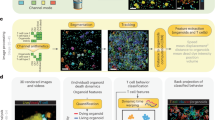
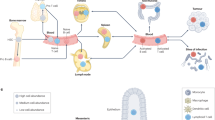
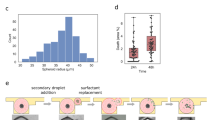
Abstract
In vitro 3D models are advanced biological tools that have been established to overcome the shortcomings of oversimplified 2D cultures and mouse models. Various in vitro 3D immuno-oncology models have been developed to mimic and recapitulate the cancer–immunity cycle, evaluate immunotherapy regimens, and explore options for optimizing current immunotherapies, including for individual patient tumours. Here, we review recent developments in this field. We focus, first, on the limitations of existing immunotherapies for solid tumours, secondly, on how in vitro 3D immuno-oncology models are established using various technologies — including scaffolds, organoids, microfluidics and 3D bioprinting — and thirdly, on the applications of these 3D models for comprehending the cancer–immunity cycle as well as for assessing and improving immunotherapies for solid tumours.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ribas, A. & Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science 359, 1350–1355 (2018).
Rosenberg, S. A. & Restifo, N. P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348, 62–68 (2015).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011).
Melero, I. et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat. Rev. Clin. Oncol. 11, 509–524 (2014).
O’Donnell, J. S., Teng, M. W. L. & Smyth, M. J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 16, 151–167 (2019).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Keskin, D. B. et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 565, 234–239 (2019).
Postow, M. A., Sidlow, R. & Hellmann, M. D. Immune-related adverse events associated with immune checkpoint blockade. N. Engl. J. Med. 378, 158–168 (2018).
Hegde, P. S. & Chen, D. S. Top 10 challenges in cancer immunotherapy. Immunity 52, 17–35 (2020).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Fridman, W. H., Pagès, F., Saut̀s-Fridman, C. & Galon, J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12, 298–306 (2012).
Hegde, P. S., Karanikas, V. & Evers, S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin. Cancer Res. 22, 1865–1874 (2016).
Joyce, A. J. & Fearon, D. T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Klemm, F. et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell 181, 1643–1660.e17 (2020).
Mlecnik, B. et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J. Clin. Oncol. 29, 610–618 (2011).
Melero, I., Rouzaut, A., Motz, G. T. & Coukos, G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. 4, 522–526 (2014).
He, B. et al. Remodeling of metastatic vasculature reduces lung colonization and sensitizes overt metastases to immunotherapy. Cell Rep. 30, 714–724.e5 (2020).
Vong, S. & Kalluri, R. The role of stromal myofibroblast and extracellular matrix in tumor angiogenesis. Genes. Cancer 2, 1139–1145 (2011).
Allen, B. M. et al. Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat. Med. 26, 1125–1134 (2020).
Krishnamurty, A. T. & Turley, S. J. Lymph node stromal cells: cartographers of the immune system. Nat. Immunol. 21, 369–380 (2020).
Cabrita, R. et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020).
Stock, K. et al. Capturing tumor complexity in vitro: comparative analysis of 2D and 3D tumor models for drug discovery. Sci. Rep. 6, 28951 (2016).
Zitvogel, L., Pitt, J. M., Daillère, R., Smyth, M. J. & Kroemer, G. Mouse models in oncoimmunology. Nat. Rev. Cancer 16, 759–773 (2016).
Meraz, I. M. et al. An improved patient-derived xenograft humanized mouse model for evaluation of lung cancer immune responses. Cancer Immunol. Res. 7, 1267–1279 (2019).
Ringquist, R., Ghoshal, D., Jain, R. & Roy, K. Understanding and improving cellular immunotherapies against cancer: from cell-manufacturing to tumor-immune models. Adv. Drug. Deliv. Rev. 179, 114003 (2021).
Riley, R. S., June, C. H., Langer, R. & Mitchell, M. J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug. Discov. 18, 175–196 (2019).
Francis, D. M. & Thomas, S. N. Progress and opportunities for enhancing the delivery and efficacy of checkpoint inhibitors for cancer immunotherapy. Adv. Drug. Deliv. Rev. 114, 33–42 (2017).
Carter, E. P., Roozitalab, R., Gibson, S. V. & Grose, R. P. Tumour microenvironment 3D-modeling: simplicity to complexity and back again. Trends Cancer 7, 1033–1046 (2021).
Hammel, J. H., Zatorski, J. M., Cook, S. R., Pompano, R. R. & Munson, J. M. Engineering in vitro immune-competent tissue models for testing and evaluation of therapeutics. Adv. Drug. Deliv. Rev. 182, 114111 (2022).
Hirt, C. et al. ‘In vitro’ 3D models of tumor-immune system interaction. Adv. Drug. Deliv. Rev. 79, 145–154 (2014).
Shelton, S. E., Nguyen, H. T., Barbie, D. A. & Kamm, R. D. Engineering approaches for studying immune-tumor cell interactions and immunotherapy. iScience 24, 101985 (2021).
Adu-Berchie, K. & Mooney, D. J. Biomaterials as local niches for immunomodulation. Acc. Chem. Res. 53, 1749–1760 (2020).
MP, M. & SN, T. Lymphatic immunomodulation using engineered drug delivery systems for cancer immunotherapy. Adv. Drug. Deliv. Rev. 160, 19–35 (2020).
Francis, D. M. et al. Blockade of immune checkpoints in lymph nodes through locoregional delivery augments cancer immunotherapy. Sci. Transl. Med. 12, eaay3575 (2020).
Yost, K. E. et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 25, 1251–1259 (2019).
Durante, M. A. et al. Single-cell analysis reveals new evolutionary complexity in uveal melanoma. Nat. Commun. 11, 496 (2020).
Jackson, H. W. et al. The single-cell pathology landscape of breast cancer. Nature 578, 615–620 (2020).
Tahmasebi, S., Elahi, R. & Esmaeilzadeh, A. Solid tumors challenges and new insights of CAR T cell engineering. Stem Cell Rev. Rep. 15, 619–636 (2019).
Hong, M., Clubb, J. D. & Chen, Y. Y. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell 38, 473–488 (2020).
Eppler, H. B. & Jewell, C. M. Biomaterials as tools to decode immunity. Adv. Mater. 32, e1903367 (2020).
AJ, N. & DJ, M. Cell and tissue engineering in lymph nodes for cancer immunotherapy. Adv. Drug. Deliv. Rev. 161, 42–62 (2020).
Tabdanov, E. D. et al. Bimodal sensing of guidance cues in mechanically distinct microenvironments. Nat. Commun. 9, 4891 (2018).
Tabdanov, E. D. et al. Engineering T cells to enhance 3D migration through structurally and mechanically complex tumor microenvironments. Nat. Commun. 12, 2815 (2021). The authors designed a nanotextured elastic platform to define how the balance between contractility localization-dependent T cell phenotypes influences migration in response to mechanical and structural cues that mimic tumour growth.
Scheetz, L. et al. Engineering patient-specific cancer immunotherapies. Nat. Biomed. Eng. 3, 768–782 (2019).
Ferber, S., Gonzalez, R. J., Cryer, A. M., von Andrian, U. H. & Artzi, N. Immunology-guided biomaterial design for mucosal cancer vaccines. Adv. Mater. 32, e1903847 (2020).
Cheung, A. S. & Mooney, D. J. Engineered materials for cancer immunotherapy. Nano Today 10, 511–531 (2015).
Kim, J. et al. Injectable, spontaneously assembling, inorganic scaffolds modulate immune cells in vivo and increase vaccine efficacy. Nat. Biotechnol. 33, 64–72 (2015).
Zhang, Y. et al. 3D printing scaffold vaccine for antitumor immunity. Adv. Mater. 33, e2106768 (2021).
Abou-el-Enein, M. et al. Scalable manufacturing of CAR T cells for cancer immunotherapy. Blood Cancer Discov. 2, 408–422 (2021).
Olweus, J. Manufacture of CAR-T cells in the body. Nat. Biotechnol. 35, 520–521 (2017).
Kaiser, A. D. et al. Towards a commercial process for the manufacture of genetically modified T cells for therapy. Cancer Gene Ther. 22, 72–78 (2015).
Hickey, J. W. et al. Adaptive nanoparticle platforms for high throughput expansion and detection of antigen-specific T cells. Nano Lett. 20, 6289–6298 (2020).
Cheung, A. S., Zhang, D. K. Y., Koshy, S. T. & Mooney, D. J. Scaffolds that mimic antigen-presenting cells enable ex vivo expansion of primary T cells. Nat. Biotechnol. 36, 160–169 (2018). These authors outline a micro-rod system that enables antigen-specific expansion of cytotoxic T cell subpopulations at a greater magnitude than is seen with the use of autologous monocyte-derived dendritic cells.
Delalat, B. et al. 3D printed lattices as an activation and expansion platform for T cell therapy. Biomaterials 140, 58–68 (2017).
Majedi, F. S. et al. Cytokine secreting microparticles engineer the fate and the effector functions of T-cells. Adv. Mater. 30, 1703178 (2018).
Lin, H. et al. Automated expansion of primary human T cells in scalable and cell-friendly hydrogel microtubes for adoptive immunotherapy. Adv. Healthc. Mater. 7, e1701297 (2018).
Hickey, J. W. et al. Engineering an artificial T-cell stimulating matrix for immunotherapy. Adv. Mater. 31, e1807359 (2019). These authors show how the ECM affects the cellular therapeutic outcome and offer a case study on how to design ECM-imitating materials for therapeutic immune stimulation.
Mellman, I. et al. De-risking immunotherapy: report of a consensus workshop of the cancer immunotherapy consortium of the cancer research institute. Cancer Immunol. Res. 4, 279–288 (2016).
Stephan, S. B. et al. Biopolymer implants enhance the efficacy of adoptive T-cell therapy. Nat. Biotechnol. 33, 97–101 (2015).
Phuengkham, H., Song, C. & Lim, Y. T. A designer scaffold with immune nanoconverters for reverting immunosuppression and enhancing immune checkpoint blockade therapy. Adv. Mater. 31, e1903242 (2019).
Wang, H. et al. Biomaterial-based scaffold for in situ chemo-immunotherapy to treat poorly immunogenic tumors. Nat. Commun. 11, 5696 (2020).
Smith, T. T. et al. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J. Clin. Invest. 127, 2176–2191 (2017).
Wallstabe, L. et al. ROR1-CAR T cells are effective against lung and breast cancer in advanced microphysiologic 3D tumor models. JCI Insight 4, e126345 (2019).
Wolf, M. T. et al. A biologic scaffold-associated type 2 immune microenvironment inhibits tumor formation and synergizes with checkpoint immunotherapy. Sci. Transl. Med. 11, eaat7973 (2019).
Anderson, A. E. et al. An immunologically active, adipose-derived extracellular matrix biomaterial for soft tissue reconstruction: concept to clinical trial. npj Regen. Med. 7, 6 (2022).
O’Melia, M. J. et al. Synthetic matrix scaffolds engineer the in vivo tumor immune microenvironment for immunotherapy screening. Adv. Mater. 34, e2108084 (2022). These authors created biomaterials to use as scaffolding to reduce the variability in immunotherapeutic testing and enable more accurate modelling of tumour immune microenvironments.
Bian, S. et al. Genetically engineered cerebral organoids model brain tumor formation. Nat. Methods 15, 631–639 (2018).
Zhao, Y. et al. Single-cell transcriptome analysis uncovers intratumoral heterogeneity and underlying mechanisms for drug resistance in hepatobiliary tumor organoids. Adv. Sci. 8, e2003897 (2021).
de Witte, C. J. et al. Patient-derived ovarian cancer organoids mimic clinical response and exhibit heterogeneous inter- and intrapatient drug responses. Cell Rep. 31, 107762 (2020).
Vlachogiannis, G. et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 359, 920–926 (2018).
Guillen, K. P. et al. A human breast cancer-derived xenograft and organoid platform for drug discovery and precision oncology. Nat. Cancer 3, 232–250 (2022).
Boj, S. F. et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 160, 324–338 (2015).
Gao, D. et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 159, 176–187 (2014).
Yuki, K., Cheng, N., Nakano, M. & Kuo, C. J. Organoid models of tumor immunology. Trends Immunol. 41, 652–664 (2020).
Neal, J. T. et al. Organoid modeling of the tumor immune microenvironment. Cell 175, 1972–1988.e16 (2018).
Ootani, A. et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 15, 701–706 (2009).
Kim, S. et al. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat. Commun. 13, 1692 (2022).
Below, C. R. et al. A microenvironment-inspired synthetic three-dimensional model for pancreatic ductal adenocarcinoma organoids. Nat. Mater. 21, 110–119 (2022).
Hernandez-Gordillo, V. et al. Fully synthetic matrices for in vitro culture of primary human intestinal enteroids and endometrial organoids. Biomaterials 254, 120125 (2020).
Broutier, L. et al. Human primary liver cancer-derived organoid cultures for disease modeling and drug screening. Nat. Med. 23, 1424–1435 (2017).
Tsai, S. et al. Development of primary human pancreatic cancer organoids, matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer 18, 335 (2018).
Jacob, F., Ming, G. L. & Song, H. Generation and biobanking of patient-derived glioblastoma organoids and their application in CAR T cell testing. Nat. Protoc. 15, 4000–4033 (2020).
Chan, I. S. et al. Cancer cells educate natural killer cells to a metastasis-promoting cell state. J. Cell Biol. 219, e202001134 (2020).
Dijkstra, K. K. et al. Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174, 1586–1598.e12 (2018).
Zhou, Z. et al. An organoid-based screen for epigenetic inhibitors that stimulate antigen presentation and potentiate T-cell-mediated cytotoxicity. Nat. Biomed. Eng. 5, 1320–1335 (2021).
Dekkers, J. F. et al. Uncovering the mode of action of engineered T cells in patient cancer organoids. Nat. Biotechnol. 41, 60–69 (2023). This group created the ‘BEHAV3D’ system to investigate the dynamic interactions between immune cells and patient-derived cancer organoids. The system can define the behavioural phenotypic heterogeneity of cellular immunotherapies in solid tumours.
Neal, J. T. & Kuo, C. J. Organoids as models for neoplastic transformation. Annu. Rev. Pathol. Mech. Dis. 11, 199–220 (2016).
Hu, Y. et al. Lung cancer organoids analyzed on microwell arrays predict drug responses of patients within a week. Nat. Commun. 12, 2581 (2021).
Sachs, N. et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell 172, 373–386.e10 (2018).
Van De Wetering, M. et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945 (2015).
Fang, G. et al. Mammary tumor organoid culture in non-adhesive alginate for luminal mechanics and high-throughput drug screening. Adv. Sci. 8, e2102418 (2021).
Gong, Z. et al. Acoustic droplet printing tumor organoids for modeling bladder tumor immune microenvironment within a week. Adv. Healthc. Mater. 10, 1–12 (2021).
Ao, Z. et al. Rapid profiling of tumor-immune interaction using acoustically assembled patient-derived cell clusters. Adv. Sci. 9, e2201478 (2022).
Jiang, X. et al. Cancer-on-a-chip for modeling immune checkpoint inhibitor and tumor interactions. Small 17, e2004282 (2021).
LeSavage, B. L., Suhar, R. A., Broguiere, N., Lutolf, M. P. & Heilshorn, S. C. Next-generation cancer organoids. Nat. Mater. 21, 143–159 (2022).
Bandaru, P. et al. A microfabricated sandwiching assay for nanoliter and high-throughput biomarker screening. Small 15, e1900300 (2019).
Cornelius, S. L. et al. Generating and imaging mouse and human epithelial organoids from normal and tumor mammary tissue without passaging. Cancer Res. 189, 10.3791/e64626 (2022).
Schuster, B. et al. Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nat. Commun. 11, 5271 (2020).
Brandenberg, N. et al. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat. Biomed. Eng. 4, 863–874 (2020).
Jiang, S. et al. An automated organoid platform with inter-organoid homogeneity and inter-patient heterogeneity. Cell Rep. Med. 1, 100161 (2020).
Font-Clos, F., Zapperi, S. & La Porta, C. A. M. Blood flow contributions to cancer metastasis. iScience 23, 101073 (2020).
Buchanan, C. F., Verbridge, S. S., Vlachos, P. P. & Rylander, M. N. Flow shear stress regulates endothelial barrier function and expression of angiogenic factors in a 3D microfluidic tumor vascular model. Cell Adh. Migr. 8, 517–524 (2014).
Silvestri, V. L. et al. A tissue-engineered 3D microvessel model reveals the dynamics of mosaic vessel formation in breast cancer. Cancer Res. 80, 4288–4301 (2020). The authors created a tissue-engineered model that replicates the tumour-vascular milieu in solid tumours and enables real-time imaging of the cellular mechanisms of mosaic vessel formation and vascular defect generation.
Wong, A. D. & Searson, P. C. Live-cell imaging of invasion and intravasation in an artificial microvessel platform. Cancer Res. 74, 4937–4945 (2014).
Rajasekar, S. et al. IFlowPlate—a customized 384-well plate for the culture of perfusable vascularized colon organoids. Adv. Mater. 32, e2002974 (2020).
Palikuqi, B. et al. Adaptable haemodynamic endothelial cells for organogenesis and tumorigenesis. Nature 585, 426–432 (2020). This group created an ‘Organ-On-VascularNet’ model that enables investigation and screening in the areas of metabolism, immunology and physiochemistry to define the interactions between organotypic endothelial cells and parenchymal cells.
Sun, X. Y. et al. Generation of vascularized brain organoids to study neurovascular interactions. eLife 11, e76707 (2022).
Bhatia, S. N. & Ingber, D. E. Microfluidic organs-on-chips. Nat. Biotechnol. 32, 760–772 (2014).
Cui, X. et al. Hacking macrophage-associated immunosuppression for regulating glioblastoma angiogenesis. Biomaterials 161, 164–178 (2018).
Kim, H. et al. Macrophages-triggered sequential remodeling of endothelium-interstitial matrix to form pre-metastatic niche in microfluidic tumor microenvironment. Adv. Sci. 6, 1900195 (2019).
Aung, A., Kumar, V., Theprungsirikul, J., Davey, S. K. & Varghese, S. An engineered tumor-on-a-chip device with breast cancer-immune cell interactions for assessing T-cell recruitment. Cancer Res. 80, 263–275 (2020).
Ando, Y. et al. Evaluating CAR-T cell therapy in a hypoxic 3D tumor model. Adv. Healthc. Mater. 8, e1900001 (2019).
Cui, X. et al. Dissecting the immunosuppressive tumor microenvironments in glioblastoma-on-a-chip for optimized PD-1 immunotherapy. eLife 9, e52253 (2020).
Jenkins, R. W. et al. Ex vivo profiling of PD-1 blockade using organotypic tumor spheroids. Cancer Discov. 8, 196–215 (2018).
Lee, S. W. L. et al. Characterizing the role of monocytes in T cell cancer immunotherapy using a 3D microfluidic model. Front. Immunol. 9, 416 (2018).
Pavesi, A. et al. A 3D microfluidic model for preclinical evaluation of TCR-engineered T cells against solid tumors. JCI Insight 2, e89762 (2017).
McAleer, C. W. et al. Multi-organ system for the evaluation of efficacy and off-target toxicity of anticancer therapeutics. Sci. Transl. Med. 11, eaav1386 (2019). The authors created an in vitro multi-organ cell-based system for effective preclinical drug testing and identifying drug metabolite effects that manifest themselves at the organ level.
Edington, C. D. et al. Interconnected microphysiological systems for quantitative biology and pharmacology studies. Sci. Rep. 8, 4530 (2018).
Mazutis, L. et al. Single-cell analysis and sorting using droplet-based microfluidics. Nat. Protoc. 8, 870–891 (2013).
Matuła, K., Rivello, F. & Huck, W. T. S. Single-cell analysis using droplet microfluidics. Adv. Biosyst. 4, e1900188 (2020).
Heath, J. R., Ribas, A. & Mischel, P. S. Single-cell analysis tools for drug discovery and development. Nat. Rev. Drug. Discov. 15, 204–216 (2016).
Tu, H. et al. Profiling of immune–cancer interactions at the single-cell level using a microfluidic well array. Analyst 145, 4138–4147 (2020).
Azizi, E. et al. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell 174, 1293–1308 (2018).
Krishna, C. et al. Single-cell sequencing links multiregional immune landscapes and tissue-resident T cells in ccRCC to tumor topology and therapy efficacy. Cancer Cell 39, 662–677.e6 (2021).
Zhang, S. Q. et al. High-throughput determination of the antigen specificities of T cell receptors in single cells. Nat. Biotechnol. 36, 1156–1159 (2018).
Guo, X. et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat. Med. 24, 978–985 (2018).
Bounab, Y. et al. Dynamic single-cell phenotyping of immune cells using the microfluidic platform DropMap. Nat. Protoc. 15, 2920–2955 (2020).
Segaliny, A. I. et al. Functional TCR T cell screening using single-cell droplet microfluidics. Lab. Chip 18, 3733–3749 (2018).
Ding, S. et al. Patient-derived micro-organospheres enable clinical precision oncology. Cell Stem Cell 29, 905–917.e6 (2022). This group developed a method to rapidly create hundreds of micro-organospheres using droplet emulsion microfluidics; the method can be used in a clinical assay to evaluate immuno-oncology treatments.
Zeming, K. K. et al. Label-free biophysical markers from whole blood microfluidic immune profiling reveal severe immune response signatures. Small 17, e2006123 (2021).
Wang, Z. et al. Efficient recovery of potent tumour-infiltrating lymphocytes through quantitative immunomagnetic cell sorting. Nat. Biomed. Eng. 6, 108–117 (2022). These authors created a reconfigurable microfluidic system that effectively recovers potent TILs from solid tumours, which is crucial for adoptive cell therapies to be effective in the long run.
Dura, B. et al. Profiling lymphocyte interactions at the single-cell level by microfluidic cell pairing. Nat. Commun. 6, 5940 (2015).
Paijens, S. T., Vledder, A., de Bruyn, M. & Nijman, H. W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol. 18, 842–859 (2021).
Simoni, Y. et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018).
Scheper, W. et al. Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat. Med. 25, 89–94 (2019).
Knowlton, S., Onal, S., Yu, C. H., Zhao, J. J. & Tasoglu, S. Bioprinting for cancer research. Trends Biotechnol. 33, 504–513 (2015).
Liu, T. K., Pang, Y., Zhou, Z. Z., Yao, R. & Sun, W. An integrated cell printing system for the construction of heterogeneous tissue models. Acta Biomater. 95, 245–257 (2019).
Lawlor, K. T. et al. Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat. Mater. 20, 260–271 (2021).
Heinrich, M. A. et al. 3D-Bioprinted mini-brain: a glioblastoma model to study cellular interactions and therapeutics. Adv. Mater. 31, 1–9 (2019).
Murphy, S. V. & Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 32, 773–785 (2014).
Ayan, B. et al. Aspiration-assisted bioprinting for precise positioning of biologics. Sci. Adv. 6, eaaw5111 (2020). These authors developed ‘aspiration-assisted bioprinting’, which enables various biofabrication schemes, such as scaffold-based or scaffold-free bioprinting, at an unprecedented placement accuracy.
Xie, F. et al. Three-dimensional bio-printing of primary human hepatocellular carcinoma for personalized medicine. Biomaterials 265, 120416 (2021).
Tang, M. et al. Three-dimensional bioprinted glioblastoma microenvironments model cellular dependencies and immune interactions. Cell Res. 30, 833–853 (2020).
Grolman, J. M., Zhang, D., Smith, A. M., Moore, J. S. & Kilian, K. A. Rapid 3D extrusion of synthetic tumor microenvironments. Adv. Mater. 27, 5512–5517 (2015).
Neufeld, L. et al. Microengineered perfusable 3D-bioprinted glioblastoma model for in vivo mimicry of tumor microenvironment. Sci. Adv. 7, eabi9119 (2021). These authors created a 3D-bioprinted model that accurately represents the heterogeneous TME. It serves as a strong platform for quick, repeatable target discovery, tailored therapeutic screening and drug development.
Burdis, R. & Kelly, D. J. Biofabrication and bioprinting using cellular aggregates, microtissues and organoids for the engineering of musculoskeletal tissues. Acta Biomater. 126, 1–14 (2021).
Kim, E. et al. Creation of bladder assembloids mimicking tissue regeneration and cancer. Nature 588, 664–669 (2020). This group reconstructed tumour organoids with surrounding stromal components to produce tumour ‘assembloids’, which better reflect the in vivo pathophysiological characteristics of urothelial carcinoma.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (2018YFA0703004), Tsinghua University Initiative Scientific Research Program (20213080030, 2022ZLB004), the Beijing Nova Program (20220484075), National Natural Science Foundation of China (52175273, 82072837, 52211540006), Beijing Natural Science Foundation (3212007) and the 111 Project (B17026).
Author information
Authors and Affiliations
Contributions
Z.Z., Y.P. and W.S. conceived the work. Z.Z. contributed to the initial version of the manuscript. All authors contributed to the continuing revisions and approved the submitted manuscript. Y.P. and W.S. provided administrative support and funding support to this work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Immunology thanks E. Carter, R. Grose and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Glossary
- Adoptive T cell therapies
-
(ATCTs). Cell therapies that involve the following processes: extraction of in vivo T cells, selection for or introduction of tumour reactive cells (such as chimeric antigen receptor- or T cell receptor-engineered T cells and tumour-infiltrating lymphocytes), in vitro expansion and delivery of the T cell product back to the patient.
- Alginate
-
A natural anionic polysaccharide that is extensively used as an instant gel for tissue engineering.
- Cancer–immunity cycle
-
(CIC). A seven-step framework used to explain how the immune system detects and destroys cancer cells. Key steps in the CIC include the production of cancer antigens and their presentation by dendritic cells, T cell priming and activation, the movement of T cells into tumours, and the detection and destruction of tumour cells by T cells.
- Cancer vaccines
-
Means to stimulate antigen (nucleic acids, proteins, peptides or patient-derived cells)-specific immune responses, especially CD8+ T cell response, to clear cancer cells.
- Collagen
-
The main structural component of tissues and an ideal candidate for engineering scaffolds.
- Decellularized extracellular matrix
-
(DECM). A native ECM obtained by removing immunogenic substances, such as residual cells, from human or animal tissues through a decellularization process.
- Drug delivery system
-
(DDS). A technical system that fully controls the dosage, timing and distribution of medications within an organism. Study objects include drugs, materials and tools used to deliver the drug, and methods for physiochemically modifying the drug or carrier.
- Fibrin
-
A self-assembling biopolymer derived from fibrinogen and thrombin, which is widely used in tissue regeneration.
- Hyaluronic acid
-
A glycosaminoglycan component of the extracellular matrix in many connective tissues.
- Immune checkpoint inhibitors
-
(ICIs). Monoclonal antibodies that block inhibitory checkpoint proteins and enable immune cells to detect and eradicate cancer.
- Immune-related adverse events
-
(IRAEs). Toxic side effects caused by the use of immunotherapy to kill tumours. IRAEs mainly cause skin, endocrine, gastrointestinal, liver, lung and skeletal muscle toxicity, as well as infusion reactions.
- Matrigel
-
A tissue-derived extracellular matrix used as a matrix biomaterial.
- Tertiary lymphoid structures
-
(TLSs). Immune cell aggregates (lymphoid structures) located in non-lymphoid tissues, which develop during an inflammatory pathological state and usually occur at the infiltrative margins of the tumour and/or interstitium.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Z., Pang, Y., Ji, J. et al. Harnessing 3D in vitro systems to model immune responses to solid tumours: a step towards improving and creating personalized immunotherapies. Nat Rev Immunol 24, 18–32 (2024). https://doi.org/10.1038/s41577-023-00896-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-023-00896-4
This article is cited by
-
Microfluidic high-throughput 3D cell culture
Nature Reviews Bioengineering (2024)
-
3D bioprinted tumor model: a prompt and convenient platform for overcoming immunotherapy resistance by recapitulating the tumor microenvironment
Cellular Oncology (2024)
-
Development and characterization of NILK-2301, a novel CEACAM5xCD3 κλ bispecific antibody for immunotherapy of CEACAM5-expressing cancers
Journal of Hematology & Oncology (2023)