Abstract
Ulcerative colitis (UC) is a chronic inflammatory bowel disease of unknown aetiology affecting the colon and rectum. Multiple factors, such as genetic background, environmental and luminal factors, and mucosal immune dysregulation, have been suggested to contribute to UC pathogenesis. UC has evolved into a global burden given its high incidence in developed countries and the substantial increase in incidence in developing countries. An improved understanding of the mechanisms underlying UC has led to the emergence of new treatments. Since the early 2000s, anti-tumour necrosis factor (TNF) treatment has significantly improved treatment outcomes. Advances in medical treatments have enabled a paradigm shift in treatment goals from symptomatic relief to endoscopic and histological healing to achieve better long-term outcomes and, consequently, diagnostic modalities have also been improved to monitor disease activity more tightly. Despite these improvements in patient care, a substantial proportion of patients, for example, those who are refractory to medical treatment or those who develop colitis-associated colorectal dysplasia or cancer, still require restorative proctocolectomy. The development of novel drugs and improvement of the treatment strategy by implementing personalized medicine are warranted to achieve optimal disease control. However, delineating the aetiology of UC is necessary to ultimately achieve disease cure.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Wilks, S. Morbid appearances in the intestine of Miss Bankes. Med. Gaz. 2, 264–265 (1859). This paper is the first historical report of UC.
Hibi, T. & Ogata, H. Novel pathophysiological concepts of inflammatory bowel disease. J. Gastroenterol. 41, 10–16 (2006).
Ungaro, R., Mehandru, S., Allen, P. B., Peyrin-Biroulet, L. & Colombel, J.-F. Ulcerative colitis. Lancet 389, 1756–1770 (2017).
Rubin, D. T., Ananthakrishnan, A. N., Siegel, C. A., Sauer, B. G. & Long, M. D. ACG clinical guideline: ulcerative colitis in adults. Am. J. Gastroenterol. 114, 384–413 (2019). This paper describes the clinical guidelines on the management of UC recommended by the American College of Gastroenterology.
Matsuoka, K. et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J. Gastroenterol. 53, 305–353 (2018).
Magro, F. et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and Ileo-anal pouch disorders. J. Crohns Colitis 11, 649–670 (2017). Consensus guidelines on the diagnosis and management of UC by the European Crohn’s and Colitis Organisation.
Truelove, S. C. & Witts, L. J. Cortisone in ulcerative colitis; final report on a therapeutic trial. BMJ 2, 1041–1048 (1955). This paper describes the results from a clinical trial on the use of steroids in UC.
Gallo, G., Kotze, P. G. & Spinelli, A. Surgery in ulcerative colitis: When? How? Best Pract. Res. Clin. Gastroenterol. 32–33, 71–78 (2018).
Ungaro, R., Colombel, J.-F., Lissoos, T. & Peyrin-Biroulet, L. A treat-to-target update in ulcerative colitis: a systematic review. Am. J. Gastroenterol. 114, 874–883 (2019).
Colombel, J.-F., D’haens, G., Lee, W.-J., Petersson, J. & Panaccione, R. Outcomes and strategies to support a treat-to-target approach in inflammatory bowel disease: a systematic review. J. Crohns Colitis 14, 254–266 (2020).
Peyrin-Biroulet, L. et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am. J. Gastroenterol. 110, 1324–1338 (2015). Consensus statements on treat-to-target strategy in the management of UC.
Ozaki, R. et al. Histological risk factors to predict clinical relapse in ulcerative colitis with endoscopically normal mucosa. J. Crohn’s Colitis 12, 1288–1294 (2018).
Peyrin-Biroulet, L., Bressenot, A. & Kampman, W. Histologic remission: the ultimate therapeutic goal in ulcerative colitis? Clin. Gastroenterol. Hepatol. 12, 929–934.e2 (2014).
Bernstein, C. N. et al. The epidemiology of inflammatory bowel disease in Canada: a population-based study. Am. J. Gastroenterol. 101, 1559–1568 (2006).
Bitton, A. et al. Epidemiology of inflammatory bowel disease in Québec: recent trends. Inflamm. Bowel Dis. 20, 1770–1776 (2014).
Leddin, D., Tamim, H. & Levy, A. R. Decreasing incidence of inflammatory bowel disease in Eastern Canada: a population database study. BMC Gastroenterol. 14, 140 (2014).
Torabi, M. et al. Geographical variation and factors associated with inflammatory bowel disease in a Central Canadian province. Inflamm. Bowel Dis. 26, 581–590 (2020).
Coward, S. et al. Past and future burden of inflammatory bowel diseases based on modeling of population-based data. Gastroenterology 156, 1345–1353.e4 (2019).
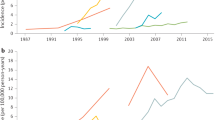
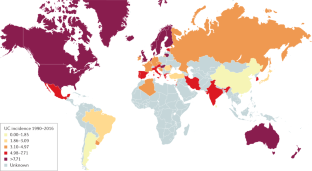
Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778 (2017). A systematic review providing an overview of the global epidemiology of IBD.
Ng, S. C. et al. Population density and risk of inflammatory bowel disease: a prospective population-based study in 13 countries or regions in Asia-Pacific. Am. J. Gastroenterol. 114, 107–115 (2019).
Banerjee, R. et al. IBD in India: similar phenotype but different demographics than the west. J. Clin. Gastroenterol. https://doi.org/10.1097/MCG.0000000000001282 (2019).
Kedia, S. & Ahuja, V. Epidemiology of inflammatory bowel disease in India: the Great Shift East. Inflamm. Intest. Dis. 2, 102–115 (2017).
Perminow, G. et al. A characterization in childhood inflammatory bowel disease, a new population-based inception cohort from South-Eastern Norway, 2005–07, showing increased incidence in Crohn’s disease. Scand. J. Gastroenterol. 44, 446–456 (2009).
Malmborg, P., Grahnquist, L., Lindholm, J., Montgomery, S. & Hildebrand, H. Increasing incidence of paediatric inflammatory bowel disease in northern Stockholm county, 2002–2007. J. Pediatr. Gastroenterol. Nutr. 57, 29–34 (2013).
Benchimol, E. I. et al. Trends in epidemiology of pediatric inflammatory bowel disease in Canada: distributed network analysis of multiple population-based provincial health administrative databases. Am. J. Gastroenterol. 112, 1120 (2017).
Muise, A. M., Snapper, S. B. & Kugathasan, S. The age of gene discovery in very early onset inflammatory bowel disease. Gastroenterology 143, 285–288 (2012).
Shah, S. C. et al. Sex-based differences in incidence of inflammatory bowel diseases—pooled analysis of population-based studies from western countries. Gastroenterology 155, 1079–1089.e3 (2018).
Benchimol, E. I. et al. Inflammatory bowel disease in immigrants to Canada and their children: a population-based cohort study. Am. J. Gastroenterol. 110, 553–563 (2015).
Ananthakrishnan, A. N. Environmental triggers for inflammatory bowel disease. Curr. Gastroenterol. Rep. 15, 302 (2013).
Bernstein, C. N. et al. World gastroenterology organisation global guidelines inflammatory bowel disease: update August 2015. J. Clin. Gastroenterol. 50, 803–818 (2016).
Wang, P. et al. Smoking and inflammatory bowel disease: a comparison of China, India, and the USA. Dig. Dis. Sci. 63, 2703–2713 (2018).
Amarapurkar, A. D. et al. Risk factors for inflammatory bowel disease: a prospective multi-center study. Indian J. Gastroenterol. 37, 189–195 (2018).
Lerner, A. & Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 14, 479–489 (2015).
Wang, Y.-F. et al. Multicenter case-control study of the risk factors for ulcerative colitis in China. World J. Gastroenterol. 19, 1827–1833 (2013).
Benchimol, E. I. et al. Rural and urban residence during early life is associated with risk of inflammatory bowel disease: a population-based inception and birth cohort study. Am. J. Gastroenterol. 112, 1412–1422 (2017).
Ungaro, R. et al. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: a meta-analysis. Am. J. Gastroenterol. 109, 1728–1738 (2014).
Lewis, J. D. & Abreu, M. T. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology 152, 398–414.e6 (2017).
Marrie, R. A. et al. Rising incidence of psychiatric disorders before diagnosis of immune-mediated inflammatory disease. Epidemiol. Psychiatr. Sci. 28, 333–342 (2019).
Bonaz, B. L. & Bernstein, C. N. Brain-gut interactions in inflammatory bowel disease. Gastroenterology 144, 36–49 (2013).
Myrelid, P., Landerholm, K., Nordenvall, C., Pinkney, T. D. & Andersson, R. E. Appendectomy and the risk of colectomy in ulcerative colitis: a national cohort study. Am. J. Gastroenterol. 112, 1311–1319 (2017).
Parian, A. et al. Appendectomy does not decrease the risk of future colectomy in UC: results from a large cohort and meta-analysis. Gut 66, 1390–1397 (2017).
Eaden, J. A., Abrams, K. R. & Mayberry, J. F. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 48, 526–535 (2001).
Olén, O. et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet 395, 123–131 (2020).
Bernstein, C. N. et al. The impact of inflammatory bowel disease in Canada 2018: extra-intestinal diseases in IBD. J. Can. Assoc. Gastroenterol. 2, S73–S80 (2019).
Desai, D. et al. Colorectal cancers in ulcerative colitis from a low-prevalence area for colon cancer. World J. Gastroenterol. 21, 3644–3649 (2015).
Wang, Y. N. et al. Clinical characteristics of ulcerative colitis-related colorectal cancer in Chinese patients. J. Dig. Dis. 18, 684–690 (2017).
Keller, D. S., Windsor, A., Cohen, R. & Chand, M. Colorectal cancer in inflammatory bowel disease: review of the evidence. Tech. Coloproctol. 23, 3–13 (2019).
Moody, G. A., Jayanthi, V., Probert, C. S., Mac Kay, H. & Mayberry, J. F. Long-term therapy with sulphasalazine protects against colorectal cancer in ulcerative colitis: a retrospective study of colorectal cancer risk and compliance with treatment in Leicestershire. Eur. J. Gastroenterol. Hepatol. 8, 1179–1183 (1996).
Eaden, J., Abrams, K., Ekbom, A., Jackson, E. & Mayberry, J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment. Pharmacol. Ther. 14, 145–153 (2000).
Bye, W. A., Nguyen, T. M., Parker, C. E., Jairath, V. & East, J. E. Strategies for detecting colon cancer in patients with inflammatory bowel disease. Cochrane Database Syst. Rev. 9, CD000279 (2017).
Turpin, W., Goethel, A., Bedrani, L. & Croitoru Mdcm, K. Determinants of IBD Heritability: Genes, Bugs, and More. Inflamm. Bowel Dis. 24, 1133–1148 (2018).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012). A study of genetics reporting 163 disease susceptibility loci for IBD.
Mirkov, M. U., Verstockt, B. & Cleynen, I. Genetics of inflammatory bowel disease: beyond NOD2. Lancet Gastroenterol. Hepatol. 2, 224–234 (2017).
Spehlmann, M. E. et al. Risk factors in German twins with inflammatory bowel disease: results of a questionnaire-based survey. J. Crohns Colitis 6, 29–42 (2012).
Halfvarson, J., Bodin, L., Tysk, C., Lindberg, E. & Järnerot, G. Inflammatory bowel disease in a Swedish twin cohort: a long-term follow-up of concordance and clinical characteristics. Gastroenterology 124, 1767–1773 (2003).
Ng, S. C., Woodrow, S., Patel, N., Subhani, J. & Harbord, M. Role of genetic and environmental factors in British twins with inflammatory bowel disease. Inflamm. Bowel Dis. 18, 725–736 (2012).
van Dongen, J., Slagboom, P. E., Draisma, H. H. M., Martin, N. G. & Boomsma, D. I. The continuing value of twin studies in the omics era. Nat. Rev. Genet. 13, 640–653 (2012).
Cleynen, I. et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: a genetic association study. Lancet 387, 156–167 (2016).
Kredel, L. I. et al. T-cell composition in ileal and colonic creeping fat - separating ileal from colonic Crohn’s disease. J. Crohns Colitis 13, 79–91 (2019).
Yoshitake, S., Kimura, A., Okada, M., Yao, T. & Sasazuki, T. HLA class II alleles in Japanese patients with inflammatory bowel disease. Tissue Antigens 53, 350–358 (1999).
Inoue, N. et al. Lack of common NOD2 variants in Japanese patients with Crohn’s disease. Gastroenterology 123, 86–91 (2002).
Martini, E., Krug, S. M., Siegmund, B., Neurath, M. F. & Becker, C. Mend your fences: the epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell Mol. Gastroenterol. Hepatol. 4, 33–46 (2017).
Van Klinken, B. J., Van der Wal, J. W., Einerhand, A. W., Büller, H. A. & Dekker, J. Sulphation and secretion of the predominant secretory human colonic mucin MUC2 in ulcerative colitis. Gut 44, 387–393 (1999).
Gitter, A. H., Wullstein, F., Fromm, M. & Schulzke, J. D. Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology 121, 1320–1328 (2001).
Büning, C. et al. Increased small intestinal permeability in ulcerative colitis: rather genetic than environmental and a risk factor for extensive disease? Inflamm. Bowel Dis. 18, 1932–1939 (2012).
Kaser, A., Adolph, T. E. & Blumberg, R. S. The unfolded protein response and gastrointestinal disease. Semin. Immunopathol. 35, 307–319 (2013).
Grootjans, J., Kaser, A., Kaufman, R. J. & Blumberg, R. S. The unfolded protein response in immunity and inflammation. Nat. Rev. Immunol. 16, 469–484 (2016).
Krug, S. M. et al. Tricellulin is regulated via interleukin-13-receptor α2, affects macromolecule uptake, and is decreased in ulcerative colitis. Mucosal Immunol. 11, 345–356 (2018).
Neurath, M. F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 20, 970–979 (2019).
Jorch, S. K. & Kubes, P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 23, 279–287 (2017).
Bennike, T. B. et al. Neutrophil extracellular traps in ulcerative colitis: a proteome analysis of intestinal biopsies. Inflamm. Bowel Dis. 21, 2052–2067 (2015).
Dinallo, V. et al. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J. Crohns Colitis 13, 772–784 (2019).
Camoglio, L., Te Velde, A. A., Tigges, A. J., Das, P. K. & Van Deventer, S. J. Altered expression of interferon-gamma and interleukin-4 in inflammatory bowel disease. Inflamm. Bowel Dis. 4, 285–290 (1998).
Heller, F. et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology 129, 550–564 (2005).
Heller, F., Fuss, I. J., Nieuwenhuis, E. E., Blumberg, R. S. & Strober, W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity 17, 629–638 (2002).
Danese, S. et al. Tralokinumab for moderate-to-severe UC: a randomised, double-blind, placebo-controlled, phase IIa study. Gut 64, 243–249 (2015).
Kindler, V., Sappino, A. P., Grau, G. E., Piguet, P. F. & Vassalli, P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell 56, 731–740 (1989).
Lissner, D. et al. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm. Bowel Dis. 21, 1297–1305 (2015).
Hanauer, S. B. et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 359, 1541–1549 (2002).
Rutgeerts, P. et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 353, 2462–2476 (2005). This study is the first phase III randomized, controlled clinical trial of an anti-TNF agent for UC.
Uhlig, H. H. et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25, 309–318 (2006).
Kobayashi, T. et al. IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn’s disease. Gut 57, 1682–1689 (2008).
Feagan, B. G. et al. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 375, 1946–1960 (2016).
Sands, B. E. et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 381, 1201–1214 (2019).
Gerlach, K. et al. TH9 cells that express the transcription factor PU.1 drive T cell-mediated colitis via IL-9 receptor signaling in intestinal epithelial cells. Nat. Immunol. 15, 676–686 (2014).
Nishida, A. et al. Increased expression of interleukin-36, a member of the interleukin-1 cytokine family, in inflammatory bowel disease. Inflamm. Bowel Dis. 22, 303–314 (2016).
Russell, S. E. et al. IL-36α expression is elevated in ulcerative colitis and promotes colonic inflammation. Mucosal Immunol. 9, 1193–1204 (2016).
Scheibe, K. et al. Inhibiting interleukin 36 receptor signaling reduces fibrosis in mice with chronic intestinal inflammation. Gastroenterology 156, 1082–1097.e11 (2019).
Gordon, I. O. et al. Fibrosis in ulcerative colitis is directly linked to severity and chronicity of mucosal inflammation. Aliment. Pharmacol. Ther. 47, 922–939 (2018).
Harusato, A. et al. IL-36γ signaling controls the induced regulatory T cell-Th9 cell balance via NFκB activation and STAT transcription factors. Mucosal Immunol. 10, 1455–1467 (2017).
Sonnenburg, J. L. & Bäckhed, F. Diet-microbiota interactions as moderators of human metabolism. Nature 535, 56–64 (2016).
Machiels, K. et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 63, 1275–1283 (2014).
Halfvarson, J. et al. Dynamics of the human gut microbiome in inflammatory bowel disease. Nat. Microbiol. 2, 17004 (2017).
Schulthess, J. et al. The short chain fatty acid butyrate imprints an antimicrobial program in macrophages. Immunity 50, 432–445.e7 (2019).
Moayyedi, P. et al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized controlled trial. Gastroenterology 149, 102–109.e6 (2015).
Paramsothy, S. et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet 389, 1218–1228 (2017).
Rossen, N. G. et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology 149, 110–118.e4 (2015).
Zheng, L. et al. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of claudin-2. J. Immunol. 199, 2976–2984 (2017).
Glauben, R. et al. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J. Immunol. 176, 5015–5022 (2006).
Fischer, A. et al. Differential effects of α4β7 and GPR15 on homing of effector and regulatory T cells from patients with UC to the inflamed gut in vivo. Gut 65, 1642–1664 (2016).
Feagan, B. G. et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 369, 699–710 (2013).
Wei, S.-C. et al. Management of ulcerative colitis in Taiwan: consensus guideline of the Taiwan society of inflammatory bowel disease. Intest. Res. 15, 266–284 (2017).
Fausel, R. A., Kornbluth, A. & Dubinsky, M. C. The first endoscopy in suspected inflammatory bowel disease. Gastrointest. Endosc. Clin. N. Am. 26, 593–610 (2016).
American Society for Gastrointestinal Endoscopy Standards of Practice Committee. et al. The role of endoscopy in inflammatory bowel disease. Gastrointest. Endosc. 81, 1101–1121.e1-13 (2015).
Chutkan, R. K., Scherl, E. & Waye, J. D. Colonoscopy in inflammatory bowel disease. Gastrointest. Endosc. Clin. N. Am. 12, 463–483 (2002).
Langner, C. et al. The histopathological approach to inflammatory bowel disease: a practice guide. Virchows Arch. 464, 511–527 (2014).
Su, H.-J. et al. Inflammatory bowel disease and its treatment in 2018: global and Taiwanese status updates. J. Formos. Med. Assoc. 118, 1083–1092 (2019).
D’Haens, G. et al. A review of activity indices and efficacy end points for clinical trials of medical therapy in adults with ulcerative colitis. Gastroenterology 132, 763–786 (2007).
Schroeder, K. W., Tremaine, W. J. & Ilstrup, D. M. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N. Engl. J. Med. 317, 1625–1629 (1987).
Peyrin-Biroulet, L. et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin. Gastroenterol. Hepatol. 14, 348–354.e17 (2016).
Fagerhol, M. K., Dale, I. & Andersson, T. A radioimmunoassay for a granulocyte protein as a marker in studies on the turnover of such cells. Bull. Eur. Physiopathol. Respir. 16, 273–282 (1980).
Guerrant, R. L. et al. Measurement of fecal lactoferrin as a marker of fecal leukocytes. J. Clin. Microbiol. 30, 1238–1242 (1992).
van Rheenen, P. F., Van de Vijver, E. & Fidler, V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 341, c3369 (2010).
Walsham, N. E. & Sherwood, R. A. Fecal calprotectin in inflammatory bowel disease. Clin. Exp. Gastroenterol. 9, 21–29 (2016).
Lin, W.-C. et al. Fecal calprotectin correlated with endoscopic remission for Asian inflammatory bowel disease patients. World J. Gastroenterol. 21, 13566–13573 (2015).
Menees, S. B., Powell, C., Kurlander, J., Goel, A. & Chey, W. D. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am. J. Gastroenterol. 110, 444–454 (2015).
Poullis, A., Foster, R., Northfield, T. C. & Mendall, M. A. Review article: faecal markers in the assessment of activity in inflammatory bowel disease. Aliment. Pharmacol. Ther. 16, 675–681 (2002).
Sipponen, T. et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment. Pharmacol. Ther. 28, 1221–1229 (2008).
Holtman, G. A. et al. Evaluation of point-of-care test calprotectin and lactoferrin for inflammatory bowel disease among children with chronic gastrointestinal symptoms. Fam. Pract. 34, 400–406 (2017).
D’Haens, G. et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm. Bowel Dis. 18, 2218–2224 (2012).
Reenaers, C. et al. Expert opinion for use of faecal calprotectin in diagnosis and monitoring of inflammatory bowel disease in daily clinical practice. United European Gastroenterol. J. 6, 1117–1125 (2018).
Ministro, P. & Martins, D. Fecal biomarkers in inflammatory bowel disease: how, when and why? Expert Rev. Gastroenterol. Hepatol. 11, 317–328 (2017).
Tibble, J. A., Sigthorsson, G., Foster, R., Forgacs, I. & Bjarnason, I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology 123, 450–460 (2002).
Hübenthal, M. et al. Sparse modeling reveals miRNA signatures for diagnostics of inflammatory bowel disease. PLoS ONE 10, e0140155 (2015).
Zhang, H., Zeng, Z., Mukherjee, A. & Shen, B. Molecular diagnosis and classification of inflammatory bowel disease. Expert Rev. Mol. Diagn. 18, 867–886 (2018).
Allocca, M. et al. Accuracy of Humanitas ultrasound criteria in assessing disease activity and severity in ulcerative colitis: a prospective study. J. Crohns Colitis 12, 1385–1391 (2018).
Kinoshita, K. et al. Usefulness of transabdominal ultrasonography for assessing ulcerative colitis: a prospective, multicenter study. J. Gastroenterol. 54, 521–529 (2019).
Sagami, S. et al. Transperineal ultrasound predicts endoscopic and histological healing in ulcerative colitis. Aliment. Pharmacol. Ther. 51, 1373–1383 (2020).
Okabayashi, S. et al. A simple 1-day colon capsule endoscopy procedure demonstrated to be a highly acceptable monitoring tool for ulcerative colitis. Inflamm. Bowel Dis. 24, 2404–2412 (2018).
Shi, H. Y. et al. A prospective study on second-generation colon capsule endoscopy to detect mucosal lesions and disease activity in ulcerative colitis (with video). Gastrointest. Endosc. 86, 1139–1146.e6 (2017).
Li, J. & Leung, W. K. Colon capsule endoscopy for inflammatory bowel disease. J. Dig. Dis. 19, 386–394 (2018).
Maeda, Y. et al. Fully automated diagnostic system with artificial intelligence using endocytoscopy to identify the presence of histologic inflammation associated with ulcerative colitis (with video). Gastrointest. Endosc. 89, 408–415 (2019).
Ullman, T. A. & Itzkowitz, S. H. Intestinal inflammation and cancer. Gastroenterology 140, 1807–1816 (2011).
Watanabe, T. et al. Comparison of targeted vs random biopsies for surveillance of ulcerative colitis-associated colorectal cancer. Gastroenterology 151, 1122–1130 (2016).
Harbord, M. et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J. Crohns Colitis 11, 769–784 (2017).
Van Assche, G., Vermeire, S. & Rutgeerts, P. Management of acute severe ulcerative colitis. Gut 60, 130–133 (2011). Consensus guidelines on treatment of UC by the European Crohn’s and Colitis Organisation.
Nguyen, G. C. et al. Consensus statements on the risk, prevention, and treatment of venous thromboembolism in inflammatory bowel disease: Canadian association of gastroenterology. Gastroenterology 146, 835–848.e6 (2014).
Travis, S. P. et al. Predicting outcome in severe ulcerative colitis. Gut 38, 905–910 (1996).
Higashiyama, M. et al. Management of elderly ulcerative colitis in Japan. J. Gastroenterol. 54, 571–586 (2019).
Ruemmele, F. M. & Turner, D. Differences in the management of pediatric and adult onset ulcerative colitis — lessons from the joint ECCO and ESPGHAN consensus guidelines for the management of pediatric ulcerative colitis. J. Crohns Colitis 8, 1–4 (2014).
Sturm, A. et al. European Crohn’s and Colitis organisation topical review on IBD in the elderly: table 1. J. Crohns Colitis https://doi.org/10.1093/ecco-jcc/jjw188 (2016).
Marteau, P. et al. Combined oral and enema treatment with Pentasa (mesalazine) is superior to oral therapy alone in patients with extensive mild/moderate active ulcerative colitis: a randomised, double blind, placebo controlled study. Gut 54, 960–965 (2005).
Sandborn, W. J. et al. Once-daily budesonide MMX® extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study. Gastroenterology 143, 1218–1226.e2 (2012).
Herfarth, H. et al. Methotrexate is not superior to placebo in maintaining steroid-free response or remission in ulcerative colitis. Gastroenterology 155, 1098–1108.e9 (2018).
Nielsen, O. H., Steenholdt, C., Juhl, C. B. & Rogler, G. Efficacy and safety of methotrexate in the management of inflammatory bowel disease: a systematic review and meta-analysis of randomized, controlled trials. EClinicalMedicine 20, 100271 (2020).
Colombel, J. F. et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology 141, 1194–1201 (2011).
Panaccione, R. et al. Combination therapy with infliximab and azathioprine is superior to monotherapy with either agent in ulcerative colitis. Gastroenterology 146, 392–400.e3 (2014).
Sandborn, W. J. et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 142, 257–265.e1–3 (2012).
Sandborn, W. J. et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 146, 85–95 (2014).
Colombel, J.-F. et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut 66, 839–851 (2017).
Sands, B. E. et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N. Engl. J. Med. 381, 1215–1226 (2019).
Sandborn, W. J. et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients with ulcerative colitis. Gastroenterology 158, 562–572.e12 (2020).
Sandborn, W. J. et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 376, 1723–1736 (2017).
FDA. FDA approves boxed warning about increased risk of blood clots and death with higher dose of arthritis and ulcerative colitis medicine tofacitinib (Xeljanz, Xeljanz XR). FDA https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-boxed-warning-about-increased-risk-blood-clots-and-death-higher-dose-arthritis-and (2019).
Sandborn, W. J. et al. Venous thromboembolic events in the tofacitinib ulcerative colitis clinical development programme. Aliment. Pharmacol. Ther. 50, 1068–1076 (2019).
Samuel, S. et al. Cumulative incidence and risk factors for hospitalization and surgery in a population-based cohort of ulcerative colitis. Inflamm. Bowel Dis. 19, 1858–1866 (2013).
Coakley, B. A., Telem, D., Nguyen, S., Dallas, K. & Divino, C. M. Prolonged preoperative hospitalization correlates with worse outcomes after colectomy for acute fulminant ulcerative colitis. Surgery 153, 242–248 (2013).
Targownik, L. E., Singh, H., Nugent, Z. & Bernstein, C. N. The epidemiology of colectomy in ulcerative colitis: results from a population-based cohort. Am. J. Gastroenterol. 107, 1228–1235 (2012).
Kaplan, G. G. et al. Decreasing colectomy rates for ulcerative colitis: a population-based time trend study. Am. J. Gastroenterol. 107, 1879–1887 (2012).
Eriksson, C. et al. Changes in medical management and colectomy rates: a population-based cohort study on the epidemiology and natural history of ulcerative colitis in Örebro, Sweden, 1963–2010. Aliment. Pharmacol. Ther. 46, 748–757 (2017).
Schineis, C. et al. Colectomy with ileostomy for severe ulcerative colitis-postoperative complications and risk factors. Int. J. Colorectal Dis. 35, 387–394 (2020).
Gu, J., Stocchi, L., Remzi, F. & Kiran, R. P. Factors associated with postoperative morbidity, reoperation and readmission rates after laparoscopic total abdominal colectomy for ulcerative colitis. Colorectal Dis. 15, 1123–1129 (2013).
Madbouly, K. M. et al. Perioperative blood transfusions increase infectious complications after ileoanal pouch procedures (IPAA). Int. J. Colorectal Dis. 21, 807–813 (2006).
Zittan, E. et al. Preoperative anti-tumor necrosis factor therapy in patients with ulcerative colitis is not associated with an increased risk of infectious and noninfectious complications after ileal pouch-anal anastomosis. Inflamm. Bowel Dis. 22, 2442–2447 (2016).
Mor, I. J. et al. Infliximab in ulcerative colitis is associated with an increased risk of postoperative complications after restorative proctocolectomy. Dis. Colon Rectum 51, 1202–1207 (2008).
Selvasekar, C. R. et al. Effect of infliximab on short-term complications in patients undergoing operation for chronic ulcerative colitis. J. Am. Coll. Surg. 204, 956–962 (2007).
Yung, D. E. et al. Systematic review and meta-analysis: vedolizumab and postoperative complications in inflammatory bowel disease. Inflamm. Bowel Dis. 24, 2327–2338 (2018).
Gu, J., Remzi, F. H., Shen, B., Vogel, J. D. & Kiran, R. P. Operative strategy modifies risk of pouch-related outcomes in patients with ulcerative colitis on preoperative anti-tumor necrosis factor-α therapy. Dis. Colon Rectum 56, 1243–1252 (2013).
Remzi, F. H. et al. Restorative proctocolectomy: an example of how surgery evolves in response to paradigm shifts in care. Colorectal Dis. 19, 1003–1012 (2017).
Ahmed Ali, U. et al. Open versus laparoscopic (assisted) ileo pouch anal anastomosis for ulcerative colitis and familial adenomatous polyposis. Cochrane Database Syst. Rev. 1, CD006267 (2009).
Shen, B., Remzi, F. H., Lavery, I. C., Lashner, B. A. & Fazio, V. W. A proposed classification of ileal pouch disorders and associated complications after restorative proctocolectomy. Clin. Gastroenterol. Hepatol. 6, 145–158 (2008).
Garrett, J. W. & Drossman, D. A. Health status in inflammatory bowel disease. Biological and behavioral considerations. Gastroenterology 99, 90–96 (1990).
De Dombal, F. T., Burton, I. & Goligher, J. C. The early and late results of surgical treatment for Crohn’s disease. Br. J. Surg. 58, 805–816 (1971).
Guyatt, G. et al. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 96, 804–810 (1989).
Love, J. R., Irvine, E. J. & Fedorak, R. N. Quality of life in inflammatory bowel disease. J. Clin. Gastroenterol. 14, 15–19 (1992).
Irvine, E. J., Zhou, Q. & Thompson, A. K. The short inflammatory bowel disease questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohn’s relapse prevention trial. Am. J. Gastroenterol. 91, 1571–1578 (1996).
Casellas, F., López-Vivancos, J., Vergara, M. & Malagelada, J. Impact of inflammatory bowel disease on health-related quality of life. Dig. Dis. 17, 208–218 (1999).
Lönnfors, S. et al. IBD and health-related quality of life – discovering the true impact. J. Crohns Colitis 8, 1281–1286 (2014).
U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research, U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research & U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims: draft guidance. Health Qual. Life Outcomes 4, 79 (2006).
WHO. International classification of functioning, disability and health (ICF). WHO http://www.who.int/classifications/icf/en/ (2018).
Cieza, A. & Stucki, G. The international classification of functioning disability and health: its development process and content validity. Eur. J. Phys. Rehabil. Med. 44, 303–313 (2008).
Peyrin-Biroulet, L. et al. Disability in inflammatory bowel diseases: developing ICF core sets for patients with inflammatory bowel diseases based on the international classification of functioning, disability, and health. Inflamm. Bowel Dis. 16, 15–22 (2010).
Ananthakrishnan, A. N. et al. Permanent work disability in Crohn’s disease. Am. J. Gastroenterol. 103, 154–161 (2008).
Thompson, P. W. & Pegley, F. S. A comparison of disability measured by the stanford health assessment questionnaire disability scales (HAQ) in male and female rheumatoid outpatients. Br. J. Rheumatol. 30, 298–300 (1991).
Dasgupta, B. et al. Patient and physician expectations of add-on treatment with golimumab for rheumatoid arthritis: relationships between expectations and clinical and quality of life outcomes. Arthritis Care Res. 66, 1799–1807 (2014).
Noseworthy, J. H., Vandervoort, M. K., Wong, C. J. & Ebers, G. C. Interrater variability with the expanded disability status scale (EDSS) and functional systems (FS) in a multiple sclerosis clinical trial. Neurology 40, 971–975 (1990).
Butzkueven, H. et al. Efficacy and safety of natalizumab in multiple sclerosis: interim observational programme results. J. Neurol. Neurosurg. Psychiatry 85, 1190–1197 (2014).
Peyrin-Biroulet, L. et al. Development of the first disability index for inflammatory bowel disease based on the international classification of functioning, disability and health. Gut 61, 241–247 (2012).
Gower-Rousseau, C. et al. Validation of the inflammatory bowel disease disability index in a population-based cohort. Gut 66, 588–596 (2017).
Lee, Y. et al. Disability in restorative proctocolectomy recipients measured using the inflammatory bowel disease disability index. J. Crohns Colitis 10, 1378–1384 (2016).
Lichtenstein, G. R., Cohen, R., Yamashita, B. & Diamond, R. H. Quality of life after proctocolectomy with ileoanal anastomosis for patients with ulcerative colitis. J. Clin. Gastroenterol. 40, 669–677 (2006).
Peyrin-Biroulet, L., Germain, A., Patel, A. S. & Lindsay, J. O. Systematic review: outcomes and post-operative complications following colectomy for ulcerative colitis. Aliment. Pharmacol. Ther. 44, 807–816 (2016).
Shafer, L. A. et al. Independent validation of a self-report version of the IBD disability index (IBDDI) in a population-based cohort of IBD patients. Inflamm. Bowel Dis. 24, 766–774 (2018).
Chiricozzi, A. et al. Quality of life of psoriatic patients evaluated by a new psychometric assessment tool: PsoDisk. Eur. J. Dermatol. 25, 64–69 (2015).
Mercuri, S. R., Gregorio, G. & Brianti, P. Quality of life of psoriasis patients measured by the PSOdisk: a new visual method for assessing the impact of the disease. G. Ital. Dermatol. Venereol. 152, 424–431 (2017).
Ghosh, S. et al. Development of the IBD Disk: a visual self-administered tool for assessing disability in inflammatory bowel diseases. Inflamm. Bowel Dis. 23, 333–340 (2017).
Levenstein, S. et al. Psychological stress and disease activity in ulcerative colitis: a multidimensional cross-sectional study. Am. J. Gastroenterol. 89, 1219–1225 (1994).
Nahon, S. et al. Risk factors of anxiety and depression in inflammatory bowel disease. Inflamm. Bowel Dis. 18, 2086–2091 (2012).
Mikocka-Walus, A. et al. Symptoms of depression and anxiety are independently associated with clinical recurrence of inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 14, 829–835.e1 (2016).
Bitton, A. et al. Psychosocial determinants of relapse in ulcerative colitis: a longitudinal study. Am. J. Gastroenterol. 98, 2203–2208 (2003).
Christensen, B. et al. Histologic normalization occurs in ulcerative colitis and is associated with improved clinical outcomes. Clin. Gastroenterol. Hepatol. 15, 1557–1564.e1 (2017).
Colombel, J.-F. et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 390, 2779–2789 (2018).
Adedokun, O. J. Association between serum concentration of infliximab and efficacy in adult patients with ulcerative colitis. Gastroenterology 147, 1296–1307.e5 (2014).
Kobayashi, T. et al. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis-results from a multicenter prospective randomized controlled trial and its post hoc analysis. J. Gastroenterol. 51, 241–251 (2016).
Papamichael, K. et al. Infliximab concentration thresholds during induction therapy are associated with short-term mucosal healing in patients with ulcerative colitis. Clin. Gastroenterol. Hepatol. 14, 543–549 (2016).
Afif, W. et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am. J. Gastroenterol. 105, 1133–1139 (2010).
Kobayashi, T. First trough level of infliximab at week 2 predicts future outcomes of induction therapy in ulcerative colitis—results from a multicenter prospective randomized controlled trial and its post hoc analysis. J. Gastroenterol. 51, 241–251 (2016).
Vande Casteele, N. et al. Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology 148, 1320–1329.e3 (2015).
Roda, G., Jharap, B., Neeraj, N. & Colombel, J.-F. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin. Transl. Gastroenterol. 7, e135 (2016).
Peyrin-Biroulet, L. et al. Loss of response to vedolizumab and ability of dose intensification to restore response in patients with Crohn’s disease or ulcerative colitis: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 17, 838–846.e2 (2019).
Baker, K. F. & Isaacs, J. D. Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn’s disease and ulcerative colitis? Ann. Rheum. Dis. 77, 175–187 (2018).
Feagan, B. G. et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 389, 1699–1709 (2017).
Ma, C., Jairath, V., Khanna, R. & Feagan, B. G. Investigational drugs in phase I and phase II clinical trials targeting interleukin 23 (IL23) for the treatment of Crohn’s disease. Expert Opin. Investig. Drugs 27, 649–660 (2018).
Vermeire, S. et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet 384, 309–318 (2014).
Vermeire, S. et al. Anti-MAdCAM antibody (PF-00547659) for ulcerative colitis (TURANDOT): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 390, 135–144 (2017).
Yoshimura, N. et al. Safety and efficacy of AJM300, an oral antagonist of α4 integrin, in induction therapy for patients with active ulcerative colitis. Gastroenterology 149, 1775–1783.e2 (2015).
Langer-Gould, A., Atlas, S. W., Green, A. J., Bollen, A. W. & Pelletier, D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 353, 375–381 (2005).
Sandborn, W. J. et al. Ozanimod induction and maintenance treatment for ulcerative colitis. N. Engl. J. Med. 374, 1754–1762 (2016).
Vermeire, S. et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet 389, 266–275 (2017).
D’Amico, F., Fiorino, G., Furfaro, F., Allocca, M. & Danese, S. Janus kinase inhibitors for the treatment of inflammatory bowel diseases: developments from phase I and phase II clinical trials. Expert Opin. Investig. Drugs 27, 595–599 (2018).
Hansen, J. J. & Sartor, R. B. Therapeutic manipulation of the microbiome in IBD: current results and future approaches. Curr. Treat. Options Gastroenterol. 13, 105–120 (2015).
Barber, G. E. et al. Genetic markers predict primary non-response and durable response to anti-TNF biologic therapies in Crohn’s disease. Am. J. Gastroenterol. 111, 1816–1822 (2016).
Boyapati, R. K., Kalla, R., Satsangi, J. & Ho, G.-T. Biomarkers in search of precision medicine in IBD. Am. J. Gastroenterol. 111, 1682–1690 (2016).
West, N. R. et al. Oncostatin M drives intestinal inflammation and predicts response to tumor necrosis factor-neutralizing therapy in patients with inflammatory bowel disease. Nat. Med. 23, 579–589 (2017).
Choung, R. S. et al. Serologic microbial associated markers can predict Crohn’s disease behaviour years before disease diagnosis. Aliment. Pharmacol. Ther. 43, 1300–1310 (2016).
Doherty, M. K. et al. Fecal microbiota signatures are associated with response to Ustekinumab therapy among Crohn’s disease patients. mBio https://doi.org/10.1128/mBio.02120-17 (2018).
Zhou, Y. et al. Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. mSystems https://doi.org/10.1128/mSystems.00188-17 (2018).
Arijs, I. et al. Predictive value of epithelial gene expression profiles for response to infliximab in Crohn’s disease. Inflamm. Bowel Dis. 16, 2090–2098 (2010).
Gamo, K. et al. Gene signature-based approach identified MEK1/2 as a potential target associated with relapse after anti-TNFα treatment for Crohn’s disease. Inflamm. Bowel Dis. 24, 1251–1265 (2018).
Kaplan, G. G. et al. The impact of inflammatory bowel disease in Canada 2018: epidemiology. J. Can. Assoc. Gastroenterol. 2, S6–S16 (2019).
Danese, S. New therapies for inflammatory bowel disease: from the bench to the bedside. Gut 61, 918–932 (2012).
Acknowledgements
B.S. acknowledges the DFG (German Research foundation) for funding her research (CRC-TRR 241 and CRC1340).
Author information
Authors and Affiliations
Contributions
Introduction (T.H., T.K. and S.D.); Epidemiology (C.N.B.); Mechanisms/pathophysiology (B. Siegmund); Diagnosis, screening and prevention (S.C.W.); Management (M.F. and B. Shen); Quality of life (C.L.B. and L.P.-B.); Outlook (T.K.); Overview of Primer (T.K. L.P.-B and T.H.).
Corresponding authors
Ethics declarations
Competing interests
T.K. receives research support from AbbVie GK, Alfresa Pharma, EA Pharma, Kyorin Pharmaceutical Co., Ltd, Mochida Pharmaceutical, Nippon Kayaku, Otsuka Holdings, Thermo Fisher Scientific and ZERIA; receives advisory fees from AbbVie GK, Activaid, Alfresa Pharma, Bristol-Myers Squibb, Celltrion, CovidienÐ, Eli Lilly, Ferring Pharmaceuticals, Gilead Sciences, Janssen, Kissei, Kyorin Pharmaceutical, Mochida Pharmaceutical, Nippon Kayaku, Pfizer, Takeda Pharmaceutical and Thermo Scientific and receives lecture fees from AbbVie GK, Astellas, Alfresa Pharma, Celltrion, EA Pharma, Gilead Sciences, Janssen, JIMRO, Kyorin Pharmaceutical, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Nippon Kayaku, Takeda Pharmaceutical and ZERIA. B. Siegmund received speaker’s fees from Abbvie, CED Service GmbH, Falk, Ferring, Janssen, Novartis, and Takeda (B. Siegmund served as representative of the Charité) and has served as consultant for AbbVie, Boehringer, Celgene, Falk, Janssen, Lilly, Pfizer, Prometheus and Takeda. C.L.B. receives honoraria from AbbVie and Ferring. S.C.W. reports consultancy fees from AbbVie, AbGenomics, Celltrion, Ferring Pharmaceuticals Inc., Gilead, Janssen, Pfizer, Takeda, and Tanabe and receives lecture fees from AbbVie, Celltrion, Eisai, Excelsior Biopharma Inc., Ferring Pharmaceuticals Inc., Janssen, Takeda, Tanabe, Tillotts Pharma, and TSPC (Taiwan Specialty Pharma Corp.). M.F. receives research grants from Amgen, Biogen, Janssen, Pfizer, and Takeda and receives consultancy fees from AbbVie, Boehringer-Ingelheim, Janssen, MSD, Pfizer, Sandoz, and Takeda and receives speaker fees from AbbVie, Amgen, Biogen, Boehringer-Ingelheim, Falk, Ferring, Janssen, Lamepro, MSD, Mylan, Pfizer, and Takeda. B. Shen receives consultant fees for AbbVie, Takeda and Janssen. C.N.B. has received educational grants from AbbVie Canada, Janssen Canada, Pfizer Canada, Shire Canada, and Takeda Canada and a research grant from AbbVie Canada. C.N.B. has performed contract research for AbbVie, Boehringer Ingelheim, Celgene, Janssen, Pfizer and Roche. He is on the advisory boards for AbbVie Canada, Janssen Canada, Pfizer Canada, Takeda Canada, and Shire Canada and consulted to Mylan Pharmaceuticals. S.D. receives consultancy fees from AbbVie, Allergan, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ely Lilly, Enthera, Ferring Pharmaceuticals Inc., Gilead, Hospira, Janssen, Johnson & Johnson, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, TiGenix, UCB Inc., and Vifor. L.P.-B. receives research grants from AbbVie, MSD, and Takeda and reports personal fees from AbbVie, Allergan, Alma, Amgen, Applied Molecular Transport, Arena, Biogen, Boehringer Ingelheim, BMS, Celltrion, Celgene, Enterome, Enthera, Ferring, Fresenius, Genentech, Gilead, Hikma, Index Pharmaceuticals, Janssen, Lilly, MSD, Mylan, Nestle, Norgine, Oppilan Pharma, OSE Immunotherapeutics, Pfizer, Pharmacosmos, Roche, Samsung Bioepis, Sandoz, Sterna, Sublimity Therapeutics, Takeda, Vifor, and Tillots and stock options from CTMA. T.H. has received research grants from AbbVie, EA Pharma, JIMRO, Otuska Holdings, and Zeria Pharmaceuticals and lecture fees from Aspen Japan KK, AbbVie GK, Ferring, Gilead Sciences, Janssen, JIMRO, Kissei Pharmaceutical, Mitsubishi-Tanabe Pharma, Mochida Pharmaceutical, Nippon Kayaku Pfizer, Takeda Pharmaceutical, and Zeria Pharmaceutical and advisory or consultancy fees from AbbVie, Bristol-Myers Squibb, Celltrion, EA Pharma, Eli Lilly, Gilead Sciences, Janssen, Kyorin, Mitsubishi-Tanabe Pharma, Nichi-Iko Pharmaceutical, Pfizer, Takeda Pharmaceutical, and Zeria Pharmaceuticals.
Additional information
Peer review information
Nature Reviews Disease Primers thanks O. H. Nielsen, D. Rubin, J. Schölmerich and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Stricture
-
An abnormal narrowing of the digestive tract.
- Fistula
-
An abnormal connection between two organs or spaces.
- Moon face
-
A medical sign of facial swelling with deposition of fat.
- Tenesmus
-
An abnormal feeling of incomplete defecation.
- Megacolon
-
Dilation of the colon without any mechanical obstruction.
- Colonic crypt
-
A repetitive invagination of colonic surface epithelium.
- Crypt abscess
-
A collection of neutrophils in an intestinal crypt.
- Basal plasmocytosis
-
The presence of plasma cells beneath the base of the crypts.
- Metaplasia
-
A transformation of one differentiated cell type to another.
- Goblet cells
-
A type of colonic epithelial cell that secrete mucus.
- Mucosal friability
-
An abnormally fragile surface of the intestine due to inflammation.
- Ileostomy
-
A surgically created opening of the small intestine in the abdominal wall.
- Sinus
-
A space or a cavity in a bone.
- Nephrolithiasis
-
The process of formation of a kidney stone.
Rights and permissions
About this article
Cite this article
Kobayashi, T., Siegmund, B., Le Berre, C. et al. Ulcerative colitis. Nat Rev Dis Primers 6, 74 (2020). https://doi.org/10.1038/s41572-020-0205-x
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-020-0205-x
This article is cited by
-
Desulfovibrio vulgaris interacts with novel gut epithelial immune receptor LRRC19 and exacerbates colitis
Microbiome (2024)
-
YAP represses intestinal inflammation through epigenetic silencing of JMJD3
Clinical Epigenetics (2024)
-
Association between bowel movement frequency and erectile dysfunction in patients with ulcerative colitis: a cross-sectional study
International Journal of Impotence Research (2024)
-
Endoscopic healing is associated with a reduced risk of biologic treatment failure in patients with ulcerative colitis
Scientific Reports (2024)
-
Longitudinal variation of serum PCSK9 in ulcerative colitis: association with disease activity, T helper 1/2/17 cells, and clinical response of tumor necrosis factor inhibitor
Irish Journal of Medical Science (1971 -) (2024)