycobacterial drugs in preclinical models. Antimicrob. Agents Chemother. 59, 6904–6912 (2015).
Byrd, T. F. & Lyons, C. R. Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection. Infect. Immun. 67, 4700–4707 (1999).
Meir, M., Grosfeld, T. & Barkan, D. Establishment and validation of Galleria mellonella as a novel model organism to study Mycobacterium abscessus infection, pathogenesis, and treatment. Antimicrob. Agents Chemother. 62, e02539-17 (2018).
Oh, C.-T., Moon, C., Jeong, M. S., Kwon, S.-H. & Jang, J. Drosophila melanogaster model for Mycobacterium abscessus infection. Microbes Infect. 15, 788–795 (2013).
Ferro, B. E. et al. Amikacin pharmacokinetics/pharmacodynamics in a novel hollow-fiber Mycobacterium abscessus disease model. Antimicrob. Agents Chemother. 60, 1242–1248 (2016).
Angenent, L. T., Kelley, S. T., St Amand, A., Pace, N. R. & Hernandez, M. T. Molecular identification of potential pathogens in water and air of a hospital therapy pool. Proc. Natl Acad. Sci. USA 102, 4860–4865 (2005).
Falkinham, J. O., Iseman, M. D., de Haas, P. & van Soolingen, D. Mycobacterium avium in a shower linked to pulmonary disease. J. Water Health 6, 209–213 (2008).
Koch, R. Die Atiologie der Tuberkulose. Berl. Klin. Wochenschr. 15, 221–230 (1882).
Glickman, M. S., Cox, J. S. & Jacobs, W. R. A novel mycolic acid cyclopropane synthetase is required for cording, persistence, and virulence of Mycobacterium tuberculosis. Mol. Cell 5, 717–727 (2000).
Julián, E. et al. Microscopic cords, a virulence-related characteristic of Mycobacterium tuberculosis, are also present in nonpathogenic mycobacteria. J. Bacteriol. 192, 1751–1760 (2010).
Sánchez-Chardi, A. et al. Demonstration of cord formation by rough Mycobacterium abscessus variants: implications for the clinical microbiology laboratory. J. Clin. Microbiol. 49, 2293–2295 (2011).
Bernut, A. et al. CFTR protects against Mycobacterium abscessus infection by fine-tuning host oxidative defenses. Cell Rep. 26, 1828–1840.e4 (2019).
Halloum, I. et al. Deletion of a dehydratase important for intracellular growth and cording renders rough Mycobacterium abscessus avirulent. Proc. Natl Acad. Sci. USA 113, E4228–E4237 (2016). This article highlights the importance of a dehydratase required for cording in M. abscessus and its role in pathogenicity.
Roux, A.-L. et al. The distinct fate of smooth and rough Mycobacterium abscessus variants inside macrophages. Open Biol. 6, 160185 (2016).
Frehel, C., Ryter, A., Rastogi, N. & David, H. The electron-transparent zone in phagocytized Mycobacterium avium and other mycobacteria: formation, persistence and role in bacterial survival. Ann. Inst. Pasteur Microbiol. 137B, 239–257 (1986).
Ramakrishnan, L. Revisiting the role of the granuloma in tuberculosis. Nat. Rev. Immunol. 12, 352–366 (2012).
Kim, B.-R., Kim, B.-J., Kook, Y.-H. & Kim, B.-J. Phagosome escape of rough Mycobacterium abscessus strains in murine macrophage via phagosomal rupture can lead to type I interferon production and their cell-to-cell spread. Front. Immunol. 10, 125 (2019).
Dubois, V. et al. MmpL8MAB controls Mycobacterium abscessus virulence and production of a previously unknown glycolipid family. Proc. Natl Acad. Sci. USA 115, E10147–E10156 (2018).
Rhoades, E. R. et al. Mycobacterium abscessus glycopeptidolipids mask underlying cell wall phosphatidyl- myo-inositol mannosides blocking induction of human macrophage TNF-α by preventing interaction with TLR2. J. Immunol. 183, 1997–2007 (2009).
Roux, A.-L. et al. Overexpression of proinflammatory TLR-2-signalling lipoproteins in hypervirulent mycobacterial variants. Cell. Microbiol. 13, 692–704 (2011). This article emphasizes the importance of surface-exposed lipoproteins in the inflammatory responses to infection with rough M. abscessus.
Davidson, L. B., Nessar, R., Kempaiah, P., Perkins, D. J. & Byrd, T. F. Mycobacterium abscessus glycopeptidolipid prevents respiratory epithelial TLR2 signaling as measured by HβD2 gene expression and IL-8 release. PLoS One 6, e29148 (2011).
Beckham, K. S. H. et al. Structure of the mycobacterial ESX-5 type VII secretion system membrane complex by single-particle analysis. Nat. Microbiol. 2, 17047 (2017). This is the first report of the X-ray structure of an ESX system in a mycobacterium.
Incandela, M. L. et al. DprE1, a new taxonomic marker in mycobacteria. FEMS Microbiol. Lett. 348, 66–73 (2013).
Acknowledgements
The authors thank the members of the L.K team (Montpellier) and the J.-L.H. team (Montigny-le-Bretonneux) for their contribution to the work presented in this Review. The authors are also grateful to the Fondation pour la Recherche Médicale (grant no. DEQ20150331719), the Association Gregory Lemarchal and Vaincre la Mucoviscidose (grants no. RIF20180502320 and no. RIF20170502057), the Labex EpiGenMed ‘Investissements d’Avenir’ programme (grant no. ANR-10-LABX-12-01) and the French National Research Agency (grant no. DIMYVIR ANR-13-BSV3-0007-01).
Author information
Authors and Affiliations
Contributions
All authors researched the data for this article, contributed substantially to discussion of the content and wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Cystic fibrosis
-
A progressive, genetic disease that causes persistent lung infections and limits the ability to breathe.
- Bronchiectasis
-
A disease in which the airways of the lungs become abnormally widened, leading to an excess of mucus that can make the lungs more vulnerable to infection.
- Granuloma
-
An aggregation of immune cells formed during inflammation in different diseases such as mycobacterial infections.
- Trophozoites
-
Protozoans in the early growth stage.
- Fomites
-
Inanimate objects which, when contaminated with infectious agents, can transmit disease to a new host.
- Minimum inhibitory concentration
-
The lowest concentration of a chemical or drug that is able to prevent visible bacterial growth.
Rights and permissions
About this article
Cite this article
Johansen, M.D., Herrmann, JL. & Kremer, L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol 18, 392–407 (2020). https://doi.org/10.1038/s41579-020-0331-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41579-020-0331-1