Abstract
Bioengineered models of human tissues have the potential to revolutionize human health by providing a translational platform to investigate disease mechanisms and develop new therapies. Recreating human immune biology is challenging owing to its unique complexity and diversity at the molecular, cellular, tissue and system levels. Advances in immunotherapy have contributed to the emergence of immuno-engineering as a new discipline that applies engineering principles to modulate and emulate the immune system. In synergy with single-cell sequencing, spatial omics, systems immunology, organoids and organ-on-a-chip technologies, these are helping to decipher and recreate human immune components and responses in vitro at a high level of biological fidelity. In this Review, we contextualize the needs for predictive non-clinical models of human immune responses and we overview recent advances in bioengineering models of lymphoid tissues and immune-competent non-lymphoid tissues. We focus on models of the thymus, lymph nodes and intestinal mucosa as examples requiring different degrees of biological and technical complexity. We highlight the experimental demonstrations of their translational value and propose avenues to boost their impact as non-clinical models for immunotherapies.
Key points
-
Preclinical studies for immunotherapies do not translate well into clinical outcomes, in part because animals or simplistic in vitro models do not sufficiently recreate complex human immune responses.
-
High-resolution spatial omics and single-cell immune repertoire sequencing can fill important knowledge gaps and guide engineering principles of the human immune system, in particular deciphering and recreating T cell and B cell diversity.
-
Recent advances in bioengineered models of lymphoid tissues, such as the thymus and lymphoid follicles, and mimicry of resident immunity in various tissue models demonstrate the possibility to recreate innate and adaptive immune responses in vitro and in vivo.
-
The translational value of models of the immune system for applications in drug research and development could be enhanced by incorporating benchmarks and readouts relevant to preclinical and clinical contexts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wouters, O. J., McKee, M. & Luyten, J. Estimated research and development investment needed to bring a new medicine to market, 2009-2018. JAMA 323, 844–853 (2020).
Hackam, D. G. & Redelmeier, D. A. Translation of research evidence from animals to humans. JAMA 296, 1731–1732 (2006).
Mak, I. W., Evaniew, N. & Ghert, M. Lost in translation: animal models and clinical trials in cancer treatment. Am. J. Transl. Res. 6, 114–118 (2014).
Pound, P. & Ritskes-Hoitinga, M. Is it possible to overcome issues of external validity in preclinical animal research? Why most animal models are bound to fail. J. Transl. Med. 16, 304 (2018).
Leenaars, C. H. C. et al. Animal to human translation: a systematic scoping review of reported concordance rates. J. Transl. Med. 17, 223 (2019).
Shultz, L. D., Ishikawa, F. & Greiner, D. L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 7, 118–130 (2007).
Yu, H. et al. A novel humanized mouse model with significant improvement of class-switched, antigen-specific antibody production. Blood 129, 959–969 (2017).
Beura, L. K. et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532, 512–516 (2016).
Perrin, S. Preclinical research: make mouse studies work. Nature 507, 423–425 (2014).
Wang, X. et al. Application of immunocompetent microphysiological systems in drug development: current perspective and recommendations. ALTEX 40, 314–336 (2022).
Rusyn, I. et al. Microphysiological systems evaluation: experience of TEX-VAL tissue chip testing consortium. Toxicol. Sci. 188, 143–152 (2022).
Baran, S. W. et al. Perspectives on the evaluation and adoption of complex in vitro models in drug development: workshop with the FDA and the pharmaceutical industry (IQ MPS Affiliate). ALTEX 39, 297–314 (2022).
Ingber, D. E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 23, 467–491 (2022).
Low, L. A., Mummery, C., Berridge, B. R., Austin, C. P. & Tagle, D. A. Organs-on-chips: into the next decade. Nat. Rev. Drug Discov. 20, 345–361 (2021).
Vulto, P. & Joore, J. Adoption of organ-on-chip platforms by the pharmaceutical industry. Nat. Rev. Drug Discov. 20, 961–962 (2021).
Hammel, J. H., Cook, S. R., Belanger, M. C., Munson, J. M. & Pompano, R. R. Modeling immunity in vitro: slices, chips, and engineered tissues. Annu. Rev. Biomed. Eng. 23, 461–491 (2021).
Bar-Ephraim, Y. E., Kretzschmar, K. & Clevers, H. Organoids in immunological research. Nat. Rev. Immunol. 20, 279–293 (2020).
Moysidou, C. M., Barberio, C. & Owens, R. M. Advances in engineering human tissue models. Front. Bioeng. Biotechnol. 8, 620962 (2020).
Najibi, A. J. & Mooney, D. J. Cell and tissue engineering in lymph nodes for cancer immunotherapy. Adv. Drug Deliv. Rev. 161-162, 42–62 (2020).
Kim, J., Koo, B. K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 21, 571–584 (2020).
Smerchansky, M. E. & Kinney, M. A. Engineering multicellular niches for pluripotent stem cell-derived immunotherapy. Curr. Opin. Biomed. Eng. 16, 19–26 (2020).
Ye, W., Luo, C., Li, C., Huang, J. & Liu, F. Organoids to study immune functions, immunological diseases and immunotherapy. Cancer Lett. 477, 31–40 (2020).
Kim, S., Shah, S. B., Graney, P. L. & Singh, A. Multiscale engineering of immune cells and lymphoid organs. Nat. Rev. Mater. 4, 355–378 (2019).
Park, S. E., Georgescu, A. & Huh, D. Organoids-on-a-chip. Science 364, 960–965 (2019).
Polini, A. et al. Towards the development of human immune-system-on-a-chip platforms. Drug Discov. Today 24, 517–525 (2019).
Coontz, R. Science’s Top 10 Breakthroughs of 2013 https://www.science.org/content/article/sciences-top-10-breakthroughs-2013 (2013).
Senior, M. Fresh from the biotech pipeline: fewer approvals, but biologics gain share. Nat. Biotechnol. 41, 174–182 (2023).
Naran, K., Nundalall, T., Chetty, S. & Barth, S. Principles of immunotherapy: implications for treatment strategies in cancer and infectious diseases. Front. Microbiol. 9, 3158 (2018).
Esfandiari, A., Cassidy, S. & Webster, R. M. Bispecific antibodies in oncology. Nat. Rev. Drug Discov. 21, 411–412 (2022).
Fares, C. M., Van Allen, E. M., Drake, C. G., Allison, J. P. & Hu-Lieskovan, S. Mechanisms of resistance to immune checkpoint blockade: why does checkpoint inhibitor immunotherapy not work for all patients? Am. Soc. Clin. Oncol. Educ. Book 39, 147–164 (2019).
Sullivan, R. J. & Weber, J. S. Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat. Rev. Drug Discov. 21, 495–508 (2022).
Ochoa de Olza, M. et al. Early-drug development in the era of immuno-oncology: are we ready to face the challenges? Ann. Oncol. 29, 1727–1740 (2018).
Sathaliyawala, T. et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 38, 187–197 (2013).
Kumar, B. V., Connors, T. J. & Farber, D. L. Human T cell development, localization, and function throughout life. Immunity 48, 202–213 (2018).
Miller, J. The function of the thymus and its impact on modern medicine. Science 369, eaba2429 (2020).
Thierry Mora, A. M. W. How many different clonotypes do immune repertoires contain? Curr. Opin. Syst. Biol. 18, 104–110 (2019).
Szabo, P. A. et al. Longitudinal profiling of respiratory and systemic immune responses reveals myeloid cell-driven lung inflammation in severe COVID-19. Immunity 54, 797–814.e6 (2021).
Farber, D. L. Tissues, not blood, are where immune cells function. Nature 593, 506–509 (2021).
Chapman, K., Pullen, N., Graham, M. & Ragan, I. Preclinical safety testing of monoclonal antibodies: the significance of species relevance. Nat. Rev. Drug Discov. 6, 120–126 (2007).
Mestas, J. & Hughes, C. C. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Medetgul-Ernar, K. & Davis, M. M. Standing on the shoulders of mice. Immunity 55, 1343–1353 (2022).
Watkins, D. I., Burton, D. R., Kallas, E. G., Moore, J. P. & Koff, W. C. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat. Med. 14, 617–621 (2008).
Estes, J. D., Wong, S. W. & Brenchley, J. M. Nonhuman primate models of human viral infections. Nat. Rev. Immunol. 18, 390–404 (2018).
Bjornson-Hooper, Z. B. et al. A comprehensive atlas of immunological differences between humans, mice, and non-human primates. Front. Immunol. 13, 867015 (2022).
No Authors Listed.Can super-antibody drugs be tamed? Nature 440, 855–856 (2006).
Horvath, C. J. & Milton, M. N. The TeGenero incident and the Duff Report conclusions: a series of unfortunate events or an avoidable event? Toxicol. Pathol. 37, 372–383 (2009).
Gogishvili, T. et al. Rapid regulatory T-cell response prevents cytokine storm in CD28 superagonist treated mice. PLoS One 4, e4643 (2009).
Eastwood, D. et al. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br. J. Pharmacol. 161, 512–526 (2010).
Panitch, H. S., Hirsch, R. L., Schindler, J. & Johnson, K. P. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology 37, 1097–1102 (1987).
Panitch, H. S., Hirsch, R. L., Haley, A. S. & Johnson, K. P. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet 1, 893–895 (1987).
Chaplin, D. D. Overview of the immune response. J. Allergy Clin. Immunol. 125, S3–S23 (2010).
Lythe, G., Callard, R. E., Hoare, R. L. & Molina-Paris, C. How many TCR clonotypes does a body maintain. J. Theor. Biol. 389, 214–224 (2016).
Karnes, J. H. et al. Applications of immunopharmacogenomics: predicting, preventing, and understanding immune-mediated adverse drug reactions. Annu. Rev. Pharmacol. Toxicol. 59, 463–486 (2019).
Chessman, D. et al. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity 28, 822–832 (2008).
Park, J. E. et al. A cell atlas of human thymic development defines T cell repertoire formation. Science 367, eaay3224 (2020).
Massoni-Badosa, R. et al. An atlas of cells in the human tonsil. Preprint at bioRxiv https://doi.org/10.1101/2022.06.24.497299 (2022).
Hurley, K. et al. Reconstructed single-cell fate trajectories define lineage plasticity windows during differentiation of human PSC-derived distal lung progenitors. Cell Stem Cell 26, 593–608.e8 (2020).
Michaels, Y. S. et al. DLL4 and VCAM1 enhance the emergence of T cell-competent hematopoietic progenitors from human pluripotent stem cells. Sci. Adv. 8, eabn5522 (2022).
Veres, A. et al. Charting cellular identity during human in vitro beta-cell differentiation. Nature 569, 368–373 (2019).
Zhang, X. et al. Harnessing matrix stiffness to engineer a bone marrow niche for hematopoietic stem cell rejuvenation. Cell Stem Cell 30, 378–395.e8 (2023).
Wagar, L. E. et al. Modeling human adaptive immune responses with tonsil organoids. Nat. Med. 27, 125–135 (2021).
Han, A., Glanville, J., Hansmann, L. & Davis, M. M. Linking T-cell receptor sequence to functional phenotype at the single-cell level. Nat. Biotechnol. 32, 684–692 (2014).
Stubbington, M. J. T. et al. T cell fate and clonality inference from single-cell transcriptomes. Nat. Methods 13, 329–332 (2016).
Zemmour, D. et al. Single-cell gene expression reveals a landscape of regulatory T cell phenotypes shaped by the TCR. Nat. Immunol. 19, 291–301 (2018).
Dominguez Conde, C. et al. Cross-tissue immune cell analysis reveals tissue-specific features in humans. Science 376, eabl5197 (2022).
Axelrod, M. L. et al. T cells specific for α-myosin drive immunotherapy-related myocarditis. Nature 611, 818–826 (2022).
Rosenberg, A. B. et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 360, 176–182 (2018).
Srivatsan, S. R. et al. Massively multiplex chemical transcriptomics at single-cell resolution. Science 367, 45–51 (2020).
Qiu, P. Embracing the dropouts in single-cell RNA-seq analysis. Nat. Commun. 11, 1169 (2020).
Vandereyken, K., Sifrim, A., Thienpont, B. & Voet, T. Methods and applications for single-cell and spatial multi-omics. Nat. Rev. Genet. 24, 494–515 (2023).
Lundberg, E. & Borner, G. H. H. Spatial proteomics: a powerful discovery tool for cell biology. Nat. Rev. Mol. Cell Biol. 20, 285–302 (2019).
Rao, A., Barkley, D., Franca, G. S. & Yanai, I. Exploring tissue architecture using spatial transcriptomics. Nature 596, 211–220 (2021).
Goltsev, Y. et al. Deep profiling of mouse splenic architecture with codex multiplexed imaging. Cell 174, 968–981.e15 (2018).
Suo, C. et al. Mapping the developing human immune system across organs. Science 376, eabo0510 (2022).
Meylan, M. et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 55, 527–541.e5 (2022).
Uzquiano, A. et al. Proper acquisition of cell class identity in organoids allows definition of fate specification programs of the human cerebral cortex. Cell 185, 3770–3788.e27 (2022).
Liu, Z. et al. Detecting tumor antigen-specific T cells via interaction-dependent fucosyl-biotinylation. Cell 183, 1117–1133.e19 (2020).
Vickovic, S. et al. SM-Omics is an automated platform for high-throughput spatial multi-omics. Nat. Commun. 13, 795 (2022).
Merritt, C. R. et al. Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat. Biotechnol. 38, 586–599 (2020).
Zhang, D. et al. Spatial epigenome-transcriptome co-profiling of mammalian tissues. Nature 616, 113–122 (2023).
He, Z. et al. Lineage recording in human cerebral organoids. Nat. Methods 19, 90–99 (2022).
Sudmeier, L. J. et al. Distinct phenotypic states and spatial distribution of CD8+ T cell clonotypes in human brain metastases. Cell Rep. Med. 3, 100620 (2022).
Gordon, J. & Manley, N. R. Mechanisms of thymus organogenesis and morphogenesis. Development 138, 3865–3878 (2011).
Blackburn, C. C. & Manley, N. R. Developing a new paradigm for thymus organogenesis. Nat. Rev. Immunol. 4, 278–289 (2004).
Klein, L., Hinterberger, M., Wirnsberger, G. & Kyewski, B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat. Rev. Immunol. 9, 833–844 (2009).
van Ewijk, W. et al. Thymic microenvironments, 3-D versus 2-D? Semin. Immunol. 11, 57–64 (1999).
Garcia-Leon, M. J., Fuentes, P., de la Pompa, J. L. & Toribio, M. L. Dynamic regulation of NOTCH1 activation and Notch ligand expression in human thymus development. Development 145, dev16597 (2018).
Klein, L., Kyewski, B., Allen, P. M. & Hogquist, K. A. Positive and negative selection of the T cell repertoire: what thymocytes see (and don’t see). Nat. Rev. Immunol. 14, 377–391 (2014).
Bayramoglu, Z., Ozturk, M., Caliskan, E., Ayyildiz, H. & Adaletli, I. Normative values of thymus in healthy children; stiffness by shear wave elastography. Diagn. Interv. Radiol. 26, 147–152 (2020).
Silva, C. S., Reis, R. L., Martins, A. & Neves, N. M. Recapitulation of thymic function by tissue engineering strategies. Adv. Healthc. Mater. 10, e2100773 (2021).
Hun, M. et al. Native thymic extracellular matrix improves in vivo thymic organoid T cell output, and drives in vitro thymic epithelial cell differentiation. Biomaterials 118, 1–15 (2017).
Asnaghi, M. A. et al. Thymus extracellular matrix-derived scaffolds support graft-resident thymopoiesis and long-term in vitro culture of adult thymic epithelial cells. Adv. Funct. Mater. 31, 2010747 (2021).
Mohtashami, M. & Zuniga-Pflucker, J. C. Three-dimensional architecture of the thymus is required to maintain delta-like expression necessary for inducing T cell development. J. Immunol. 176, 730–734 (2006).
Anderson, G. & Jenkinson, E. J. Investigating central tolerance with reaggregate thymus organ cultures. Methods Mol. Biol. 380, 185–196 (2007).
Pinto, S. et al. An organotypic coculture model supporting proliferation and differentiation of medullary thymic epithelial cells and promiscuous gene expression. J. Immunol. 190, 1085–1093 (2013).
Tajima, A. et al. Bioengineering mini functional thymic units with EAK16-II/EAKIIH6 self-assembling hydrogel. Clin. Immunol. 160, 82–89 (2015).
Chung, B. et al. Engineering the human thymic microenvironment to support thymopoiesis in vivo. Stem Cell 32, 2386–2396 (2014).
Seet, C. S. et al. Generation of mature T cells from human hematopoietic stem and progenitor cells in artificial thymic organoids. Nat. Methods 14, 521–530 (2017).
Fan, Y. et al. Bioengineering thymus organoids to restore thymic function and induce donor-specific immune tolerance to allografts. Mol. Ther. 23, 1262–1277 (2015).
Tajima, A., Pradhan, I., Geng, X., Trucco, M. & Fan, Y. Construction of thymus organoids from decellularized thymus scaffolds. Methods Mol. Biol. 1576, 33–42 (2019).
Campinoti, S. et al. Reconstitution of a functional human thymus by postnatal stromal progenitor cells and natural whole-organ scaffolds. Nat. Commun. 11, 6372 (2020).
Zeleniak, A. et al. De novo construction of T cell compartment in humanized mice engrafted with iPSC-derived thymus organoids. Nat. Methods 19, 1306–1319 (2022).
Sharma, H. & Moroni, L. Recent advancements in regenerative approaches for thymus rejuvenation. Adv. Sci. 8, 2100543 (2021).
Parent, A. V. et al. Generation of functional thymic epithelium from human embryonic stem cells that supports host T cell development. Cell Stem Cell 13, 219–229 (2013).
Sun, X. et al. Directed differentiation of human embryonic stem cells into thymic epithelial progenitor-like cells reconstitutes the thymic microenvironment in vivo. Cell Stem Cell 13, 230–236 (2013).
Okabe, M., Ito, S., Nishio, N., Tanaka, Y. & Isobe, K. Thymic epithelial cells induced from pluripotent stem cells by a three-dimensional spheroid culture system regenerates functional T cells in nude mice. Cell. Reprogram. 17, 368–375 (2015).
Montel-Hagen, A. et al. Organoid-induced differentiation of conventional t cells from human pluripotent stem cells. Cell Stem Cell 24, 376–389.e8 (2019).
Chhatta, A. R. et al. De novo generation of a functional human thymus from induced pluripotent stem cells. J. Allergy Clin. Immunol. 144, 1416–1419.e7 (2019).
Ramos, S. A. et al. Generation of functional human thymic cells from induced pluripotent stem cells. J. Allergy Clin. Immunol. 149, 767–781.e6 (2021).
Ramos, S. A. et al. Generation of functional thymic organoids from human pluripotent stem cells. Stem Cell Rep. 18, 829–840 (2023).
Duke-Cohan, J. S. et al. Pre-T cell receptor self-MHC sampling restricts thymocyte dedifferentiation. Nature 613, 565–574 (2023).
Amadori, A. et al. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat. Med. 1, 1279–1283 (1995).
Garrido-Rodriguez, V. et al. Immunological features beyond CD4/CD8 ratio values in older individuals. Aging 13, 13443–13459 (2021).
Junt, T., Scandella, E. & Ludewig, B. Form follows function: lymphoid tissue microarchitecture in antimicrobial immune defence. Nat. Rev. Immunol. 8, 764–775 (2008).
Grant, S. M., Lou, M., Yao, L., Germain, R. N. & Radtke, A. J. The lymph node at a glance - how spatial organization optimizes the immune response. J. Cell Sci. 133, jcs241828 (2020).
Delves, P. J. & Roitt, I. M. The immune system. First two parts. N. Engl. J. Med. 343, 37–49 (2000).
Turley, S. J., Fletcher, A. L. & Elpek, K. G. The stromal and haematopoietic antigen-presenting cells that reside in secondary lymphoid organs. Nat. Rev. Immunol. 10, 813–825 (2010).
Mueller, S. N. & Germain, R. N. Stromal cell contributions to the homeostasis and functionality of the immune system. Nat. Rev. Immunol. 9, 618–629 (2009).
Trombetta, E. S. & Mellman, I. Cell biology of antigen processing in vitro and in vivo. Annu. Rev. Immunol. 23, 975–1028 (2005).
Allen, C. D., Okada, T. & Cyster, J. G. Germinal-center organization and cellular dynamics. Immunity 27, 190–202 (2007).
De Silva, N. S. & Klein, U. Dynamics of B cells in germinal centres. Nat. Rev. Immunol. 15, 137–148 (2015).
McHeyzer-Williams, M., Okitsu, S., Wang, N. & McHeyzer-Williams, L. Molecular programming of B cell memory. Nat. Rev. Immunol. 12, 24–34 (2011).
Friedman, R. S., Jacobelli, J. & Krummel, M. F. Surface-bound chemokines capture and prime T cells for synapse formation. Nat. Immunol. 7, 1101–1108 (2006).
Zeng, Y. et al. Substrate stiffness regulates B-cell activation, proliferation, class switch, and T-cell-independent antibody responses in vivo. Eur. J. Immunol. 45, 1621–1634 (2015).
Krishnamurty, A. T. & Turley, S. J. Lymph node stromal cells: cartographers of the immune system. Nat. Immunol. 21, 369–380 (2020).
Prados, A. et al. Fibroblastic reticular cell lineage convergence in Peyer’s patches governs intestinal immunity. Nat. Immunol. 22, 510–519 (2021).
Lutge, M., Pikor, N. B. & Ludewig, B. Differentiation and activation of fibroblastic reticular cells. Immunol. Rev. 302, 32–46 (2021).
Rodda, L. B. et al. Single-cell RNA sequencing of lymph node stromal cells reveals niche-associated heterogeneity. Immunity 48, 1014–1028.e6 (2018).
Tomei, A. A., Siegert, S., Britschgi, M. R., Luther, S. A. & Swartz, M. A. Fluid flow regulates stromal cell organization and CCL21 expression in a tissue-engineered lymph node microenvironment. J. Immunol. 183, 4273–4283 (2009).
Kim, J. et al. Characterizing natural hydrogel for reconstruction of three-dimensional lymphoid stromal network to model T-cell interactions. J. Biomed. Mater. Res. A 103, 2701–2710 (2015).
Murakami, T. et al. Secondary lymphoid organ fibroblastic reticular cells mediate trans-infection of HIV-1 via CD44-hyaluronan interactions. Nat. Commun. 9, 2436 (2018).
Mitra, B. et al. Microdevice integrating innate and adaptive immune responses associated with antigen presentation by dendritic cells. RSC Adv. 3, 16002–16010 (2013).
Gopalakrishnan, N. et al. Infection and immunity on a chip: a compartmentalised microfluidic platform to monitor immune cell behaviour in real time. Lab. Chip 15, 1481–1487 (2015).
Moura Rosa, P., Gopalakrishnan, N., Ibrahim, H., Haug, M. & Halaas, O. The intercell dynamics of T cells and dendritic cells in a lymph node-on-a-chip flow device. Lab. Chip 16, 3728–3740 (2016).
Byers, A. M., Tapia, T. M., Sassano, E. R. & Wittman, V. In vitro antibody response to tetanus in the MIMIC system is a representative measure of vaccine immunogenicity. Biologicals 37, 148–151 (2009).
Higbee, R. G. et al. An immunologic model for rapid vaccine assessment — a clinical trial in a test tube. Altern. Lab. Anim. 37, 19–27 (2009).
Giese, C. et al. Immunological substance testing on human lymphatic micro-organoids in vitro. J. Biotechnol. 148, 38–45 (2010).
Kraus, T. et al. Evaluation of a 3D human artificial lymph node as test model for the assessment of immunogenicity of protein aggregates. J. Pharm. Sci. 108, 2358–2366 (2019).
Purwada, A. et al. Ex vivo engineered immune organoids for controlled germinal center reactions. Biomaterials 63, 24–34 (2015).
Purwada, A. & Singh, A. Immuno-engineered organoids for regulating the kinetics of B-cell development and antibody production. Nat. Protoc. 12, 168–182 (2017).
Purwada, A., Shah, S. B., Beguelin, W., Melnick, A. M. & Singh, A. Modular immune organoids with integrin ligand specificity differentially regulate ex vivo B cell activation. ACS Biomater. Sci. Eng. 3, 214–225 (2017).
Purwada, A. et al. Ex vivo synthetic immune tissues with T cell signals for differentiating antigen-specific, high affinity germinal center B cells. Biomaterials 198, 27–36 (2019).
Apoorva, F. N. U. et al. Lymph node stiffness-mimicking hydrogels regulate human B-cell lymphoma growth and cell surface receptor expression in a molecular subtype-specific manner. J. Biomed. Mater. Res. A 105, 1833–1844 (2017).
Beguelin, W. et al. EZH2 enables germinal centre formation through epigenetic silencing of CDKN1A and an Rb-E2F1 feedback loop. Nat. Commun. 8, 877 (2017).
Roh, K. H. et al. A synthetic stroma-free germinal center niche for efficient generation of humoral immunity ex vivo. Biomaterials 164, 106–120 (2018).
Kramer, L. et al. Lipid membrane-based antigen presentation to B cells using a fully synthetic ex vivo germinal center model. Adv. Nanobiomed. Res. 2, 2100137 (2022).
Jackson, K. J. et al. Human responses to influenza vaccination show seroconversion signatures and convergent antibody rearrangements. Cell Host Microbe 16, 105–114 (2014).
Goyal, G. et al. Ectopic lymphoid follicle formation and human seasonal influenza vaccination responses recapitulated in an organ-on-a-chip. Adv. Sci. 9, e2103241 (2022).
Martin, C. H., Woll, P. S., Ni, Z., Zuniga-Pflucker, J. C. & Kaufman, D. S. Differences in lymphocyte developmental potential between human embryonic stem cell and umbilical cord blood-derived hematopoietic progenitor cells. Blood 112, 2730–2737 (2008).
Carpenter, L. et al. Human induced pluripotent stem cells are capable of B-cell lymphopoiesis. Blood 117, 4008–4011 (2011).
French, A., Yang, C. T., Taylor, S., Watt, S. M. & Carpenter, L. Human induced pluripotent stem cell-derived B lymphocytes express sIgM and can be generated via a hemogenic endothelium intermediate. Stem Cell Dev. 24, 1082–1095 (2015).
Richardson, S. E., Ghazanfari, R., Chhetri, J., Enver, T. & Boiers, C. In vitro differentiation of human pluripotent stem cells into the B lineage using OP9-MS5 co-culture. STAR Protoc. 2, 100420 (2021).
Maharjan, S., Cecen, B. & Zhang, Y. S. 3D immunocompetent organ-on-a-chip models. Small Methods 4, 2000235 (2020).
de Waal, A. M., Hiemstra, P. S., Ottenhoff, T. H., Joosten, S. A. & van der Does, A. M. Lung epithelial cells interact with immune cells and bacteria to shape the microenvironment in tuberculosis. Thorax 77, 408–416 (2022).
Harrington, H. et al. Immunocompetent 3D model of human upper airway for disease modeling and in vitro drug evaluation. Mol. Pharm. 11, 2082–2091 (2014).
Seo, H. R. et al. Human pluripotent stem cell-derived alveolar organoid with macrophages. Int. J. Mol. Sci. 23, 9211 (2022).
Pupovac, A. et al. Toward immunocompetent 3D skin models. Adv. Healthc. Mater. 7, e1701405 (2018).
Ormel, P. R. et al. Microglia innately develop within cerebral organoids. Nat. Commun. 9, 4167 (2018).
Rogal, J. et al. Autologous human immunocompetent white adipose tissue-on-chip. Adv. Sci. 9, e2104451 (2022).
Peterson, L. W. & Artis, D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14, 141–153 (2014).
Johansson, M. E. et al. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl Acad. Sci. USA 105, 15064–15069 (2008).
Bevins, C. L. & Salzman, N. H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 9, 356–368 (2011).
Benjamin, J. L., Sumpter, R. Jr., Levine, B. & Hooper, L. V. Intestinal epithelial autophagy is essential for host defense against invasive bacteria. Cell Host Microbe 13, 723–734 (2013).
Cadwell, K. et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–263 (2008).
Mabbott, N. A., Donaldson, D. S., Ohno, H., Williams, I. R. & Mahajan, A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 6, 666–677 (2013).
Mowat, A. M. & Agace, W. W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 14, 667–685 (2014).
Yip, J. L. K., Balasuriya, G. K., Spencer, S. J. & Hill-Yardin, E. L. The role of intestinal macrophages in gastrointestinal homeostasis: heterogeneity and implications in disease. Cell Mol. Gastroenterol. Hepatol. 12, 1701–1718 (2021).
Luciani, C., Hager, F. T., Cerovic, V. & Lelouard, H. Dendritic cell functions in the inductive and effector sites of intestinal immunity. Mucosal Immunol. 15, 40–50 (2022).
Mueller, S. N. & Mackay, L. K. Tissue-resident memory T cells: local specialists in immune defence. Nat. Rev. Immunol. 16, 79–89 (2016).
Cheroutre, H., Lambolez, F. & Mucida, D. The light and dark sides of intestinal intraepithelial lymphocytes. Nat. Rev. Immunol. 11, 445–456 (2011).
Eberl, G., Colonna, M., Di Santo, J. P. & McKenzie, A. N. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science 348, aaa6566 (2015).
Sato, T. et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Sato, T. et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141, 1762–1772 (2011).
Ootani, A. et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat. Med. 15, 701–706 (2009).
Rossi, G., Manfrin, A. & Lutolf, M. P. Progress and potential in organoid research. Nat. Rev. Genet. 19, 671–687 (2018).
Spence, J. R. et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470, 105–109 (2011).
Leslie, J. L. et al. Persistence and toxin production by Clostridium difficile within human intestinal organoids result in disruption of epithelial paracellular barrier function. Infect. Immun. 83, 138–145 (2015).
Puschhof, J. et al. Intestinal organoid cocultures with microbes. Nat. Protoc. 16, 4633–4649 (2021).
Jalili-Firoozinezhad, S. et al. A complex human gut microbiome cultured in an anaerobic intestine-on-a-chip. Nat. Biomed. Eng. 3, 520–531 (2019).
Weller, A. et al. Quantifying the transport of biologics across intestinal barrier models in real-time by fluorescent imaging. Front. Bioeng. Biotechnol. 10, 965200 (2022).
Kasendra, M. et al. Development of a primary human small intestine-on-a-chip using biopsy-derived organoids. Sci. Rep. 8, 2871 (2018).
Kasendra, M. et al. Duodenum intestine-chip for preclinical drug assessment in a human relevant model. eLife 9, e50135 (2020).
Gjorevski, N. et al. Neutrophilic infiltration in Organ-on-a-Chip model of tissue inflammation. Lab. Chip 20, 3365–3374 (2020).
Kerns, S. J. et al. Human immunocompetent Organ-on-Chip platforms allow safety profiling of tumor-targeted T-cell bispecific antibodies. eLife 10, e67106 (2021).
Nikolaev, M. et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 585, 574–578 (2020).
Gjorevski, N. et al. Tissue geometry drives deterministic organoid patterning. Science 375, eaaw9021 (2022).
Shah, P. et al. A microfluidics-based in vitro model of the gastrointestinal human-microbe interface. Nat. Commun. 7, 11535 (2016).
Siwczak, F. et al. Intestinal stem cell-on-chip to study human host-microbiota interaction. Front. Immunol. 12, 798552 (2021).
Maurer, M. et al. A three-dimensional immunocompetent intestine-on-chip model as in vitro platform for functional and microbial interaction studies. Biomaterials 220, 119396 (2019).
Noel, G. et al. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci. Rep. 7, 45270 (2017).
Jowett, G. M. et al. Organoids capture tissue-specific innate lymphoid cell development in mice and humans. Cell Rep. 40, 111281 (2022).
Jung, J. M. et al. KIR3DS1 directs NK cell-mediated protection against human adenovirus infections. Sci. Immunol. 6, eabe2942 (2021).
Nozaki, K. et al. Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J. Gastroenterol. 51, 206–213 (2016).
Rogoz, A., Reis, B. S., Karssemeijer, R. A. & Mucida, D. A 3-D enteroid-based model to study T-cell and epithelial cell interaction. J. Immunol. Methods 421, 89–95 (2015).
Bouffi, C. et al. In vivo development of immune tissue in human intestinal organoids transplanted into humanized mice. Nat. Biotechnol. 41, 824–831 (2023).
Zhou, J. et al. Infection of bat and human intestinal organoids by SARS-CoV-2. Nat. Med. 26, 1077–1083 (2020).
Lamers, M. M. et al. SARS-CoV-2 productively infects human gut enterocytes. Science 369, 50–54 (2020).
Giobbe, G. G. et al. SARS-CoV-2 infection and replication in human gastric organoids. Nat. Commun. 12, 6610 (2021).
Bein, A. et al. Enteric coronavirus infection and treatment modeled with an immunocompetent human intestine-on-a-chip. Front. Pharmacol. 12, 718484 (2021).
Labrijn, A. F., Janmaat, M. L., Reichert, J. M. & Parren, P. Bispecific antibodies: a mechanistic review of the pipeline. Nat. Rev. Drug Discov. 18, 585–608 (2019).
Crawford, A. & Chiu, D. Targeting solid tumors using cd3 bispecific antibodies. Mol. Cancer Ther. 20, 1350–1358 (2021).
Luoma, A. M. et al. Molecular pathways of colon inflammation induced by cancer immunotherapy. Cell 182, 655–671.e22 (2020).
Coutzac, C. et al. Colon immune-related adverse events: anti-CTLA-4 and anti-PD-1 blockade induce distinct immunopathological entities. J. Crohns Colitis 11, 1238–1246 (2017).
Grover, S. & Srivastava, A. Lymphocytic colitis-like pattern of mucosal injury and the challenges in diagnosing cancer immunotherapy-related toxicity. Cancer 125, 1768–1770 (2019).
Badran, Y. R. et al. Concurrent therapy with immune checkpoint inhibitors and TNFα blockade in patients with gastrointestinal immune-related adverse events. J. Immunother. Cancer 7, 226 (2019).
Edgar, J. M., Michaels, Y. S. & Zandstra, P. W. Multi-objective optimization reveals time- and dose-dependent inflammatory cytokine-mediated regulation of human stem cell derived T-cell development. NPJ Regen. Med. 7, 11 (2022).
Khan, A. O. et al. Human bone marrow organoids for disease modeling, discovery, and validation of therapeutic targets in hematologic malignancies. Cancer Discov. 13, 364–385 (2023).
Taylor, C. J., Peacock, S., Chaudhry, A. N., Bradley, J. A. & Bolton, E. M. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient HLA types. Cell Stem Cell 11, 147–152 (2012).
Lee, S. et al. Repurposing the cord blood bank for haplobanking of HLA-homozygous iPSCs and their usefulness to multiple populations. Stem Cell 36, 1552–1566 (2018).
Hudson, D., Fernandes, R. A., Basham, M., Ogg, G. & Koohy, H. Can we predict T cell specificity with digital biology and machine learning? Nat. Rev. Immunol. 23, 511–521 (2023).
Yamauchi, T. & Moroishi, T. Hippo pathway in mammalian adaptive immune system. Cells 8, 398 (2019).
Tsuruta, S. et al. Development of human gut organoids with resident tissue macrophages as a model of intestinal immune responses. Cell Mol. Gastroenterol. Hepatol. 14, 726–729.e5 (2022).
Waldman, A. D., Fritz, J. M. & Lenardo, M. J. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668 (2020).
Sauna, Z. E., Lagasse, D., Pedras-Vasconcelos, J., Golding, B. & Rosenberg, A. S. Evaluating and mitigating the immunogenicity of therapeutic proteins. Trends Biotechnol. 36, 1068–1084 (2018).
Rosenberg, A. S. & Sauna, Z. E. Immunogenicity assessment during the development of protein therapeutics. J. Pharm. Pharmacol. 70, 584–594 (2018).
Vaisman-Mentesh, A., Gutierrez-Gonzalez, M., DeKosky, B. J. & Wine, Y. The molecular mechanisms that underlie the immune biology of anti-drug antibody formation following treatment with monoclonal antibodies. Front. Immunol. 11, 1981 (2020).
Ito, S. et al. In vitro human helper T-cell assay to screen antibody drug candidates for immunogenicity. J. Immunotoxicol. 16, 125–132 (2019).
Wagar, L. E., DiFazio, R. M. & Davis, M. M. Advanced model systems and tools for basic and translational human immunology. Genome Med. 10, 73 (2018).
Giavridis, T. et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 24, 731–738 (2018).
Norelli, M. et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 24, 739–748 (2018).
Tonegawa, S. Somatic generation of antibody diversity. Nature 302, 575–581 (1983).
Nikolich-Zugich, J., Slifka, M. K. & Messaoudi, I. The many important facets of T-cell repertoire diversity. Nat. Rev. Immunol. 4, 123–132 (2004).
Briney, B., Inderbitzin, A., Joyce, C. & Burton, D. R. Commonality despite exceptional diversity in the baseline human antibody repertoire. Nature 566, 393–397 (2019).
Alberts, B. et al. In Molecular Biology of the Cell 4th ed (Garland Science, 2002).
Warren, R. L. et al. Exhaustive T-cell repertoire sequencing of human peripheral blood samples reveals signatures of antigen selection and a directly measured repertoire size of at least 1 million clonotypes. Genome Res. 21, 790–797 (2011).
Robinson, J. et al. IPD-IMGT/HLA database. Nucleic Acids Res. 48, D948–D955 (2020).
Vrisekoop, N., Monteiro, J. P., Mandl, J. N. & Germain, R. N. Revisiting thymic positive selection and the mature T cell repertoire for antigen. Immunity 41, 181–190 (2014).
Derbinski, J., Schulte, A., Kyewski, B. & Klein, L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat. Immunol. 2, 1032–1039 (2001).
Mishra, A. K. & Mariuzza, R. A. Insights into the structural basis of antibody affinity maturation from next-generation sequencing. Front. Immunol. 9, 117 (2018).
Xu, Z., Zan, H., Pone, E. J., Mai, T. & Casali, P. Immunoglobulin class-switch DNA recombination: induction, targeting and beyond. Nat. Rev. Immunol. 12, 517–531 (2012).
Muramatsu, M. et al. Specific expression of activation-induced cytidine deaminase (AID), a novel member of the RNA-editing deaminase family in germinal center B cells. J. Biol. Chem. 274, 18470–18476 (1999).
Seok, J. et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl Acad. Sci. USA 110, 3507–3512 (2013).
Valatas, V., Vakas, M. & Kolios, G. The value of experimental models of colitis in predicting efficacy of biological therapies for inflammatory bowel diseases. Am. J. Physiol. Gastrointest. Liver Physiol 305, G763–G785 (2013).
Baydi, Z. et al. An update of research animal models of inflammatory bowel disease. ScientificWorldJournal 2021, 7479540 (2021).
Knights, D., Lassen, K. G. & Xavier, R. J. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut 62, 1505–1510 (2013).
Xavier, R. J. & Podolsky, D. K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 (2007).
Zhou, G. X. & Liu, Z. J. Potential roles of neutrophils in regulating intestinal mucosal inflammation of inflammatory bowel disease. J. Dig. Dis. 18, 495–503 (2017).
Acknowledgements
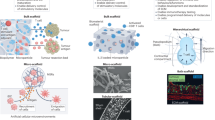
We thank D. Mooney, A. Khalil, B. Lowe, A. Singh and Z. Zhong for providing unpublished immunofluorescence images of thymus and tonsil primary tissues and organoids (Fig. 2). Y.S.M. acknowledges support from the CancerCare Manitoba Foundation.
Author information
Authors and Affiliations
Contributions
A.M. and N.G. conceptualized the manuscript. A.M., N.G., C.F.B. and Y.S.M. wrote the manuscript. A.M. and Y.S.M. edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Bioengineering thanks Yong Fan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Michaels, Y.S., Buchanan, C.F., Gjorevski, N. et al. Bioengineering translational models of lymphoid tissues. Nat Rev Bioeng 1, 731–748 (2023). https://doi.org/10.1038/s44222-023-00101-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44222-023-00101-0