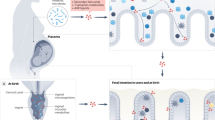
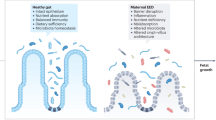
Abstract
Inflammatory bowel disease (IBD) has a peak age of diagnosis before the age of 35 years. Concerns about infertility, adverse pregnancy outcomes, and heritability of IBD have influenced decision-making for patients of childbearing age and their care providers. The interplay between the complex physiology in pregnancy and IBD can affect placental development, microbiome composition and responses to therapy. Current evidence has shown that effective disease management, including pre-conception counselling, multidisciplinary care and therapeutic agents to minimize disease activity, can improve pregnancy outcomes. This Review outlines the management of IBD in pregnancy and the safety of IBD therapies, including novel agents, with regard to both maternal and fetal health. The vast majority of IBD therapies can be used with low risk during pregnancy and lactation without substantial effects on neonatal outcomes.
Key points
-
Placental and lactation physiology involves a complicated milieu of cells, and includes immune-mediated effects, with implications for inflammatory bowel disease (IBD) and medication pharmacokinetics.
-
Both maternal and fetal microbiomes are influenced by pregnancy and IBD, and investigation into the role these interactions play in disease pathogenesis is ongoing.
-
Patients with no prior abdominal surgery and quiescent disease can anticipate similar rates of fertility and pregnancy outcomes to the general population.
-
Pre-conception counselling and multidisciplinary care, including maternal–fetal medicine where available, can help address patient concerns and improve pregnancy outcomes.
-
The vast majority of IBD therapies, including biologic therapies, can be used with low risk during pregnancy and breastfeeding, and uninterrupted therapy can help mitigate the risk of adverse outcomes.
-
Investigation to address knowledge gaps regarding the safety of new and evolving therapies is needed, particularly small-molecule medications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Shivashankar, R., Tremaine, W. J., Harmsen, W. S. & Loftus, E. V. Incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin. Gastroenterol. Hepatol. 15, 857–863 (2017).
Coward, S. et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology 156, 1345–1353.e4 (2019).
Erlebacher, A. & Fisher, S. J. Baby’s first organ. Sci. Am. 317, 46–53 (2017).
Huhn, O. et al. How do uterine natural killer and innate lymphoid cells contribute to successful pregnancy? Front. Immunol. 12, 607669 (2021).
Romanowska-Próchnicka, K. et al. The role of TNF-α and anti-TNF-α agents during preconception, pregnancy, and breastfeeding. Int. J. Mol. Sci. https://doi.org/10.3390/ijms22062922 (2021).
Pavlov, O., Selutin, A., Pavlova, O. & Selkov, S. Macrophages are a source of IL-17 in the human placenta. Am. J. Reprod. Immunol. 80, e13016 (2018).
Alijotas-Reig, J., Esteve-Valverde, E., Ferrer-Oliveras, R., Llurba, E. & Gris, J. M. Tumor necrosis factor-alpha and pregnancy: focus on biologics. An updated and comprehensive review. Clin. Rev. Allergy Immunol. 53, 40–53 (2017).
Blaisdell, A., Zhou, Y., Kattah, M. G., Fisher, S. J. & Mahadevan, U. Vedolizumab antagonizes MAdCAM-1-dependent human placental cytotrophoblast adhesion and invasion in vitro. Inflamm. Bowel Dis. https://doi.org/10.1093/ibd/izac056 (2022).
Maltepe, E. & Fisher, S. J. Placenta: the forgotten organ. Annu. Rev. Cell Dev. Biol. 31, 523–552 (2015).
Simister, N. E. Placental transport of immunoglobulin G. Vaccine 21, 3365–3369 (2003).
Roopenian, D. C. & Akilesh, S. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7, 715–725 (2007).
Porter, C. et al. Certolizumab pegol does not bind the neonatal Fc receptor (FcRn): consequences for FcRn-mediated in vitro transcytosis and ex vivo human placental transfer. J. Reprod. Immunol. 116, 7–12 (2016).
Gisbert, J. P. & Chaparro, M. Safety of new biologics (vedolizumab and ustekinumab) and small molecules (tofacitinib) during pregnancy: a review. Drugs 80, 1085–1100 (2020).
Datta, P., Baker, T. & Hale, T. W. Balancing the use of medications while maintaining breastfeeding. Clin. Perinatol. 46, 367–382 (2019).
Anderson, G. D. Using pharmacokinetics to predict the effects of pregnancy and maternal-infant transfer of drugs during lactation. Expert. Opin. Drug Metab. Toxicol. 2, 947–960 (2006).
Fleishaker, J. C., Desai, N. & McNamara, P. J. Factors affecting the milk-to-plasma drug concentration ratio in lactating women: physical interactions with protein and fat. J. Pharm. Sci. 76, 189–193 (1987).
Hunt, K. M. et al. Mastitis is associated with increased free fatty acids, somatic cell count, and interleukin-8 concentrations in human milk. Breastfeed. Med. 8, 105–110 (2013).
Tuaillon, E. et al. Subclinical mastitis occurs frequently in association with dramatic changes in inflammatory/anti-inflammatory breast milk components. Pediatr. Res. 81, 556–564 (2017).
Ansell, C., Moore, A. & Barrie, H. Electrolyte pH changes in human milk. Pediatr. Res. 11, 1177–1179 (1977).
Syversen, G. B. & Ratkje, S. K. Drug distribution within human milk phases. J. Pharm. Sci. 74, 1071–1074 (1985).
Koshimichi, H., Ito, K., Hisaka, A., Honma, M. & Suzuki, H. Analysis and prediction of drug transfer into human milk taking into consideration secretion and reuptake clearances across the mammary epithelia. Drug Metab. Dispos. 39, 2370–2380 (2011).
Anderson, P. O. Drugs in lactation. Pharm. Res. 35, 45 (2018).
Matro, R., Martin, C. F., Wolf, D., Shah, S. A. & Mahadevan, U. Exposure concentrations of infants breastfed by women receiving biologic therapies for inflammatory bowel diseases and effects of breastfeeding on infections and development. Gastroenterology 155, 696–704 (2018).
Atyeo, C. & Alter, G. The multifaceted roles of breast milk antibodies. Cell 184, 1486–1499 (2021).
Sartor, R. B. Microbial influences in inflammatory bowel diseases. Gastroenterology 134, 577–594 (2008).
Glover, L. E., Fennimore, B. & Wingfield, M. Inflammatory bowel disease: influence and implications in reproduction. Inflamm. Bowel Dis. 22, 2724–2732 (2016).
Edwards, S. M., Cunningham, S. A., Dunlop, A. L. & Corwin, E. J. The maternal gut microbiome during pregnancy. MCN Am. J. Matern. Child. Nurs. 42, 310–317 (2017).
Koren, O. et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150, 470–480 (2012).
Santacruz, A. et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 104, 83–92 (2010).
Mulak, A., Taché, Y. & Larauche, M. Sex hormones in the modulation of irritable bowel syndrome. World J. Gastroenterol. 20, 2433–2448 (2014).
Fuhler, G. M. The immune system and microbiome in pregnancy. Best. Pract. Res. Clin. Gastroenterol. 44-45, 101671 (2020).
Machiels, K. et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63, 1275–1283 (2014).
Torres, J. et al. Infants born to mothers with IBD present with altered gut microbiome that transfers abnormalities of the adaptive immune system to germ-free mice. Gut 69, 42–51 (2020).
Satokari, R., Grönroos, T., Laitinen, K., Salminen, S. & Isolauri, E. Bifidobacterium and Lactobacillus DNA in the human placenta. Lett. Appl. Microbiol. 48, 8–12 (2009).
Aagaard, K. et al. The placenta harbors a unique microbiome. Sci. Transl. Med. 6, 237ra265 (2014).
Ferretti, P. et al. Mother-to-infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host Microbe 24, 133–145.e5 (2018).
Mountifield, R., Bampton, P., Prosser, R., Muller, K. & Andrews, J. M. Fear and fertility in inflammatory bowel disease: a mismatch of perception and reality affects family planning decisions. Inflamm. Bowel Dis. 15, 720–725 (2009).
Walldorf, J., Brunne, S., Gittinger, F. S. & Michl, P. Family planning in inflammatory bowel disease: childlessness and disease-related concerns among female patients. Eur. J. Gastroenterol. Hepatol. 30, 310–315 (2018).
Aboubakr, A., Riggs, A. R., Jimenez, D., Mella, M. T. & Dubinsky, M. C. Identifying patient priorities for preconception and pregnancy counseling in IBD. Dig. Dis. Sci. 66, 1829–1835 (2021).
de Lima, A., Zelinkova, Z., Mulders, A. G. & van der Woude, C. J. Preconception care reduces relapse of inflammatory bowel disease during pregnancy. Clin. Gastroenterol. Hepatol. 14, 1285–1292.e1 (2016).
Laube, R., Paramsothy, S. & Leong, R. W. Review of pregnancy in Crohn’s disease and ulcerative colitis. Ther. Adv. Gastroenterol. 14, 17562848211016242 (2021).
De Dombal, F. T., Watts, J. M., Watkinson, G. & Goligher, J. C. Ulcerative colitis and pregnancy. Lancet 2, 599–602 (1965).
Hudson, M. et al. Fertility and pregnancy in inflammatory bowel disease. Int. J. Gynaecol. Obstet. 58, 229–237 (1997).
Korelitz, B. I. Inflammatory bowel disease and pregnancy. Gastroenterol. Clin. North. Am. 27, 213–224 (1998).
Ørding Olsen, K., Juul, S., Berndtsson, I., Oresland, T. & Laurberg, S. Ulcerative colitis: female fecundity before diagnosis, during disease, and after surgery compared with a population sample. Gastroenterology 122, 15–19 (2002).
Waljee, A., Waljee, J., Morris, A. M. & Higgins, P. D. Threefold increased risk of infertility: a meta-analysis of infertility after ileal pouch anal anastomosis in ulcerative colitis. Gut 55, 1575–1580 (2006).
Oresland, T. et al. Gynaecological and sexual function related to anatomical changes in the female pelvis after restorative proctocolectomy. Int. J. Colorectal Dis. 9, 77–81 (1994).
Friedman, S., Nielsen, J., Nøhr, E. A., Jølving, L. R. & Nørgård, B. M. Comparison of time to pregnancy in women with and without inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 18, 1537–1544.e1 (2020).
Ban, L., Tata, L. J., Humes, D. J., Fiaschi, L. & Card, T. Decreased fertility rates in 9639 women diagnosed with inflammatory bowel disease: a United Kingdom population-based cohort study. Aliment. Pharmacol. Ther. 42, 855–866 (2015).
Zhao, Y. et al. Risk factors associated with impaired ovarian reserve in young women of reproductive age with Crohn’s disease. Intest. Res. 18, 200–209 (2020).
Hernandez-Nieto, C. et al. Infertile patients with inflammatory bowel disease have comparable in vitro fertilization clinical outcomes to the general infertile population. Gynecol. Endocrinol. 36, 554–557 (2020).
Friedman, S., Larsen, P. V., Fedder, J. & Nørgård, B. M. The efficacy of assisted reproduction in women with inflammatory bowel disease and the impact of surgery – a nationwide cohort study. Inflamm. Bowel Dis. 23, 208–217 (2017).
Laube, R., Tran, Y., Paramsothy, S. & Leong, R. W. Assisted reproductive technology in Crohn’s disease and ulcerative colitis: a systematic review and meta-analysis. Am. J. Gastroenterol. 116, 2334–2344 (2021).
Kim, M. A. et al. The influence of disease activity on pregnancy outcomes in women with inflammatory bowel disease: a systematic review and meta-analysis. J. Crohns Colitis 15, 719–732 (2021).
Mahadevan, U. et al. Pregnancy outcomes in women with inflammatory bowel disease: a large community-based study from Northern California. Gastroenterology 133, 1106–1112 (2007).
Shand, A. W., Chen, J. S., Selby, W., Solomon, M. & Roberts, C. L. Inflammatory bowel disease in pregnancy: a population-based study of prevalence and pregnancy outcomes. BJOG 123, 1862–1870 (2016).
Mahadevan, U. et al. Pregnancy and neonatal outcomes after fetal exposure to biologics and thiopurines among women with inflammatory bowel disease. Gastroenterology 160, 1131–1139 (2021).
Abdul Sultan, A. et al. Adverse pregnancy outcomes among women with inflammatory bowel disease: a population-based study from England. Inflamm. Bowel Dis. 22, 1621–1630 (2016).
Boyd, H. A., Basit, S., Harpsøe, M. C., Wohlfahrt, J. & Jess, T. Inflammatory bowel disease and risk of adverse pregnancy outcomes. PLoS ONE 10, e0129567 (2015).
Lee, H. H. et al. Pregnancy outcomes in women with inflammatory bowel disease: a 10-year nationwide population-based cohort study. Aliment. Pharmacol. Ther. 51, 861–869 (2020).
Bröms, G. et al. Birth outcomes in women with inflammatory bowel disease: effects of disease activity and drug exposure. Inflamm. Bowel Dis. 20, 1091–1098 (2014).
Miller, J. P. Inflammatory bowel disease in pregnancy: a review. J. R. Soc. Med. 79, 221–225 (1986).
Abhyankar, A., Ham, M. & Moss, A. C. Meta-analysis: the impact of disease activity at conception on disease activity during pregnancy in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 38, 460–466 (2013).
de Lima-Karagiannis, A., Zelinkova-Detkova, Z. & van der Woude, C. J. The effects of active IBD during pregnancy in the era of novel IBD therapies. Am. J. Gastroenterol. 111, 1305–1312 (2016).
Rottenstreich, A. et al. Factors associated with inflammatory bowel disease flare during pregnancy among women with preconception remission. Dig. Dis. Sci. 66, 1189–1194 (2021).
Nguyen, G. C. et al. The Toronto consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology 150, 734–757.e1 (2016).
Mahadevan, U. et al. Inflammatory bowel disease in pregnancy clinical care pathway: a report from the American Gastroenterological Association IBD Parenthood Project Working Group. Gastroenterology 156, 1508–1524 (2019).
Jharap, B. et al. Intrauterine exposure and pharmacology of conventional thiopurine therapy in pregnant patients with inflammatory bowel disease. Gut 63, 451–457 (2014).
Flanagan, E. et al. Maternal thiopurine metabolism during pregnancy in inflammatory bowel disease and clearance of thiopurine metabolites and outcomes in exposed neonates. Aliment. Pharmacol. Ther. 53, 810–820 (2021).
Flanagan, E. et al. Infliximab, adalimumab and vedolizumab concentrations across pregnancy and vedolizumab concentrations in infants following intrauterine exposure. Aliment. Pharmacol. Ther. 52, 1551–1562 (2020).
Bell, S. J. & Flanagan, E. K. Updates in the management of inflammatory bowel disease during pregnancy. Med. J. Aust. 210, 276–280 (2019).
Klajnbard, A. et al. Laboratory reference intervals during pregnancy, delivery and the early postpartum period. Clin. Chem. Lab. Med. 48, 237–248 (2010).
Friedman, S., McElrath, T. F. & Wolf, J. L. Management of fertility and pregnancy in women with inflammatory bowel disease: a practical guide. Inflamm. Bowel Dis. 19, 2937–2948 (2013).
Julsgaard, M. et al. Fecal calprotectin is not affected by pregnancy: clinical implications for the management of pregnant patients with inflammatory bowel disease. Inflamm. Bowel Dis. 23, 1240–1246 (2017).
Rottenstreich, A. et al. Clinical utility of fecal calprotectin in monitoring disease activity and predicting relapse in pregnant patients with inflammatory bowel diseases. Eur. J. Intern. Med. 77, 105–110 (2020).
de Lima, A., Zelinkova, Z. & van der Woude, C. J. A prospective study of the safety of lower gastrointestinal endoscopy during pregnancy in patients with inflammatory bowel disease. J. Crohns Colitis 9, 519–524 (2015).
De Voogd, F. et al. Intestinal ultrasound to evaluate treatment response during pregnancy in patients with inflammatory bowel disease. Inflamm. Bowel Dis. https://doi.org/10.1093/ibd/izab216 (2021).
Ko, M. S., Rudrapatna, V. A., Avila, P. & Mahadevan, U. Safety of flexible sigmoidoscopy in pregnant patients with known or suspected inflammatory bowel disease. Dig. Dis. Sci. 65, 2979–2985 (2020).
Shergill, A. K. et al. Guidelines for endoscopy in pregnant and lactating women. Gastrointest. Endosc. 76, 18–24 (2012).
Savas, N. Gastrointestinal endoscopy in pregnancy. World J. Gastroenterol. 20, 15241–15252 (2014).
Bernstein, C. N. et al. Increased burden of psychiatric disorders in inflammatory bowel disease. Inflamm. Bowel Dis. 25, 360–368 (2019).
Vigod, S. N. et al. Inflammatory bowel disease and new-onset psychiatric disorders in pregnancy and post partum: a population-based cohort study. Gut 68, 1597–1605 (2019).
Selinger, C. et al. Standards for the provision of antenatal care for patients with inflammatory bowel disease: guidance endorsed by the British Society of Gastroenterology and the British Maternal and Fetal Medicine Society. Frontline Gastroenterol. 12, 182–187 (2021).
Ota, K. et al. Vitamin D deficiency may be a risk factor for recurrent pregnancy losses by increasing cellular immunity and autoimmunity. Hum. Reprod. 29, 208–219 (2014).
Holick, M. F. et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 96, 1911–1930 (2011).
Lee, S. et al. Pregnant women with inflammatory bowel disease are at increased risk of vitamin D insufficiency: a cross-sectional study. J. Crohns Colitis 12, 702–709 (2018).
van der Woude, C. J. et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J. Crohns Colitis 9, 107–124 (2015).
Hernández-Díaz, S., Werler, M. M., Walker, A. M. & Mitchell, A. A. Folic acid antagonists during pregnancy and the risk of birth defects. N. Engl. J. Med. 343, 1608–1614 (2000).
Sauk, J. & Kane, S. The use of medications for inflammatory bowel disease during pregnancy and nursing. Expert. Opin. Pharmacother. 6, 1833–1839 (2005).
Milman, N. Prepartum anaemia: prevention and treatment. Ann. Hematol. 87, 949–959 (2008).
Lopez, A., Cacoub, P., Macdougall, I. C. & Peyrin-Biroulet, L. Iron deficiency anaemia. Lancet 387, 907–916 (2016).
Pavord, S. et al. UK guidelines on the management of iron deficiency in pregnancy. Br. J. Haematol. 188, 819–830 (2020).
Auerbach, M. et al. Results of the first American prospective study of intravenous iron in oral iron-intolerant iron-deficient gravidas. Am. J. Med. 130, 1402–1407 (2017).
US Food and Drug Administration. Pregnancy and lactation labeling (drugs) final rule. FDA https://www.fda.gov/drugs/labeling-information-drug-products/pregnancy-and-lactation-labeling-drugs-final-rule (2021).
Caro-Patón, T. et al. Is metronidazole teratogenic? A meta-analysis. Br. J. Clin. Pharmacol. 44, 179–182 (1997).
Bar-Oz, B., Moretti, M. E., Boskovic, R., O’Brien, L. & Koren, G. The safety of quinolones–a meta-analysis of pregnancy outcomes. Eur. J. Obstet. Gynecol. Reprod. Biol. 143, 75–78 (2009).
Afzali, A. Inflammatory bowel disease during pregnancy: management of a disease flare or remission. Curr. Opin. Gastroenterol. 35, 281–287 (2019).
Khanna, S., Shin, A. & Kelly, C. P. Management of Clostridium difficile infection in inflammatory bowel disease: expert review from the Clinical Practice Updates Committee of the AGA Institute. Clin. Gastroenterol. Hepatol. 15, 166–174 (2017).
ViroPharma. Highlights of prescribing information: Vancocin capsules (vancomycin hydrochloride). FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/050606s028lbl.pdf (2011).
Restellini, S. et al. Update on the management of inflammatory bowel disease during pregnancy and breastfeeding. Digestion 101, 27–42 (2020).
Jarde, A. et al. Pregnancy outcomes in women taking probiotics or prebiotics: a systematic review and meta-analysis. BMC Pregnancy Childbirth 18, 14 (2018).
De Felice, K. M. & Kane, S. V. Inflammatory bowel disease in women of reproductive age. Expert. Rev. Gastroenterol. Hepatol. 8, 417–425 (2014).
Nørgård, B., Fonager, K., Pedersen, L., Jacobsen, B. A. & Sørensen, H. T. Birth outcome in women exposed to 5-aminosalicylic acid during pregnancy: a Danish cohort study. Gut 52, 243–247 (2003).
Nørgård, B., Pedersen, L., Christensen, L. A. & Sørensen, H. T. Therapeutic drug use in women with Crohn’s disease and birth outcomes: a Danish nationwide cohort study. Am. J. Gastroenterol. 102, 1406–1413 (2007).
Mahadevan, U. Fertility and pregnancy in the patient with inflammatory bowel disease. Gut 55, 1198–1206 (2006).
Rahimi, R., Nikfar, S., Rezaie, A. & Abdollahi, M. Pregnancy outcome in women with inflammatory bowel disease following exposure to 5-aminosalicylic acid drugs: a meta-analysis. Reprod. Toxicol. 25, 271–275 (2008).
Katz, J. A. & Pore, G. Inflammatory bowel disease and pregnancy. Inflamm. Bowel Dis. 7, 146–157 (2001).
Hashash, J. G. & Kane, S. Pregnancy and inflammatory bowel disease. Gastroenterol. Hepatol. 11, 96–102 (2015).
Bandoli, G., Palmsten, K., Forbess Smith, C. J. & Chambers, C. D. A review of systemic corticosteroid use in pregnancy and the risk of select pregnancy and birth outcomes. Rheum. Dis. Clin. North. Am. 43, 489–502 (2017).
Park-Wyllie, L. et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta-analysis of epidemiological studies. Teratology 62, 385–392 (2000).
Odufalu, F. D., Long, M., Lin, K. & Mahadevan, U. Exposure to corticosteroids in pregnancy is associated with adverse perinatal outcomes among infants of mothers with inflammatory bowel disease: results from the PIANO registry. Gut https://doi.org/10.1136/gutjnl-2021-325317 (2021).
Beaulieu, D. B. et al. Budesonide induction and maintenance therapy for Crohn’s disease during pregnancy. Inflamm. Bowel Dis. 15, 25–28 (2009).
Murphy, V. E. et al. Metabolism of synthetic steroids by the human placenta. Placenta 28, 39–46 (2007).
Gluck, P. A. & Gluck, J. C. A review of pregnancy outcomes after exposure to orally inhaled or intranasal budesonide. Curr. Med. Res. Opin. 21, 1075–1084 (2005).
de Boer, N. K. et al. Azathioprine use during pregnancy: unexpected intrauterine exposure to metabolites. Am. J. Gastroenterol. 101, 1390–1392 (2006).
Kanis, S. L. et al. Health outcomes of 1000 children born to mothers with inflammatory bowel disease in their first 5 years of life. Gut 70, 1266–1274 (2021).
Nasser, R. et al. Thiopurine hepatotoxicity can mimic intrahepatic cholestasis of pregnancy. Clin. Res. Hepatol. Gastroenterol. 44, e29–e31 (2020).
Akbari, M., Shah, S., Velayos, F. S., Mahadevan, U. & Cheifetz, A. S. Systematic review and meta-analysis on the effects of thiopurines on birth outcomes from female and male patients with inflammatory bowel disease. Inflamm. Bowel Dis. 19, 15–22 (2013).
Hutson, J. R., Matlow, J. N., Moretti, M. E. & Koren, G. The fetal safety of thiopurines for the treatment of inflammatory bowel disease in pregnancy. J. Obstet. Gynaecol. 33, 1–8 (2013).
Casanova, M. J. et al. Safety of thiopurines and anti-TNF-α drugs during pregnancy in patients with inflammatory bowel disease. Am. J. Gastroenterol. 108, 433–440 (2013).
Koslowsky, B. et al. Thiopurine therapy for inflammatory bowel disease during pregnancy is not associated with anemia in the infant. Dig. Dis. Sci. 64, 2286–2290 (2019).
Sheikh, M., Nelson-Piercy, C., Duley, J., Florin, T. & Ansari, A. Successful pregnancies with thiopurine-allopurinol co-therapy for inflammatory bowel disease. J. Crohns Colitis 9, 680–684 (2015).
van den Berg, S. A. et al. Safety of tioguanine during pregnancy in inflammatory bowel disease. J. Crohns Colitis 10, 159–165 (2016).
Martínez Lopez, J. A., Loza, E. & Carmona, L. Systematic review on the safety of methotrexate in rheumatoid arthritis regarding the reproductive system (fertility, pregnancy, and breastfeeding). Clin. Exp. Rheumatol. 27, 678–684 (2009).
Weber-Schoendorfer, C. et al. Pregnancy outcome after methotrexate treatment for rheumatic disease prior to or during early pregnancy: a prospective multicenter cohort study. Arthritis Rheumatol. 66, 1101–1110 (2014).
Feldkamp, M. & Carey, J. C. Clinical teratology counseling and consultation case report: low dose methotrexate exposure in the early weeks of pregnancy. Teratology 47, 533–539 (1993).
Bar Oz, B., Hackman, R., Einarson, T. & Koren, G. Pregnancy outcome after cyclosporine therapy during pregnancy: a meta-analysis. Transplantation 71, 1051–1055 (2001).
Jain, A. B. et al. Pregnancy after liver transplantation with tacrolimus immunosuppression: a single center’s experience update at 13 years. Transplantation 76, 827–832 (2003).
Branche, J. et al. Cyclosporine treatment of steroid-refractory ulcerative colitis during pregnancy. Inflamm. Bowel Dis. 15, 1044–1048 (2009).
Baumgart, D. C., Sturm, A., Wiedenmann, B. & Dignass, A. U. Uneventful pregnancy and neonatal outcome with tacrolimus in refractory ulcerative colitis. Gut 54, 1822–1823 (2005).
Ponticelli, C. & Glassock, R. J. Prevention of complications from use of conventional immunosuppressants: a critical review. J. Nephrol. 32, 851–870 (2019).
Chaparro, M. et al. Long-term safety of in utero exposure to anti-TNFα drugs for the treatment of inflammatory bowel disease: results from the multicenter European TEDDY study. Am. J. Gastroenterol. 113, 396–403 (2018).
Luu, M. et al. Continuous anti-TNFα use throughout pregnancy: possible complications for the mother but not for the fetus. a retrospective cohort on the French National Health Insurance Database (EVASION). Am. J. Gastroenterol. 113, 1669–1677 (2018).
Torres, J. et al. European Crohn’s and colitis guidelines on sexuality, fertility, pregnancy, and lactation. J. Crohns Colitis https://doi.org/10.1093/ecco-jcc/jjac115 (2022).
Julsgaard, M. et al. Concentrations of adalimumab and infliximab in mothers and newborns, and effects on infection. Gastroenterology 151, 110–119 (2016).
Hellwig, K., Haghikia, A. & Gold, R. Pregnancy and natalizumab: results of an observational study in 35 accidental pregnancies during natalizumab treatment. Mult. Scler. 17, 958–963 (2011).
Landi, D. et al. Continuation of natalizumab versus interruption is associated with lower risk of relapses during pregnancy and postpartum in women with MS [abstract 338]. Mult. Scler. J. 25 (Suppl. 2), 892–894 (2019).
Moens, A. et al. Pregnancy outcomes in inflammatory bowel disease patients treated with vedolizumab, anti-TNF or conventional therapy: results of the European CONCEIVE study. Aliment. Pharmacol. Ther. 51, 129–138 (2020).
Wils, P. et al. Safety of ustekinumab or vedolizumab in pregnant inflammatory bowel disease patients: a multicentre cohort study. Aliment. Pharmacol. Ther. 53, 460–470 (2021).
Bar-Gil Shitrit, A. et al. Exposure to vedolizumab in IBD pregnant women appears of low risk for mother and neonate: a first prospective comparison study. Am. J. Gastroenterol. 114, 1172–1175 (2019).
Bell, C., Tandon, P., Lentz, E., Marshall, J. K. & Narula, N. Systematic review and meta-analysis: safety of vedolizumab during pregnancy in patients with inflammatory bowel disease. J. Gastroenterol. Hepatol. 36, 2640–2648 (2021).
Ferrante, M. et al. Long-term safety and efficacy of risankizumab treatment in patients with Crohn’s disease: results from the phase 2 open-label extension study. J. Crohns Colitis 15, 2001–2010 (2021).
Volger, S. et al. Pregnancy outcomes in women with psoriasis, psoriatic arthritis, Crohn’s disease and ulcerative colitis treated with ustekinumab [abstract Sa1827]. Gastroenterology 158 (6 Suppl. 1), S-442 (2020).
Chugh, R. et al. Maternal and neonatal outcomes in vedolizumab and ustekinumab exposed pregnancies: results from the PIANO registry [abstract S719]. Am. J. Gastroenterol. 117(10S), e502–e503 (2022).
Owczarek, W. et al. The use of biological drugs in psoriasis patients prior to pregnancy, during pregnancy and lactation: a review of current clinical guidelines. Postepy Dermatol. Alergol. 37, 821–830 (2020).
Danese, S. et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet 399, 2113–2128 (2022).
Pfizer. Highlights of prescribing information: Xeljanz/Xeljanz XR (tofacitinib). FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/203214s018lbl.pdf (2018).
Mahadevan, U. et al. Outcomes of pregnancies with maternal/paternal exposure in the tofacitinib safety databases for ulcerative colitis. Inflamm. Bowel Dis. 24, 2494–2500 (2018).
Abbvie. Highlights of prescribing information: Rinvoq (upadacitinib). FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211675s000lbl.pdf (2019).
Dubinsky, M. C. et al. Pregnancy outcomes in the ozanimod clinical development program in relapsing multiple sclerosis, ulcerative colitis, and Crohn’s disease [abstract DOP53]. J. Crohns Colitis 15 (Suppl. 1), S088–S089 (2021).
Celgene. Highlights of prescribing information: Zeposia (ozanimod). FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209899s000lbl.pdf (2020).
Lopez-Leon, S. et al. A systematic review and meta-analyses of pregnancy and fetal outcomes in women with multiple sclerosis: a contribution from the IMI2 ConcePTION project. J. Neurol. 267, 2721–2731 (2020).
Seah, D. & De Cruz, P. Review article: the practical management of acute severe ulcerative colitis. Aliment. Pharmacol. Ther. 43, 482–513 (2016).
Ollech, J. E. et al. Effective treatment of acute severe ulcerative colitis in pregnancy is associated with good maternal and fetal outcomes. Clin. Gastroenterol. Hepatol. 19, 2444–2446.e2 (2021).
Chaparro, M. et al. Surgery due to inflammatory bowel disease during pregnancy: mothers and offspring outcomes from an Ecco Confer Multicentre Case Series (Scar Study). J. Crohns Colitis https://doi.org/10.1093/ecco-jcc/jjac050 (2022).
Killeen, S., Gunn, J. & Hartley, J. Surgical management of complicated and medically refractory inflammatory bowel disease during pregnancy. Colorectal Dis. 19, 123–138 (2017).
Novacek, G. et al. Inflammatory bowel disease is a risk factor for recurrent venous thromboembolism. Gastroenterology 139, 779–787 (2010).
Nguyen, G. C., Boudreau, H., Harris, M. L. & Maxwell, C. V. Outcomes of obstetric hospitalizations among women with inflammatory bowel disease in the United States. Clin. Gastroenterol. Hepatol. 7, 329–334 (2009).
Foulon, A. et al. Defining the most appropriate delivery mode in women with inflammatory bowel disease: a systematic review. Inflamm. Bowel Dis. 23, 712–720 (2017).
Friedman, S., Zegers, F. D., Jølving, L. R., Nielsen, J. & Nørgård, B. M. Postpartum surgical complications in women with inflammatory bowel disease after caesarian section: a Danish nationwide cohort study. J. Crohns Colitis https://doi.org/10.1093/ecco-jcc/jjab187 (2021).
Lever, G., Chipeta, H., Glanville, T. & Selinger, C. Perineal outcomes after delivery in 179 mothers with inflammatory bowel disease compared to 31 258 controls: a single-centre cohort study. J. Crohns Colitis 16, 511–514 (2022).
Remzi, F. H. et al. Vaginal delivery after ileal pouch–anal anastomosis: a word of caution. Dis. Colon. Rectum 48, 1691–1699 (2005).
Hahnloser, D. et al. Pregnancy and delivery before and after ileal pouch–anal anastomosis for inflammatory bowel disease: immediate and long-term consequences and outcomes. Dis. Colon. Rectum 47, 1127–1135 (2004).
Van Horn, C. & Barrett, P. Pregnancy, delivery, and postpartum experiences of fifty-four women with ostomies. J. Wound Ostomy Cont. Nurs. 24, 151–162 (1997).
PAPooSE Study Group. Pregnancy outcomes after stoma surgery for inflammatory bowel disease: the results of a retrospective multicentre audit. Colorectal Dis. 24, 838–844 (2022).
Aukamp, V. & Sredl, D. Collaborative care management for a pregnant woman with an ostomy. Complement. Ther. Nurs. Midwifery 10, 5–12 (2004).
Peeters, M. et al. Familial aggregation in Crohn’s disease: increased age-adjusted risk and concordance in clinical characteristics. Gastroenterology 111, 597–603 (1996).
Laharie, D. et al. Inflammatory bowel disease in spouses and their offspring. Gastroenterology 120, 816–819 (2001).
Barclay, A. R. et al. Systematic review: the role of breastfeeding in the development of pediatric inflammatory bowel disease. J. Pediatr. 155, 421–426 (2009).
Penders, J. et al. Gut microbiota composition and development of atopic manifestations in infancy: the KOALA Birth Cohort Study. Gut 56, 661–667 (2007).
Turfkruyer, M. & Verhasselt, V. Breast milk and its impact on maturation of the neonatal immune system. Curr. Opin. Infect. Dis. 28, 199–206 (2015).
Meng, X. et al. The profile of human milk metabolome, cytokines, and antibodies in inflammatory bowel diseases versus healthy mothers, and potential impact on the newborn. J. Crohns Colitis 13, 431–441 (2019).
Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 129, e827–e841 (2012).
Passmore, C. M., McElnay, J. C., Rainey, E. A. & D’Arcy, P. F. Metronidazole excretion in human milk and its effect on the suckling neonate. Br. J. Clin. Pharmacol. 26, 45–51 (1988).
Biedermann, L., Rogler, G., Vavricka, S. R., Seibold, F. & Seirafi, M. Pregnancy and breastfeeding in inflammatory bowel disease. Digestion 86, 45–54 (2012).
Drugs and Lactation Database (LactMed). Vancomycin (National Institute of Child Health and Human Development, 2022).
Klotz, U. & Harings-Kaim, A. Negligible excretion of 5-aminosalicylic acid in breast milk. Lancet 342, 618–619 (1993).
Ost, L., Wettrell, G., Björkhem, I. & Rane, A. Prednisolone excretion in human milk. J. Pediatr. 106, 1008–1011 (1985).
Christensen, L. A., Dahlerup, J. F., Nielsen, M. J., Fallingborg, J. F. & Schmiegelow, K. Azathioprine treatment during lactation. Aliment. Pharmacol. Ther. 28, 1209–1213 (2008).
Moretti, M. E. et al. Cyclosporine excretion into breast milk. Transplantation 75, 2144–2146 (2003).
Lahiff, C. & Moss, A. C. Cyclosporine in the management of severe ulcerative colitis while breast-feeding. Inflamm. Bowel Dis. 17, E78 (2011).
Drugs and Lactation Database (LactMed). Cyclosporine (National Institute of Child Health and Human Development, 2021).
Fritzsche, J., Pilch, A., Mury, D., Schaefer, C. & Weber-Schoendorfer, C. Infliximab and adalimumab use during breastfeeding. J. Clin. Gastroenterol. 46, 718–719 (2012).
Bar-Gil Shitrit, A. et al. Detection of ustekinumab in breast milk of nursing mothers with Crohn disease. Inflamm. Bowel Dis. 27, 742–745 (2021).
Flanagan, E. et al. Ustekinumab levels in pregnant women with inflammatory bowel disease and infants exposed in utero. Aliment. Pharmacol. Ther. 55, 700–704 (2022).
Angelberger, S. et al. Long-term follow-up of babies exposed to azathioprine in utero and via breastfeeding. J. Crohns Colitis 5, 95–100 (2011).
Beaulieu, D. B. et al. Use of biologic therapy by pregnant women with inflammatory bowel disease does not affect infant response to vaccines. Clin. Gastroenterol. Hepatol. 16, 99–105 (2018).
Cheent, K. et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J. Crohns Colitis 4, 603–605 (2010).
Takeda. Highlights of prescribing information: Entyvio (vedolizumab). FDA https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/125476Orig1s046lbl.pdf (2022).
European Medicines Agency. Direct healthcare professional communication (DHPC): Infliximab (Remicade, Flixabi, Inflectra, Remsima and Zessly): Use of live vaccines in infants exposed in utero or during breastfeeding. EMA https://www.ema.europa.eu/en/documents/dhpc/direct-healthcare-professional-communication-dhpc-infliximab-remicade-flixabi-inflectra-remsima_en.pdf (2022).
Lim, J., Hammami, M. B. & Mahadevan, U. Infliximab use in a child with ulcerative colitis and prior in utero exposure to infliximab. Gastroenterology 158, 1834–1835 (2020).
Ban, L., Tata, L. J., Fiaschi, L. & Card, T. Limited risks of major congenital anomalies in children of mothers with IBD and effects of medications. Gastroenterology 146, 76–84 (2014).
Bengtson, M. B. et al. Relationships between inflammatory bowel disease and perinatal factors: both maternal and paternal disease are related to preterm birth of offspring. Inflamm. Bowel Dis. 16, 847–855 (2010).
Bortoli, A. et al. Pregnancy before and after the diagnosis of inflammatory bowel diseases: retrospective case-control study. J. Gastroenterol. Hepatol. 22, 542–549 (2007).
Bortoli, A. et al. Pregnancy outcome in inflammatory bowel disease: prospective European case-control ECCO-EpiCom study, 2003-2006. Aliment. Pharmacol. Ther. 34, 724–734 (2011).
Bröms, G., Granath, F., Stephansson, O. & Kieler, H. Preterm birth in women with inflammatory bowel disease – the association with disease activity and drug treatment. Scand. J. Gastroenterol. 51, 1462–1469 (2016).
Bush, M. C., Patel, S., Lapinski, R. H. & Stone, J. L. Perinatal outcomes in inflammatory bowel disease. J. Matern. Fetal Neonatal Med. 15, 237–241 (2004).
Cleary, B. J. & Källén, B. Early pregnancy azathioprine use and pregnancy outcomes. Birth Defects Res. A Clin. Mol. Teratol. 85, 647–654 (2009).
Clowse, M. E. et al. Pregnancy outcomes in subjects exposed to certolizumab pegol. J. Rheumatol. 42, 2270–2278 (2015).
Coelho, J. et al. Pregnancy outcome in patients with inflammatory bowel disease treated with thiopurines: cohort from the CESAME study. Gut 60, 198–203 (2011).
Dominitz, J. A., Young, J. C. & Boyko, E. J. Outcomes of infants born to mothers with inflammatory bowel disease: a population-based cohort study. Am. J. Gastroenterol. 97, 641–648 (2002).
Elbaz, G., Fich, A., Levy, A., Holcberg, G. & Sheiner, E. Inflammatory bowel disease and preterm delivery. Int. J. Gynaecol. Obstet. 90, 193–197 (2005).
Francella, A. et al. The safety of 6-mercaptopurine for childbearing patients with inflammatory bowel disease: a retrospective cohort study. Gastroenterology 124, 9–17 (2003).
Goldstein, L. H. et al. Pregnancy outcome of women exposed to azathioprine during pregnancy. Birth Defects Res. A Clin. Mol. Teratol. 79, 696–701 (2007).
Kammerlander, H. et al. The effect of disease activity on birth outcomes in a nationwide cohort of women with moderate to severe inflammatory bowel disease. Inflamm. Bowel Dis. 23, 1011–1018 (2017).
Kanis, S. L., de Lima-Karagiannis, A., de Boer, N. K. H. & van der Woude, C. J. Use of thiopurines during conception and pregnancy is not associated with adverse pregnancy outcomes or health of infants at one year in a prospective study. Clin. Gastroenterol. Hepatol. 15, 1232–1241.e1 (2017).
Koslowsky, B. et al. Pregnancy-onset inflammatory bowel disease: a subtle diagnosis. Inflamm. Bowel Dis. 24, 1826–1832 (2018).
Lichtenstein, G. R. et al. Pregnancy outcomes reported during the 13-year TREAT registry: a descriptive report. Am. J. Gastroenterol. 113, 1678–1688 (2018).
Lin, H. C. et al. Ulcerative colitis and pregnancy outcomes in an Asian population. Am. J. Gastroenterol. 105, 387–394 (2010).
Meyer, A., Drouin, J., Weill, A., Carbonnel, F. & Dray-Spira, R. Pregnancy in women with inflammatory bowel disease: a French nationwide study 2010-2018. Aliment. Pharmacol. Ther. 52, 1480–1490 (2020).
Meyer, A., Drouin, J., Weill, A., Carbonnel, F. & Dray-Spira, R. Comparative study of pregnancy outcomes in women with inflammatory bowel disease treated with thiopurines and/or anti-TNF: a French nationwide study 2010-2018. Aliment. Pharmacol. Ther. 54, 302–311 (2021).
Moskovitz, D. N. et al. The effect on the fetus of medications used to treat pregnant inflammatory bowel-disease patients. Am. J. Gastroenterol. 99, 656–661 (2004).
Naganuma, M. et al. Conception and pregnancy outcome in women with inflammatory bowel disease: a multicentre study from Japan. J. Crohns Colitis 5, 317–323 (2011).
Nørgård, B., Fonager, K., Sørensen, H. T. & Olsen, J. Birth outcomes of women with ulcerative colitis: a nationwide Danish cohort study. Am. J. Gastroenterol. 95, 3165–3170 (2000).
Padhan, R. K. et al. Long-term disease course and pregnancy outcomes in women with inflammatory bowel disease: an Indian cohort study. Dig. Dis. Sci. 62, 2054–2062 (2017).
Raatikainen, K., Mustonen, J., Pajala, M. O., Heikkinen, M. & Heinonen, S. The effects of pre- and post-pregnancy inflammatory bowel disease diagnosis on birth outcomes. Aliment. Pharmacol. Ther. 33, 333–339 (2011).
Schnitzler, F. et al. Outcome of pregnancy in women with inflammatory bowel disease treated with antitumor necrosis factor therapy. Inflamm. Bowel Dis. 17, 1846–1854 (2011).
Seirafi, M. et al. Factors associated with pregnancy outcome in anti-TNF treated women with inflammatory bowel disease. Aliment. Pharmacol. Ther. 40, 363–373 (2014).
Smink, M., Lotgering, F. K., Albers, L. & de Jong, D. J. Effect of childbirth on the course of Crohn’s disease; results from a retrospective cohort study in the Netherlands. BMC Gastroenterol. 11, 6 (2011).
Stephansson, O. et al. Crohn’s disease is a risk factor for preterm birth. Clin. Gastroenterol. Hepatol. 8, 509–515 (2010).
Stephansson, O. et al. Congenital abnormalities and other birth outcomes in children born to women with ulcerative colitis in Denmark and Sweden. Inflamm. Bowel Dis. 17, 795–801 (2011).
Tandon, P., Diong, C., Chong, R. Y. & Nguyen, G. C. Regional variation in pregnancy outcomes amongst women in inflammatory bowel disease: a population-based cohort study. Can. J. Gastroenterol. Hepatol. 2021, 3037128 (2021).
Acknowledgements
The authors thank D. Johnson and J. Sauberan for their clinical insights.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
U.M. has acted as a consultant for Abbvie, Bristol Myers Squibb, Gilead, Janssen and Pfizer, Takeda. M.N.B. declares no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Vivian Huang, Parul Tandon, Sally Bell, Ralley Prentice and Christian Selinger for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brondfield, M.N., Mahadevan, U. Inflammatory bowel disease in pregnancy and breastfeeding. Nat Rev Gastroenterol Hepatol 20, 504–523 (2023). https://doi.org/10.1038/s41575-023-00758-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-023-00758-3
This article is cited by
-
Pharmacokinetics of Monoclonal Antibodies Throughout Pregnancy: A Systematic Literature Review
Clinical Pharmacokinetics (2024)