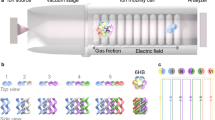
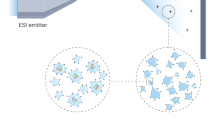
Abstract
Despite their structural and functional differences, synthetic supramolecular assemblies share many similarities with biological assemblies, especially enzymes. The assemblies can be on the same length scale, and their structures and guest binding are typically governed by non-covalent interactions. Thus, only relatively weak interactions define the shape of a synthetic supramolecule or the secondary and tertiary structure of a protein, such that the resulting dynamism makes structure elucidation challenging. For biomolecules such as peptides, proteins, glycans and lipids this has often been tackled using ion mobility–mass spectrometry (IM-MS), whereby analyte ions are separated according to their gas-phase mobility and their mass-to-charge ratio. IM-MS is an established method in omics, separation sciences and small-molecule structural chemistry, but has only recently grown in popularity for the study of synthetic supramolecular assemblies in the gas phase. This Review describes IM-MS techniques and how they can help us understand the structures of molecular self-assemblies, host–guest complexes and metallosupramolecular complexes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rissanen, K. Crystallography of encapsulated molecules. Chem. Soc. Rev. 46, 2638–2648 (2017).
Mali, K. S., Pearce, N., De Feyter, S. & Champness, N. R. Frontiers of supramolecular chemistry at solid surfaces. Chem. Soc. Rev. 46, 2520–2542 (2017).
Avram, L. & Cohen, Y. Diffusion NMR of molecular cages and capsules. Chem. Soc. Rev. 44, 586–602 (2015).
Avram, L. & Cohen, Y. Self-assembly of resorcin[4]arene in the presence of small alkylammonium guests in solution. Org. Lett. 10, 1505–1508 (2008).
Evan-Salem, T. & Cohen, Y. Octahydroxypyridine[4]arene self-assembles spontaneously to form hexameric capsules and dimeric aggregates. Chem. Eur. J. 13, 7659–7663 (2007).
Cohen, Y., Evan-Salem, T. & Avram, L. Hydrogen-bonded hexameric capsules of resorcin[4]arene, pyrogallol[4]arene and octahydroxypyridine[4]arene are abundant structures in organic solvents: a view from diffusion NMR. Supramol. Chem. 20, 71–79 (2008).
Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 40, 1305–1323 (2011).
von Krbek, L. K. S., Schalley, C. A. & Thordarson, P. Assessing cooperativity in supramolecular systems. Chem. Soc. Rev. 46, 2622–2637 (2017).
Hill, H. J., Eiceman, G. A. & Karpas, Z. Ion Mobility Spectrometry 3rd edn (CRC Press, 2013).
May, J. C., Morris, C. B. & McLean, J. A. Ion mobility collision cross section compendium. Anal. Chem. 89, 1032–1044 (2017).
Kanu, A. B., Dwivedi, P., Tam, M., Matz, L. & Hill, H. H. Ion mobility–mass spectrometry. J. Mass Spectrom. 43, 1–22 (2008).
Lanucara, F., Holman, S. W., Gray, C. J. & Eyers, C. E. The power of ion mobility-mass spectrometry for structural characterization and the study of conformational dynamics. Nat. Chem. 6, 281–294 (2014).
Konijnenberg, A., Butterer, A. & Sobott, F. Native ion mobility-mass spectrometry and related methods in structural biology. Biochim. Biophys. Acta. 1834, 1239–1256 (2013).
Uetrecht, C., Rose, R. J., van Duijn, E., Lorenzen, K. & Heck, A. J. R. Ion mobility mass spectrometry of proteins and proteinassemblies. Chem. Soc. Rev. 39, 1633–1655 (2010).
Van Berkel, G. J., Pasilis, S. P. & Ovchinnikova, O. Established and emerging atmospheric pressure surface sampling/ionization techniques for mass spectrometry. J. Mass Spectrom. 43, 1161–1180 (2008).
Bowers, M. T. Re-print of “ion mobility spectrometry: a personal view of its development at UCSB”. Int. J. Mass Spectrom. 377, 625–645 (2015).
May, J. C. et al. Conformational ordering of biomolecules in the gas phase: nitrogen collision cross sections measured on a prototype high resolution drift tube ion mobility-mass spectrometer. Anal. Chem. 86, 2107–2116 (2014).
Michelmann, K., Silveira, J. A., Ridgeway, M. E. & Park, M. A. Fundamentals of trapped ion mobility spectrometry. J. Am. Soc. Mass Spectrom. 26, 14–24 (2014).
Kaplan, K. et al. Resistive glass IM-TOFMS. Anal. Chem. 82, 9336–9343 (2010).
Giles, K., Williams, J. P. & Campuzano, I. Enhancements in travelling wave ion mobility resolution. Rapid Commun. Mass Spectrom. 25, 1559–1566 (2011).
May, J. C. & McLean, J. A. Ion mobility-mass spectrometry: time-dispersive instrumentation. Anal. Chem. 87, 1422–1436 (2015).
Wyttenbach, T., Bleiholder, C. & Bowers, M. T. Factors contributing to the collision cross section of polyatomic ions in the kilodalton to gigadalton range: application to ion mobility measurements. Anal. Chem. 85, 2191–2199 (2013).
Wyttenbach, T., Bleiholder, C., Anderson, S. E. & Bowers, M. T. A new algorithm to characterise the degree of concaveness of a molecular surface relevant in ion mobility spectrometry. Mol. Phys. 113, 2344–2349 (2015).
Larriba, C. & Hogan, C. J. Free molecular collision cross section calculation methods for nanoparticles and complex ions with energy accommodation. J. Comput. Phys. 251, 344–336 (2013).
Shvartsburg, A. A. & Jarrold, M. F. An exact hard-spheres scattering model for the mobilities of polyatomic ions. Chem. Phys. Lett. 261, 86–91 (1996).
Siems, W. F., Viehland, L. A. & Hill, H. H. Correcting the fundamental ion mobility equation for field effects. Analyst 141, 6396–6407 (2016).
Giles, K. et al. Applications of a travelling wave-based radio-frequency-only stacked ring ion guide. Rapid Commun. Mass Spectrom. 18, 2401–2414 (2004).
Hines, K. M., May, J. C., McLean, J. A. & Xu, L. Evaluation of collision cross section calibrants for structural analysis of lipids by traveling wave ion mobility-mass spectrometry. Anal. Chem. 88, 7329–7336 (2016).
Silveira, J. A., Michelmann, K., Ridgeway, M. E. & Park, M. A. Fundamentals of trapped ion mobility spectrometry part II: fluid dynamics. J. Am. Soc. Mass Spectrom. 27, 585–595 (2016).
Benigni, P. et al. Analysis of geologically relevant metal porphyrins using trapped ion mobility spectrometry–mass spectrometry and theoretical calculations. Energy Fuels 30, 10341–10347 (2016).
Groessl, M., Graf, S. & Knochenmuss, R. High resolution ion mobility-mass spectrometry for separation and identification of isomeric lipids. Analyst 140, 6904–6911 (2015).
Wyttenbach, T., Pierson, N. A., Clemmer, D. E. & Bowers, M. T. Ion mobility analysis of molecular dynamics. Annu. Rev. Phys. Chem. 65, 175–196 (2014).
Allison, T. M. et al. Quantifying the stabilizing effects of protein–ligand interactions in the gas phase. Nat. Commun. 6, 8551 (2015).
Stojko, J. et al. Ion mobility coupled to native mass spectrometry as a relevant tool to investigate extremely small ligand-induced conformational changes. Analyst 140, 7234–7245 (2015).
Jurneczko, E. & Barran, P. E. How useful is ion mobility mass spectrometry for structural biology? The relationship between protein crystal structures and their collision cross sections in the gas phase. Analyst 136, 20–28 (2011).
Lee, J. W., Davidson, K. L., Bush, M. F. & Kim, H. I. Collision cross sections and ion structures: development of a general calculation method via high-quality ion mobility measurements and theoretical modeling. Analyst 142, 4289–4298 (2017).
Albrecht, M. & Hahn, F. E. (eds) Topics in Current Chemistry: Chemistry of Nanocontainers Vol. 319 (Springer-Verlag, Berlin, Heidelberg, 2012).
Pinalli, R. & Dalcanale, E. Supramolecular sensing with phosphonate cavitands. Acc. Chem. Res. 46, 399–411 (2013).
Beer, P. & Gale, P. Anion recognition and sensing: the state of the art and future perspectives. Angew. Chem. Int. Ed. 40, 486–516 (2001).
Brotherhood, P. R. & Davis, A. P. Steroid-based anion receptors and transporters. Chem. Soc. Rev. 39, 3633–3647 (2010).
Zhang, H., Grabenauer, M., Bowers, M. T. & Dearden, D. V. Supramolecular modification of ion chemistry: modulation of peptide charge state and dissociation behavior through complexation with cucurbit[n]uril (n=5, 6) or α-cyclodextrin. J. Phys. Chem. A 113, 1508–1517 (2009).
Lee, S. J. C. et al. Host-guest chemistry from solution to the gas phase: an essential role of direct interaction with water for high-affinity binding of cucurbit[n]urils. J. Phys. Chem. B 117, 8855–8864 (2013).
Wu, G., Olesin´ska, M., Wu, Y., Matak-Vinkovic, D. & Scherman, O. A. Mining 2:2 complexes from 1:1 stoichiometry: formation of cucurbit[8]uril–diarylviologen quaternary complexes favored by electron-donating substituents. J. Am. Chem. Soc. 139, 3202–3208 (2017).
Kovalenko, E. et al. Supramolecular adducts of cucurbit[7]uril and amino acids in the gas phase. J. Am. Soc. Mass Spectrom. 27, 265–276 (2016).
Lee, J. W., Shin, M. H., Mobley, W., Urbach, A. R. & Kim, H. I. Supramolecular enhancement of protein analysis via the recognition of phenylalanine with cucurbit[7]uril. J. Am. Chem. Soc. 137, 15322–15329 (2015).
Lee, T.-C. et al. Chemistry inside molecular containers in the gas phase. Nat. Chem. 5, 376–382 (2013).
Carroy, G. et al. Influence of equilibration time in solution on the inclusion/exclusion topology ratio of host–guest complexes probed by ion mobility and collision-induced dissociation. Chem. Eur. J. 22, 4528–4534 (2016).
Kiesilä, A. et al. Simultaneous endo and exo complex formation of pyridine[4]arene dimers with neutral and anionic guests. Angew. Chem. Int. Ed. 56, 10942–10946 (2017).
Schröder, H. V., Wollschläger, J. M. & Schalley, C. A. Redox-controlled self-inclusion of a lasso-type pseudo[1]rotaxane. Chem. Commun. 53, 9218–9221 (2017).
Brocker, E. R., Anderson, S. E., Northrop, B. H., Stang, P. J. & Bowers, M. T. Structures of metallosupramolecular coordination assemblies can be obtained by ion mobility spectrometry–mass spectrometry. J. Am. Chem. Soc. 132, 13486–13494 (2010).
Guo, K. et al. Characterization of metallosupramolecular polymers by top-down multidimensional mass spectrometry methods. Macromol. Rapid Commun. 36, 1539–1552 (2015).
Wang, L. et al. Self-assembly of tetrameric and hexameric terpyridine-based macrocycles using Cd(II), Zn(II), and Fe(II). Inorg. Chem. 57, 3548–3558 (2018).
Myung, S., Julian, R. R., Nanita, S. C., Cooks, R. G. & Clemmer, D. E. Formation of nanometer-scale serine clusters by sonic spray. J. Phys. Chem. B 108, 6105–6111 (2004).
Turunen, L. et al. [N···I+···N] Halogen-bonded dimeric capsules from tetrakis(3-pyridyl)ethylene cavitands. Angew. Chem. Int. Ed. 55, 14033–14036 (2016).
Chan, Y.-T. et al. Self-assembly and traveling wave ion mobility mass spectrometry analysis of hexacadmium macrocycles. J. Am. Chem. Soc. 131, 16395–16397 (2009).
Perera, S. et al. Hexameric palladium(II) terpyridyl metallomacrocycles: assembly with 4,4’-bipyridine and characterization by TWIM mass spectrometry. Angew. Chem. Int. Ed. 49, 6539–6544 (2010).
Chan, Y.-T. et al. Design, synthesis, and traveling wave ion mobility mass spectrometry characterization of iron(II)– and ruthenium(II)–terpyridine metallomacrocycles. J. Am. Chem. Soc. 133, 11967–11976 (2011).
Li, Y. et al. Giant, hollow 2D metalloarchitecture: stepwise self-assembly of a hexagonal supramolecular nut. J. Am. Chem. Soc. 138, 10041–10046 (2016).
Bonakdarzadeh, P. et al. DOSY NMR, x-ray structural and ion-mobility mass spectrometric studies on electron-deficient and electron-rich M6L4 coordination cages. Inorg. Chem. 54, 6055–6061 (2015).
Song, B. et al. Direct self-assembly of a 2D and 3D Star of David. Angew. Chem. Int. Ed. 56, 5258–5262 (2017).
Baker, E. S. et al. Probing shapes of bichromophoric metal–organic complexes using ion mobility mass spectrometry. J. Am. Chem. Soc. 127, 18222–18228 (2005).
Baksi, A. et al. Isomerism in monolayer protected silver cluster ions: an ion mobility-mass spectrometry approach. J. Phys. Chem. C 121, 13421–13427 (2017).
Jurc˘ek, O. et al. Superchiral Pd3L6 coordination complex and its reversible structural conversion into Pd3L3Cl6 metallocycles. Angew. Chem. Int. Ed. 54, 15462–15467 (2015).
Ujma, J. et al. Shapes of supramolecular cages by ion mobility mass spectrometry. Chem. Commun. 48, 4423–4425 (2012).
Wang, C. et al. Self-assembly of giant supramolecular cubes with terpyridine ligands as vertices and metals on edges. Chem. Sci. 5, 1221–1226 (2014).
Acknowledgements
The authors thank the Academy of Finland (project numbers 284562 and 312514) and the University of Jyväskylä for financial support.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to the preparation of this manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kalenius, E., Groessl, M. & Rissanen, K. Ion mobility–mass spectrometry of supramolecular complexes and assemblies. Nat Rev Chem 3, 4–14 (2019). https://doi.org/10.1038/s41570-018-0062-2
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-018-0062-2
This article is cited by
-
High-resolution separation of bioisomers using ion cloud profiling
Nature Communications (2023)
-
Increasing the size and complexity of discrete 2D metallosupramolecules
Nature Reviews Materials (2020)
-
Chemical mimicry of viral capsid self-assembly via corannulene-based pentatopic tectons
Nature Communications (2019)