Abstract
Crohn’s disease is an inflammatory bowel disease that is characterized by chronic inflammation of any part of the gastrointestinal tract, has a progressive and destructive course and is increasing in incidence worldwide. Several factors have been implicated in the cause of Crohn’s disease, including a dysregulated immune system, an altered microbiota, genetic susceptibility and environmental factors, but the cause of the disease remains unknown. The onset of the disease at a young age in most cases necessitates prompt but long-term treatment to prevent disease flares and disease progression with intestinal complications. Thus, earlier, more aggressive treatment with biologic therapies or novel small molecules could profoundly change the natural history of the disease and decrease complications and the need for hospitalization and surgery. Although less invasive biomarkers are in development, diagnosis still relies on endoscopy and histological assessment of biopsy specimens. Crohn’s disease is a complex disease, and treatment should be personalized to address the underlying pathogenetic mechanism. In the future, disease management might rely on severity scores that incorporate prognostic factors, bowel damage assessment and non-invasive close monitoring of disease activity to reduce the severity of complications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
19 June 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
20 May 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
06 April 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
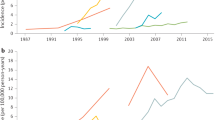
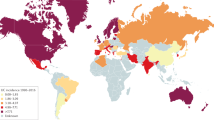
Ng, S. C. et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet 390, 2769–2778 (2018). This study provides a comprehensive analysis of the global IBD epidemiology.
Torres, J., Mehandru, S., Colombel, J.-F. & Peyrin-Biroulet, L. Crohn’s disease. Lancet 389, 1741–1755 (2017).
Thia, K. T., Sandborn, W. J., Harmsen, W. S., Zinsmeister, A. R. & Loftus, E. V. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology 139, 1147–1155 (2010).
Fiorino, G., Bonifacio, C., Peyrin-Biroulet, L. & Danese, S. Preventing collateral damage in Crohn’s disease: the Lémann index. J. Crohns Colitis 10, 495–500 (2016). This study clearly shows the importance of assessing bowel damage in a very early inflammatory stage of CD. The authors demonstrate that the presence of bowel damage in early CD is associated with a worse outcome.
Zeng, Z. et al. Incidence and clinical characteristics of inflammatory bowel disease in a developed region of Guangdong province, China: a prospective population-based study. J. Gastroenterol. Hepatol. 28, 1148–1153 (2013).
Zhao, J. et al. First prospective, population-based inflammatory bowel disease incidence study in mainland of China: the emergence of ‘western’ disease. Inflamm. Bowel Dis. 19, 1839–1845 (2013).
Ng, S. C. et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-Pacific Crohn’s and Colitis Epidemiology Study. Gastroenterology 145, 158–165.e2 (2013).
Kim, H. J. et al. Incidence and natural course of inflammatory bowel disease in Korea, 2006-2012: a nationwide population-based study. Inflamm. Bowel Dis. 21, 623–630 (2015).
Park, S. H. et al. A 30-year trend analysis in the epidemiology of inflammatory bowel disease in the Songpa-Kangdong district of Seoul, Korea in 1986–2015. J. Crohns Colitis 13, 1410–1417 (2019).
Ananthakrishnan, A. N. et al. Environmental triggers in IBD: a review of progress and evidence. Nat. Rev. Gastroenterol. Hepatol. 15, 39–49 (2018).
Bernstein, C. N. et al. Increased burden of psychiatric disorders in inflammatory bowel disease. Inflamm. Bowel Dis. 25, 360–368 (2019).
Moradkhani, A., Beckman, L. J. & Tabibian, J. H. Health-related quality of life in inflammatory bowel disease: psychosocial, clinical, socioeconomic, and demographic predictors. J. Crohns Colitis 7, 467–473 (2013).
Shah, S. C., Colombel, J.-F., Sands, B. E. & Narula, N. Systematic review with meta-analysis: mucosal healing is associated with improved long-term outcomes in Crohn’s disease. Aliment. Pharmacol. Ther. 43, 317–333 (2016).
Kaplan, G. G. & Ng, S. C. Globalisation of inflammatory bowel disease: perspectives from the evolution of inflammatory bowel disease in the UK and China. Lancet Gastroenterol. Hepatol. 1, 307–316 (2016).
Ng, S. C. et al. Geographical variability and environmental risk factors in inflammatory bowel disease. Gut 62, 630–649 (2013).
Yen, H.-H. et al. Epidemiological trend in inflammatory bowel disease in Taiwan from 2001 to 2015: a nationwide population-based study. Intest. Res. 17, 54–62 (2019).
Ng, S. C. et al. Epidemiology of inflammatory bowel disease from 1981 to 2014: results from a territory-wide population-based registry in Hong Kong. Inflamm. Bowel Dis. 22, 1954–1960 (2016).
Mansour-Ghanaei, F. et al. Epidemiologic features of inflammatory bowel disease in Guilan province, north of Iran, during 2002-2012. Middle East. J. Dig. Dis. 7, 69–74 (2015).
Linares de la Cal, J. A., Cantón, C., Hermida, C., Pérez-Miranda, M. & Maté-Jiménez, J. Estimated incidence of inflammatory bowel disease in Argentina and Panama (1987–1993). Rev. Esp. Enferm. Dig. 91, 277–286 (1999).
Piovani, D. et al. Environmental risk factors for inflammatory bowel diseases: an umbrella review of meta-analyses. Gastroenterology 157, 647–659.e4 (2019).
Lakatos, P. L. et al. Is current smoking still an important environmental factor in inflammatory bowel diseases? Results from a population-based incident cohort. Inflamm. Bowel Dis. 19, 1010–1017 (2013).
Kondo, K. et al. The association between environmental factors and the development of Crohn’s disease with focusing on passive smoking: a multicenter case-control study in Japan. PLoS One 14, e0216429 (2019).
Ng, S. C. et al. Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut 64, 1063–1071 (2015).
Levine, A., Sigall Boneh, R. & Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 67, 1726–1738 (2018).
Khalili, H. et al. Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: results from two large prospective cohort studies. Gut https://doi.org/10.1136/gutjnl-2019-319505 (2020).
Ortizo, R. et al. Exposure to oral contraceptives increases the risk for development of inflammatory bowel disease: a meta-analysis of case-controlled and cohort studies. Eur. J. Gastroenterol. Hepatol. 29, 1064–1070 (2017).
Ananthakrishnan, A. N. et al. Aspirin, nonsteroidal anti-inflammatory drug use, and risk for Crohn disease and ulcerative colitis: a cohort study. Ann. Intern. Med. 156, 350–359 (2012).
Moninuola, O. O., Milligan, W., Lochhead, P. & Khalili, H. Systematic review with meta-analysis: association between acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) and risk of Crohn’s disease and ulcerative colitis exacerbation. Aliment. Pharmacol. Ther. 47, 1428–1439 (2018).
Ungaro, R. et al. Statins associated with decreased risk of new onset inflammatory bowel disease. Am. J. Gastroenterol. 111, 1416–1423 (2016).
Green, N., Miller, T., Suskind, D. & Lee, D. A review of dietary therapy for IBD and a vision for the future. Nutrients 11, E947 (2019).
Halfvarson, J., Bodin, L., Tysk, C., Lindberg, E. & Järnerot, G. Inflammatory bowel disease in a Swedish twin cohort: a long-term follow-up of concordance and clinical characteristics. Gastroenterology 124, 1767–1773 (2003).
Hugot, J. P. et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 411, 599–603 (2001).
Ogura, Y. et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 411, 603–606 (2001).
Yamazaki, K. et al. Single nucleotide polymorphisms in TNFSF15 confer susceptibility to Crohn’s disease. Hum. Mol. Genet. 14, 3499–3506 (2005).
Huang, H. et al. Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature 547, 173–178 (2017).
Ellinghaus, D. et al. Analysis of five chronic inflammatory diseases identifies 27 new associations and highlights disease-specific patterns at shared loci. Nat. Genet. 48, 510–518 (2016).
Jostins, L. et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012).
Ogura, Y. et al. Expression of NOD2 in Paneth cells: a possible link to Crohn’s ileitis. Gut 52, 1591–1597 (2003).
Sidiq, T., Yoshihama, S., Downs, I. & Kobayashi, K. S. Nod2: a critical regulator of ileal microbiota and Crohn’s disease. Front. Immunol. 7, 367 (2016).
Hampe, J. et al. A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat. Genet. 39, 207–211 (2007).
Liu, J. Z. et al. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat. Genet. 47, 979–986 (2015).
Hong, M. et al. Immunochip meta-analysis of inflammatory bowel disease identifies three novel loci and four novel associations in previously reported loci. J. Crohns Colitis 12, 730–741 (2018).
Zhu, L. et al. IL-10 and IL-10 receptor mutations in very early onset inflammatory bowel disease. Gastroenterology Res. 10, 65–69 (2017).
Uniken Venema, W. T., Voskuil, M. D., Dijkstra, G., Weersma, R. K. & Festen, E. A. The genetic background of inflammatory bowel disease: from correlation to causality. J. Pathol. 241, 146–158 (2017).
Cleynen, I. et al. Inherited determinants of Crohn’s disease and ulcerative colitis phenotypes: a genetic association study. Lancet 387, 156–167 (2016).
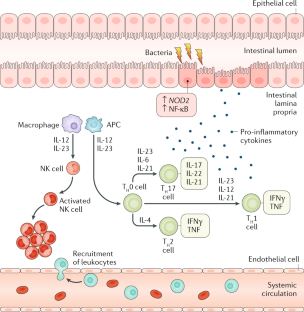
Peterson, L. W. & Artis, D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 14, 141–153 (2014).
Zeissig, S. et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut 56, 61–72 (2007).
Weber, C. R., Nalle, S. C., Tretiakova, M., Rubin, D. T. & Turner, J. R. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab. Invest. 88, 1110–1120 (2008).
Odenwald, M. A. & Turner, J. R. The intestinal epithelial barrier: a therapeutic target? Nat. Rev. Gastroenterol. Hepatol. 14, 9–21 (2017).
Wehkamp, J. et al. NOD2 (CARD15) mutations in Crohn’s disease are associated with diminished mucosal alpha-defensin expression. Gut 53, 1658–1664 (2004).
Cadwell, K. et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456, 259–263 (2008).
Thachil, E. et al. Abnormal activation of autophagy-induced crinophagy in Paneth cells from patients with Crohn’s disease. Gastroenterology 142, 1097–1099.e4 (2012).
Zhang, Q. et al. Commensal bacteria direct selective cargo sorting to promote symbiosis. Nat. Immunol. 16, 918–926 (2015).
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 (2008).
Adolph, T. E. et al. Paneth cells as a site of origin for intestinal inflammation. Nature 503, 272–276 (2013).
Tschurtschenthaler, M. et al. Defective ATG16L1-mediated removal of IRE1α drives Crohn’s disease-like ileitis. J. Exp. Med. 214, 401–422 (2017).
Willson, T. A., Jurickova, I., Collins, M. & Denson, L. A. Deletion of intestinal epithelial cell STAT3 promotes T-lymphocyte STAT3 activation and chronic colitis following acute dextran sodium sulfate injury in mice. Inflamm. Bowel Dis. 19, 512–525 (2013).
Diamanti, M. A. et al. IKKα controls ATG16L1 degradation to prevent ER stress during inflammation. J. Exp. Med. 214, 423–437 (2017).
Zhou, C., Qiu, Y. & Yang, H. CD4CD8αα IELs: they have something to say. Front. Immunol. 10, 2269 (2019).
Regner, E. H. et al. Functional intraepithelial lymphocyte changes in inflammatory bowel disease and spondyloarthritis have disease specific correlations with intestinal microbiota. Arthritis Res. Ther. 20, 149 (2018).
Catalan-Serra, I., Sandvik, A. K., Bruland, T. & Andreu-Ballester, J. C. Gammadelta T cells in Crohn’s disease: a new player in the disease pathogenesis? J. Crohns Colitis 11, 1135–1145 (2017).
Hosomi, S. et al. Intestinal epithelial cell endoplasmic reticulum stress promotes MULT1 up-regulation and NKG2D-mediated inflammation. J. Exp. Med. 214, 2985–2997 (2017).
Allez, M., Skolnick, B. E., Wisniewska-Jarosinska, M., Petryka, R. & Overgaard, R. V. Anti-NKG2D monoclonal antibody (NNC0142-0002) in active Crohn’s disease: a randomised controlled trial. Gut 66, 1918–1925 (2017).
Kaser, A., Zeissig, S. & Blumberg, R. S. Inflammatory bowel disease. Annu. Rev. Immunol. 28, 573–621 (2010).
Abraham, C. & Cho, J. H. Inflammatory bowel disease. N. Engl. J. Med. 361, 2066–2078 (2009).
Ouellette, A. J. Paneth cells and innate mucosal immunity. Curr. Opin. Gastroenterol. 26, 547–553 (2010).
de Souza, H. S. P. & Fiocchi, C. Immunopathogenesis of IBD: current state of the art. Nat. Rev. Gastroenterol. Hepatol. 13, 13–27 (2016).
Uhlig, H. H. & Powrie, F. Translating immunology into therapeutic concepts for inflammatory bowel disease. Annu. Rev. Immunol. 36, 755–781 (2018).
Pazmandi, J., Kalinichenko, A., Ardy, R. C. & Boztug, K. Early-onset inflammatory bowel disease as a model disease to identify key regulators of immune homeostasis mechanisms. Immunol. Rev. 287, 162–185 (2019).
Cooney, R. et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat. Med. 16, 90–97 (2010).
Travassos, L. H. et al. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat. Immunol. 11, 55–62 (2010).
Segal, A. W. The role of neutrophils in the pathogenesis of Crohn’s disease. Eur. J. Clin. Invest. 48, e12983 (2018).
Geremia, A. & Arancibia-Cárcamo, C. V. Innate lymphoid cells in intestinal inflammation. Front. Immunol. 8, 1296 (2017).
Bernink, J. H. et al. Interleukin-12 and -23 control plasticity of CD127+ group 1 and group 3 innate lymphoid cells in the intestinal lamina propria. Immunity 43, 146–160 (2015).
van der Gracht, E., Zahner, S. & Kronenberg, M. When insult is added to injury: cross talk between ILCs and intestinal epithelium in IBD. Mediators Inflamm. 2016, 9765238 (2016).
Uhlig, H. H. et al. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity 25, 309–318 (2006).
Feagan, B. G. et al. Ustekinumab as Induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 375, 1946–1960 (2016).
Feagan, B. G. et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet 389, 1699–1709 (2017).
Sands, B. E. et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology 153, 77–86.e6 (2017).
Sarin, R., Wu, X. & Abraham, C. Inflammatory disease protective R381Q IL23 receptor polymorphism results in decreased primary CD4+ and CD8+ human T-cell functional responses. Proc. Natl Acad. Sci. USA 108, 9560–9565 (2011).
Duerr, R. H. et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science 314, 1461–1463 (2006).
Fantini, M. C. et al. Smad7 controls resistance of colitogenic T cells to regulatory T cell-mediated suppression. Gastroenterology 136, 1308–1316 (2009).
Lo Presti, A. et al. Fecal and mucosal microbiota profiling in irritable bowel syndrome and inflammatory bowel disease. Front. Microbiol. 10, 1655 (2019).
Vich Vila, A. et al. Gut microbiota composition and functional changes in inflammatory bowel disease and irritable bowel syndrome. Sci. Transl Med. 10, eaap8914 (2018).
Pascal, V. et al. A microbial signature for Crohn’s disease. Gut 66, 813–822 (2017).
Palmela, C. et al. Adherent-invasive Escherichia coli in inflammatory bowel disease. Gut 67, 574–587 (2018).
Sokol, H. et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl Acad. Sci. USA 105, 16731–16736 (2008).
Barnich, N. & Darfeuille-Michaud, A. Adherent-invasive Escherichia coli and Crohn’s disease. Curr. Opin. Gastroenterol. 23, 16–20 (2007).
Simpson, K. W. et al. Adherent and invasive Escherichia coli is associated with granulomatous colitis in boxer dogs. Infect. Immun. 74, 4778–4792 (2006).
Yilmaz, B. et al. Microbial network disturbances in relapsing refractory Crohn’s disease. Nat. Med. 25, 323–336 (2019).
Libertucci, J. et al. Inflammation-related differences in mucosa-associated microbiota and intestinal barrier function in colonic Crohn’s disease. Am. J. Physiol. Gastrointest. Liver Physiol. 315, G420–G431 (2018).
Vieira-Silva, S. et al. Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat. Microbiol. 4, 1826–1831 (2019).
Norman, J. M. et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 160, 447–460 (2015).
Pérez-Brocal, V. et al. Study of the viral and microbial communities associated with Crohn’s disease: a metagenomic approach. Clin. Transl. Gastroenterol. 4, e36 (2013).
Imai, T. et al. Characterization of fungal dysbiosis in Japanese patients with inflammatory bowel disease. J. Gastroenterol. 54, 149–159 (2019).
Feuerstein, J. D. & Cheifetz, A. S. Crohn disease: epidemiology, diagnosis, and management. Mayo Clin. Proc. 92, 1088–1103 (2017).
Gomollón, F. et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J. Crohns Colitis 11, 3–25 (2017).
Kuriyama, M. et al. Specific gastroduodenoscopic findings in Crohn’s disease: comparison with findings in patients with ulcerative colitis and gastroesophageal reflux disease. Dig. Liver Dis. 40, 468–475 (2008).
Sawczenko, A. & Sandhu, B. K. Presenting features of inflammatory bowel disease in Great Britain and Ireland. Arch. Dis. Child. 88, 995–1000 (2003).
Peyrin-Biroulet, L., Loftus, E. V., Colombel, J.-F. & Sandborn, W. J. The natural history of adult Crohn’s disease in population-based cohorts. Am. J. Gastroenterol. 105, 289–297 (2010). This comprehensive article describes the natural history of CD.
Fiorino, G. et al. Prevalence of bowel damage assessed by cross-sectional imaging in early Crohn’s disease and its impact on disease outcome. J. Crohns Colitis 11, 274–280 (2017).
Safroneeva, E. et al. Impact of the early use of immunomodulators or TNF antagonists on bowel damage and surgery in Crohn’s disease. Aliment. Pharmacol. Ther. 42, 977–989 (2015).
Peyrin-Biroulet, L. et al. Perianal Crohn’s disease findings other than fistulas in a population-based cohort. Inflamm. Bowel Dis. 18, 43–48 (2012).
Ott, C. & Schölmerich, J. Extraintestinal manifestations and complications in IBD. Nat. Rev. Gastroenterol. Hepatol. 10, 585–595 (2013).
Park, S. H. et al. Update on the natural course of fistulizing perianal Crohn’s disease in a population-based cohort. Inflamm. Bowel Dis. 25, 1054–1060 (2019).
Freeman, H. J. Natural history and long-term clinical course of Crohn’s disease. World J. Gastroenterol. 20, 31–36 (2014).
Danese, S. et al. Development of red flags index for early referral of adults with symptoms and signs suggestive of Crohn’s disease: an IOIBD initiative. J. Crohns Colitis 9, 601–606 (2015).
Vavricka, S. R. et al. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am. J. Gastroenterol. 106, 110–119 (2011).
Jang, H.-J., Kang, B. & Choe, B.-H. The difference in extraintestinal manifestations of inflammatory bowel disease for children and adults. Transl. Pediatr. 8, 4–15 (2019).
Peyrin-Biroulet, L., Loftus, E. V., Colombel, J.-F. & Sandborn, W. J. Long-term complications, extraintestinal manifestations, and mortality in adult Crohn’s disease in population-based cohorts. Inflamm. Bowel Dis. 17, 471–478 (2011). This comprehensive article describes long-term outcomes in patients with CD.
Pennazio, M. et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy 47, 352–376 (2015).
Koulaouzidis, A., Rondonotti, E. & Karargyris, A. Small-bowel capsule endoscopy: a ten-point contemporary review. World J. Gastroenterol. 19, 3726–3746 (2013).
Dionisio, P. M. et al. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am. J. Gastroenterol. 105, 1240–1248 (2010).
Magro, F. et al. European consensus on the histopathology of inflammatory bowel disease. J. Crohns Colitis 7, 827–851 (2013).
Annese, V. et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J. Crohns Colitis 7, 982–1018 (2013).
Tontini, G. E., Vecchi, M., Neurath, M. F. & Neumann, H. Advanced endoscopic imaging techniques in Crohn’s disease. J. Crohns Colitis 8, 261–269 (2014).
Allocca, M., Fiorino, G. & Danese, S. Cross-sectional imaging modalities in Crohn’s disease. Dig. Dis. 31, 199–201 (2013).
Chatu, S., Subramanian, V. & Pollok, R. C. G. Meta-analysis: diagnostic medical radiation exposure in inflammatory bowel disease. Aliment. Pharmacol. Ther. 35, 529–539 (2012).
Horsthuis, K., Bipat, S., Bennink, R. J. & Stoker, J. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 247, 64–79 (2008).
Panés, J. et al. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment. Pharmacol. Ther. 34, 125–145 (2011).
Sahni, V. A., Ahmad, R. & Burling, D. Which method is best for imaging of perianal fistula? Abdom. Imaging 33, 26–30 (2008).
Allocca, M. et al. Comparative accuracy of bowel ultrasound versus magnetic resonance enterography in combination with colonoscopy in assessing Crohn’s disease and guiding clinical decision-making. J. Crohns Colitis 12, 1280–1287 (2018).
Magro, F. et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J. Crohns Colitis 11, 649–670 (2017).
Vermeire, S., Schreiber, S., Sandborn, W. J., Dubois, C. & Rutgeerts, P. Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin. Gastroenterol. Hepatol. 8, 357–363 (2010).
Best, W. R. Predicting the Crohn’s disease activity index from the Harvey-Bradshaw index. Inflamm. Bowel Dis. 12, 304–310 (2006).
Mitsuyama, K. et al. Antibody markers in the diagnosis of inflammatory bowel disease. World J. Gastroenterol. 22, 1304–1310 (2016).
Gu, P. et al. Serological, genetic and clinical associations with increased health-care resource utilization in inflammatory bowel disease. J. Dig. Dis. 19, 15–23 (2018).
Plevy, S. et al. Combined serological, genetic, and inflammatory markers differentiate non-IBD, Crohn’s disease, and ulcerative colitis patients. Inflamm. Bowel Dis. 19, 1139–1148 (2013).
Maaser, C. et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J. Crohns Colitis 13, 144–164 (2019).
Vermeire, S., Van Assche, G. & Rutgeerts, P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm. Bowel Dis. 10, 661–665 (2004).
Vermeire, S., Van Assche, G. & Rutgeerts, P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut 55, 426–431 (2006).
Solem, C. A. et al. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm. Bowel Dis. 11, 707–712 (2005).
Cellier, C. et al. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn’s disease. A prospective multicentre study of 121 cases. The Groupe d’Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut 35, 231–235 (1994).
Lakatos, P. L. et al. Serum lipopolysaccharide-binding protein and soluble CD14 are markers of disease activity in patients with Crohn’s disease. Inflamm. Bowel Dis. 17, 767–777 (2011).
Kwon, J. H. et al. Disease phenotype, activity and clinical course prediction based on C-reactive protein levels at diagnosis in patients with Crohn’s disease: results from the CONNECT study. Gut Liver 10, 595–603 (2016).
Carroccio, A. et al. Diagnostic accuracy of fecal calprotectin assay in distinguishing organic causes of chronic diarrhea from irritable bowel syndrome: a prospective study in adults and children. Clin. Chem. 49, 861–867 (2003).
Diamanti, A. et al. Diagnostic work-up of inflammatory bowel disease in children: the role of calprotectin assay. Inflamm. Bowel Dis. 16, 1926–1930 (2010).
Goutorbe, F. et al. Endoscopic factors influencing fecal calprotectin value in Crohn’s disease. J. Crohns Colitis 9, 1113–1119 (2015).
van Rheenen, P. F., Van de Vijver, E. & Fidler, V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta-analysis. BMJ 341, c3369 (2010).
Suray, N. de et al. Close monitoring of CRP and fecal calprotectin is able to predict clinical relapse in patients with Crohn’s disease in remission after infliximab withdrawal. a sub-analysis of the Stori study. Gastroenterology 142, S-149 (2012).
Orlando, A. et al. The role of calprotectin in predicting endoscopic post-surgical recurrence in asymptomatic Crohn’s disease: a comparison with ultrasound. Eur. Rev. Med. Pharmacol. Sci. 10, 17–22 (2006).
Guo, S. et al. A simple fecal bacterial marker panel for the diagnosis of Crohn’s disease. Front. Microbiol. 10, 1306 (2019).
Marlicz, W., Skonieczna-Żydecka, K., Dabos, K. J., Łoniewski, I. & Koulaouzidis, A. Emerging concepts in non-invasive monitoring of Crohn’s disease. Ther. Adv. Gastroenterol. 11, 1756284818769076 (2018).
Somineni, H. K. et al. Blood-derived DNA methylation signatures of Crohn’s disease and severity of intestinal inflammation. Gastroenterology 156, 2254–2265.e3 (2019).
Leong, R. W. et al. Full-spectrum endoscopy improves surveillance for dysplasia in patients with inflammatory bowel diseases. Gastroenterology 152, 1337–1344.e3 (2017).
Stidham, R. W. & Higgins, P. D. R. Colorectal cancer in inflammatory bowel disease. Clin. Colon. Rectal Surg. 31, 168–178 (2018).
Tontini, G. E., Vecchi, M., Pastorelli, L., Neurath, M. F. & Neumann, H. Differential diagnosis in inflammatory bowel disease colitis: state of the art and future perspectives. World J. Gastroenterol. 21, 21–46 (2015).
He, Y. et al. Development and validation of a novel diagnostic nomogram to differentiate between intestinal tuberculosis and Crohn’s disease: a 6-year prospective multicenter study. Am. J. Gastroenterol. 114, 490–499 (2019).
Bae, J. H. et al. Development and validation of a novel prediction model for differential diagnosis between Crohn’s disease and intestinal tuberculosis. Inflamm. Bowel Dis. 23, 1614–1623 (2017).
Lee, S. K., Kim, B. K., Kim, T. I. & Kim, W. H. Differential diagnosis of intestinal Behçet’s disease and Crohn’s disease by colonoscopic findings. Endoscopy 41, 9–16 (2009).
Valenti, S., Gallizzi, R., De Vivo, D. & Romano, C. Intestinal Behçet and Crohn’s disease: two sides of the same coin. Pediatr. Rheumatol. Online J. 15, 33 (2017).
Kedia, S. et al. Differentiating Crohn’s disease from intestinal tuberculosis. World J. Gastroenterol. 25, 418–432 (2019).
Oliveira, S. B. & Monteiro, I. M. Diagnosis and management of inflammatory bowel disease in children. BMJ 357, j2083 (2017).
Amre, D. K., Lu, S.-E., Costea, F. & Seidman, E. G. Utility of serological markers in predicting the early occurrence of complications and surgery in pediatric Crohn’s disease patients. Am. J. Gastroenterol. 101, 645–652 (2006).
Gisbert, J. P., Marín, A. C. & Chaparro, M. Systematic review: factors associated with relapse of inflammatory bowel disease after discontinuation of anti-TNF therapy. Aliment. Pharmacol. Ther. 42, 391–405 (2015).
Peyrin-Biroulet, L. et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am. J. Gastroenterol. 110, 1324–1338 (2015).
van Deen, W. K. et al. Value redefined for inflammatory bowel disease patients: a choice-based conjoint analysis of patients’ preferences. Qual. Life Res. 26, 455–465 (2017).
Loy, L. et al. Detection and management of early stage inflammatory bowel disease: an update for clinicians. Expert Rev. Gastroenterol. Hepatol. 13, 547–555 (2019).
Bewtra, M. et al. Inflammatory bowel disease patients’ willingness to accept medication risk to avoid future disease relapse. Am. J. Gastroenterol. 110, 1675–1681 (2015).
Torres, J. et al. Predicting outcomes to optimize disease management in inflammatory bowel diseases. J. Crohns Colitis 10, 1385–1394 (2016).
Beaugerie, L., Seksik, P., Nion-Larmurier, I., Gendre, J.-P. & Cosnes, J. Predictors of Crohn’s disease. Gastroenterology 130, 650–656 (2006).
Loly, C., Belaiche, J. & Louis, E. Predictors of severe Crohn’s disease. Scand. J. Gastroenterol. 43, 948–954 (2008).
Beaugerie, L. & Sokol, H. Clinical, serological and genetic predictors of inflammatory bowel disease course. World J. Gastroenterol. 18, 3806–3813 (2012).
Mao, R. et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: a meta-analysis of prospective studies. Inflamm. Bowel Dis. 18, 1894–1899 (2012).
Ghaly, S. et al. High vitamin D-binding protein concentration, low albumin, and mode of remission predict relapse in Crohn’s disease. Inflamm. Bowel Dis. 22, 2456–2464 (2016).
Qin, G. et al. Serum albumin and C-reactive protein/albumin ratio are useful biomarkers of Crohn’s Disease activity. Med. Sci. Monit. 22, 4393–4400 (2016).
Allez, M. et al. Long term outcome of patients with active Crohn’s disease exhibiting extensive and deep ulcerations at colonoscopy. Am. J. Gastroenterol. 97, 947–953 (2002).
Nahon, S. et al. Diagnostic delay in a French cohort of Crohn’s disease patients. J. Crohns Colitis 8, 964–969 (2014).
Maconi, G. et al. The impact of symptoms, irritable bowel syndrome pattern and diagnostic investigations on the diagnostic delay of Crohn’s disease: a prospective study. Dig. Liver Dis. 47, 646–651 (2015).
Vavricka, S. R. et al. Systematic evaluation of risk factors for diagnostic delay in inflammatory bowel disease. Inflamm. Bowel Dis. 18, 496–505 (2012).
Schoepfer, A. M. et al. Diagnostic delay in Crohn’s disease is associated with a complicated disease course and increased operation rate. Am. J. Gastroenterol. 108, 1744–1753 (2013).
Peyrin-Biroulet, L. et al. Development of the Paris definition of early Crohn’s disease for disease-modification trials: results of an international expert opinion process. Am. J. Gastroenterol. 107, 1770–1776 (2012). This is the first description of early CD, a category of the disease defined by prognostic factors that predict a favourable response to early aggressive treatment.
Danese, S., Fiorino, G., Fernandes, C. & Peyrin-Biroulet, L. Catching the therapeutic window of opportunity in early Crohn’s disease. Curr. Drug Targets 15, 1056–1063 (2014).
Høivik, M. L. et al. Work disability in inflammatory bowel disease patients 10 years after disease onset: results from the IBSEN study. Gut 62, 368–375 (2013).
Frøslie, K. F., Jahnsen, J., Moum, B. A., Vatn, M. H. & IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 133, 412–422 (2007).
Colombel, J.-F. et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 390, 2779–2789 (2018).
Peyrin-Biroulet, L. et al. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut 63, 88–95 (2014).
Louis, E. et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology 142, 63–70.e5 (2012).
Doherty, G. et al. European Crohn’s and Colitis Organisation topical review on treatment withdrawal [‘exit strategies’] in inflammatory bowel disease. J. Crohns Colitis 12, 17–31 (2018).
Munkholm, P., Langholz, E., Davidsen, M. & Binder, V. Frequency of glucocorticoid resistance and dependency in Crohn’s disease. Gut 35, 360–362 (1994).
Modigliani, R. et al. Clinical, biological, and endoscopic picture of attacks of Crohn’s disease. Evolution on prednisolone. Gastroenterology 98, 811–818 (1990).
Lamb, C. A. et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 68, s1–s106 (2019).
Panés, J. et al. Early azathioprine therapy is no more effective than placebo for newly diagnosed Crohn’s disease. Gastroenterology 145, 766–774.e1 (2013).
Beaugerie, L. et al. Risk of new or recurrent cancer under immunosuppressive therapy in patients with IBD and previous cancer. Gut 63, 1416–1423 (2014).
Cosnes, J. et al. Early administration of azathioprine vs conventional management of Crohn’s disease: a randomized controlled trial. Gastroenterology 145, 758–765.e2 (2013).
Chande, N., Townsend, C. M., Parker, C. E. & MacDonald, J. K. Azathioprine or 6-mercaptopurine for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 10, CD000545 (2016).
Chatu, S., Subramanian, V., Saxena, S. & Pollok, R. C. G. The role of thiopurines in reducing the need for surgical resection in Crohn’s disease: a systematic review and meta-analysis. Am. J. Gastroenterol. 109, 23–34 (2014).
Herfarth, H. H., Kappelman, M. D., Long, M. D. & Isaacs, K. L. Use of methotrexate in the treatment of inflammatory bowel diseases. Inflamm. Bowel Dis. 22, 224–233 (2016).
Colombel, J. F. et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 362, 1383–1395 (2010).
Dulai, P. S. et al. The real-world effectiveness and safety of vedolizumab for moderate-severe Crohn’s disease: results from the US VICTORY consortium. Am. J. Gastroenterol. 111, 1147–1155 (2016).
Kariburyo, M. F., Xie, L., Teeple, A., Tan, H. & Ingham, M. Predicting pre-emptive discussions of biologic treatment: results from an openness and preference survey of inflammatory bowel disease patients and their prescribers. Adv. Ther. 34, 1398–1410 (2017).
Sands, B. E. et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N. Engl. J. Med. 381, 1215–1226 (2019).
Vande Casteele, N. et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut 64, 1539–1545 (2015).
Nanda, K. S., Cheifetz, A. S. & Moss, A. C. Impact of antibodies to infliximab on clinical outcomes and serum infliximab levels in patients with inflammatory bowel disease (IBD): a meta-analysis. Am. J. Gastroenterol. 108, 40–47; quiz 48 (2013).
Seinen, M. L., De Boer, N. K. & van Bodegraven, A. A. Key insights from therapeutic drug monitoring in Crohn’s disease patients. Expert Opin. Drug Metab. Toxicol. 15, 399–406 (2019).
Restellini, S., Khanna, R. & Afif, W. Therapeutic drug monitoring with ustekinumab and vedolizumab in inflammatory bowel disease. Inflamm. Bowel Dis. 24, 2165–2172 (2018).
D’Amico, F., Fiorino, G., Furfaro, F., Allocca, M. & Danese, S. Janus kinase inhibitors for the treatment of inflammatory bowel diseases: developments from phase I and phase II clinical trials. Expert Opin. Investig. Drugs 27, 595–599 (2018).
Peyrin-Biroulet, L., Christopher, R., Behan, D. & Lassen, C. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun. Rev. 16, 495–503 (2017).
Ma, C., Jairath, V., Khanna, R. & Feagan, B. G. Investigational drugs in phase I and phase II clinical trials targeting interleukin 23 (IL23) for the treatment of Crohn’s disease. Expert Opin. Investig. Drugs 27, 649–660 (2018).
Prideaux, L., Kamm, M. A., De Cruz, P. P., Chan, F. K. L. & Ng, S. C. Inflammatory bowel disease in Asia: a systematic review. J. Gastroenterol. Hepatol. 27, 1266–1280 (2012).
Prideaux, L. et al. Comparison of clinical characteristics and management of inflammatory bowel disease in Hong Kong versus Melbourne. J. Gastroenterol. Hepatol. 27, 919–927 (2012).
Gálvez, J. Role of Th17 cells in the pathogenesis of human IBD. ISRN Inflamm. 2014, 928461 (2014).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
van der Giessen, J. et al. Modulation of cytokine patterns and microbiome during pregnancy in IBD. Gut 69, 473–486 (2020).
van der Giessen, J., Huang, V. W., van der Woude, C. J. & Fuhler, G. M. Modulatory effects of pregnancy on inflammatory bowel disease. Clin. Transl. Gastroenterol. 10, e00009 (2019).
Maunder, R. G., Cohen, Z., McLeod, R. S. & Greenberg, G. R. Effect of intervention in inflammatory bowel disease on health-related quality of life: a critical review. Dis. Colon Rectum 38, 1147–1161 (1995).
Chen, X.-L. et al. Inflammatory bowel disease-specific health-related quality of life instruments: a systematic review of measurement properties. Health Qual. Life Outcomes 15, 177 (2017).
Kaplan, G. G. The global burden of IBD: from 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 12, 720–727 (2015).
Cohen, R. D. The quality of life in patients with Crohn’s disease. Aliment. Pharmacol. Ther. 16, 1603–1609 (2002).
López Blanco, B., Moreno-Jiménez, B., Devesa Múgica, J. M. & Rodríguez Muñoz, A. Relationship between socio-demographic and clinical variables, and health-related quality of life in patients with inflammatory bowel disease. Rev. Esp. Enferm. Dig. 97, 887–898 (2005). This study reveals the effect of IBD on QOL, which needs to be considered in clinical practice.
Blondel-Kucharski, F. et al. Health-related quality of life in Crohn’s disease: a prospective longitudinal study in 231 patients. Am. J. Gastroenterol. 96, 2915–2920 (2001).
Andersson, P., Olaison, G., Bendtsen, P., Myrelid, P. & Sjödahl, R. Health related quality of life in Crohn’s proctocolitis does not differ from a general population when in remission. Colorectal Dis. 5, 56–62 (2003).
Bernklev, T. et al. Course of disease, drug treatment and health-related quality of life in patients with inflammatory bowel disease 5 years after initial diagnosis. Eur. J. Gastroenterol. Hepatol. 17, 1037–1045 (2005).
Casellas, F., López-Vivancos, J., Badia, X., Vilaseca, J. & Malagelada, J. R. Impact of surgery for Crohn’s disease on health-related quality of life. Am. J. Gastroenterol. 95, 177–182 (2000).
Romberg-Camps, M. J. L. et al. Fatigue and health-related quality of life in inflammatory bowel disease: results from a population-based study in the Netherlands: the IBD-South Limburg cohort. Inflamm. Bowel Dis. 16, 2137–2147 (2010).
Schirbel, A. et al. Impact of pain on health-related quality of life in patients with inflammatory bowel disease. World J. Gastroenterol. 16, 3168–3177 (2010).
Katz, L. et al. Mechanisms of quality of life and social support in inflammatory bowel disease. J. Clin. Psychol. Med. Settings 23, 88–98 (2016).
Reinink, A. R., Lee, T. C. & Higgins, P. D. R. Endoscopic mucosal healing predicts favorable clinical outcomes in inflammatory bowel disease: a meta-analysis. Inflamm. Bowel Dis. 22, 1859–1869 (2016).
Peyrin-Biroulet, L. et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin. Gastroenterol. Hepatol. 14, 348–354.e17 (2016).
Pariente, B. et al. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm. Bowel Dis. 17, 1415–1422 (2011).
Siegel, C. A. et al. Development of an index to define overall disease severity in IBD. Gut 67, 244–254 (2018). This study discusses the development of severity indices to allow assessment of disease severity and bowel damage progression in the future.
Rieder, F. & Fiocchi, C. Intestinal fibrosis in inflammatory bowel disease - current knowledge and future perspectives. J. Crohns Colitis 2, 279–290 (2008).
Burke, J. P. et al. Fibrogenesis in Crohn’s disease. Am. J. Gastroenterol. 102, 439–448 (2007).
Allen, P. B., Gower-Rousseau, C., Danese, S. & Peyrin-Biroulet, L. Preventing disability in inflammatory bowel disease. Ther. Adv. Gastroenterol. 10, 865–876 (2017).
McGovern, D. Personalized medicine in inflammatory bowel disease. Gastroenterol. Hepatol. 10, 662–664 (2014).
Siegel, C. A. et al. A validated web-based tool to display individualised Crohn’s disease predicted outcomes based on clinical, serologic and genetic variables. Aliment. Pharmacol. Ther. 43, 262–271 (2016).
Acknowledgements
Work in the laboratory of A.K. is supported by the Wellcome Trust (Senior Investigator Award 106260/Z/14/Z) and the European Research Council (Consolidator Grant 648889).
Author information
Authors and Affiliations
Contributions
Introduction (G.R., L.P.-B. and S.D.); Epidemiology (S.C.N.); Mechanisms/pathophysiology (A.K.); Diagnosis, screening and prevention (P.G.K. and M.A.); Management (R.P.); Quality of life (G.R., A.S., L.P.-B. and S.D.); Outlook (G.R., A.S., L.P.-B. and S.D.).
Corresponding author
Ethics declarations
Competing interests
S.D. has served as a speaker, consultant and advisory board member for Schering-Plough, AbbVie, Merck Sharp & Dohme, UCB Pharma, Ferring, Cellerix, Takeda Pharmaceutical Company, Nycomed, Pharmacosmos, Actelion, Alpha Wasserman, Genentech, Grünenthal, Pfizer, AstraZeneca, Novo Nordisk, Cosmo Pharmaceuticals, Vifor, Johnson & Johnson and Nikkiso Europe GmbH. L.P.-B. has received consulting fees from AbbVie, Amgen, Biogaran, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Ferring, Genentech, HAC Pharma, Hospira, Index Pharmaceuticals, Janssen, Lilly, Merck, Mitsubishi, Norgine, Pfizer, Pharmacosmos, Pilege, Sandoz, Takeda, Therakos, Tillotts, UCB Pharma and Vifor and lecture fees from AbbVie, Ferring, HAC Pharma, Janssen, Merck, Mitsubishi, Norgine, Takeda, Therakos, Tillotts and Vifor. A.S. has acted as a consultant or speaker for Ethicon, Olympus, Frankenman, Transenterix (not active), Tigenyx, Pfizer, Takeda and Sandoz. P.G.K has been a lecturer for AbbVie, Janssen, Pfizer and Takeda and is a member of the advisory board of AbbVie, Pfizer and Takeda. A.K. has served as an adviser to Boehringer Ingelheim, Ferring, Genentech, GlaxoSmithKline, Gilead, Hospira, Janssen, Pfizer, and VHSquared. R.P. has received consultant and/or lecture fees from AbbVie, Amgen, AstraZeneca, Axcan Pharma (now Aptalis), Biogen Idec, Bristol-Myers Squibb, Centocor, ChemoCentryx, Eisai Medical Research Inc., Elan Pharmaceuticals, Ferring, Genentech, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Millennium Pharmaceuticals (now Takeda Oncology), Ocera Therapeutics Inc., Otsuka America Pharmaceutical, Pfizer, Shire Pharmaceuticals, Prometheus Laboratories, Schering-Plough Corporation, Synta Pharmaceuticals Corp., Teva, UCB Pharma and Warner Chilcott. S.C.N. has received consulting and speaker fees from AbbVie, Ferring, Janssen, Menarini and Takeda, has served as a scientific advisory board member for AbbVie, Ferring and Takeda and has received research grants from AbbVie, Ferring and Janssen. The other authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Disease Primers thanks B.D. Ye, A. Forbes, J. Gisbert, G. Monteleone, A. Regueiro and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Roda, G., Chien Ng, S., Kotze, P.G. et al. Crohn’s disease. Nat Rev Dis Primers 6, 22 (2020). https://doi.org/10.1038/s41572-020-0156-2
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-020-0156-2
This article is cited by
-
Efficacy and safety of stem cell therapy for Crohn’s disease: a meta-analysis of randomized controlled trials
Stem Cell Research & Therapy (2024)
-
Increased CpG methylation at the CDH1 locus in inflamed ileal mucosa of patients with Crohn disease
Clinical Epigenetics (2024)
-
YAP represses intestinal inflammation through epigenetic silencing of JMJD3
Clinical Epigenetics (2024)
-
Efficacy and safety of gut microbiota-based therapies in autoimmune and rheumatic diseases: a systematic review and meta-analysis of 80 randomized controlled trials
BMC Medicine (2024)
-
Etrolizumab-s fails to control E-Cadherin-dependent co-stimulation of highly activated cytotoxic T cells
Nature Communications (2024)