Abstract
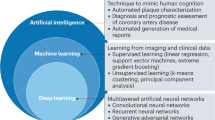
Research into artificial intelligence (AI) has made tremendous progress over the past decade. In particular, the AI-powered analysis of images and signals has reached human-level performance in many applications owing to the efficiency of modern machine learning methods, in particular deep learning using convolutional neural networks. Research into the application of AI to medical imaging is now very active, especially in the field of cardiovascular imaging because of the challenges associated with acquiring and analysing images of this dynamic organ. In this Review, we discuss the clinical questions in cardiovascular imaging that AI can be used to address and the principal methodological AI approaches that have been developed to solve the related image analysis problems. Some approaches are purely data-driven and rely mainly on statistical associations, whereas others integrate anatomical and physiological information through additional statistical, geometric and biophysical models of the human heart. In a structured manner, we provide representative examples of each of these approaches, with particular attention to the underlying computational imaging challenges. Finally, we discuss the remaining limitations of AI approaches in cardiovascular imaging (such as generalizability and explainability) and how they can be overcome.
Key points
-
Artificial intelligence (AI) algorithms have shown impressive results in specific and often time-consuming cardiovascular imaging tasks such as image segmentation, anomaly detection and patient selection; however, these applications are limited to specific tasks in the clinical workflow.
-
In cardiovascular imaging, AI algorithms are often purely data-driven but can be improved when associated with biophysical models of the heart, which enables the integration of pre-existing knowledge of human anatomy and physiology.
-
A bottleneck in AI applications often lies in the collection of imaging data and their annotation by experts, which is limited by the lack of resources and expertise; therefore, the creation of large databases must be a community effort.
-
The appropriate integration of AI algorithms into clinical workflows remains an unresolved problem; important security, privacy and explainability issues must be resolved to achieve a sufficiently high level of trust.
-
AI algorithms have the potential to enrich the amount and the robustness of information extracted from cardiac images, while at the same time redistributing physician time and work towards patient interaction and complex decision-making tasks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Greenspan, H., van Ginneken, B. & Summers, R. M. Guest editorial deep learning in medical imaging: overview and future promise of an exciting new technique. IEEE Trans. Med. Imaging 35, 1153–1159 (2016).
LeCun, Y., Bengio, Y. & Hinton, G. Deep learning. Nature 521, 436–444 (2015).
Dey, D. et al. Artificial intelligence in cardiovascular imaging. J. Am. Coll. Cardiol. 73, 1317–1335 (2019).
Siegersma, K. R. et al. Artificial intelligence in cardiovascular imaging: state of the art and implications for the imaging cardiologist. Neth. Heart J. 27, 403–413 (2019).
Henglin, M. et al. Machine learning approaches in cardiovascular imaging. Circ. Cardiovasc. Imaging 10, e005614 (2017).
O’Regan, D. P. Putting machine learning into motion: applications in cardiovascular imaging. Clin. Radiol. 75, 33–37 (2019).
Seetharam, K., Shrestha, S. & Sengupta, P. P. Artificial intelligence in cardiovascular medicine. Curr. Treat. Options Cardiovasc. Med. 21, 25 (2019).
Litjens, G. et al. State-of-the-art deep learning in cardiovascular image analysis. JACC Cardiovasc. Imaging 12, 1549–1565 (2019).
Leiner, T. et al. Machine learning in cardiovascular magnetic resonance: basic concepts and applications. J. Cardiovasc. Magn. Reson. 21, 61 (2019).
Lundervold, A. S. & Lundervold, A. An overview of deep learning in medical imaging focusing on MRI. Z. Med. Phys. 29, 102–127 (2019).
Hampe, N., Wolterink, J. M., van Velzen, S. G. M., Leiner, T. & Išgum, I. Machine learning for assessment of coronary artery disease in cardiac CT: a survey. Front. Cardiovasc. Med. 6, 172 (2019).
Alsharqi, M. et al. Artificial intelligence and echocardiography. Echo Res. Pract. 5, R115–R125 (2018).
van Sloun, R. J. G., Cohen, R. & Eldar, Y. C. Deep learning in ultrasound imaging. Proc. IEEE 108, 11–29 (2020).
Cluitmans, M. et al. Validation and opportunities of electrocardiographic imaging: from technical achievements to clinical applications. Front. Physiol. 9, 1305 (2018).
Alawad, M. & Wang, L. Learning domain shift in simulated and clinical data: localizing the origin of ventricular activation from 12-lead electrocardiograms. IEEE Trans. Med. Imaging 38, 1172–1184 (2019).
Bacoyannis, T., Krebs, J., Cedilnik, N., Cochet, H. & Sermesant, M. in Functional Imaging and Modeling of the Heart Ch. 3 (eds Coudière, Y., Ozenne, V., Vigmond, E. & Zemzemi, N.) 20–28 (Springer, 2019).
Bai, W. et al. A population-based phenome-wide association study of cardiac and aortic structure and function. Nat. Med. 26, 1654–1662 (2020).
Petersen, S. E., Abdulkareem, M. & Leiner, T. Artificial intelligence will transform cardiac imaging — opportunities and challenges. Front. Cardiovasc. Med. 6, 169 (2019).
Attia, Z. I. et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat. Med. 25, 70–74 (2019).
Bernard, O. et al. Deep learning techniques for automatic MRI cardiac multi-structures segmentation and diagnosis: is the problem solved? IEEE Trans. Med. Imaging 37, 2514–2525 (2018).
Zhang, N. et al. Deep learning for diagnosis of chronic myocardial infarction on nonenhanced cardiac cine MRI. Radiology 291, 606–617 (2019).
Bello, G. A. et al. Deep learning cardiac motion analysis for human survival prediction. Nat. Mach. Intell. 1, 95–104 (2019).
Bruse, J. L. et al. in Statistical Atlases and Computational Models of the Heart. Imaging and Modelling Challenges Ch. 3 (eds Camara, O. et al.) 21–29 (Springer, 2016).
Leonardi, B. et al. Computational modelling of the right ventricle in repaired tetralogy of Fallot: can it provide insight into patient treatment? Eur. Heart J. Cardiovasc. Imaging 14, 381–386 (2013).
Grbic, S. et al. Personalized mitral valve closure computation and uncertainty analysis from 3D echocardiography. Med. Image Anal. 35, 238–249 (2017).
European Society of Radiology. What the radiologist should know about artificial intelligence — an ESR white paper. Insights Imaging 10, 44 (2019).
James, G., Witten, D., Hastie, T. & Tibshirani, R. in An Introduction to Statistical Learning Ch. 2 26–28 (Springer, 2013).
Hu, S.-Y. et al. Can machine learning improve patient selection for cardiac resynchronization therapy? PLoS ONE 14, e0222397 (2019).
Hosny, A., Parmar, C., Quackenbush, J., Schwartz, L. H. & Aerts, H. J. W. L. Artificial intelligence in radiology. Nat. Rev. Cancer 18, 500–510 (2018).
Cheplygina, V., de Bruijne, M. & Pluim, J. P. W. Not-so-supervised: a survey of semi-supervised, multi-instance, and transfer learning in medical image analysis. Med. Image Anal. 54, 280–296 (2019).
Mlynarski, P., Delingette, H., Criminisi, A. & Ayache, N. Deep learning with mixed supervision for brain tumor segmentation. J. Med. Imaging 6, 034002 (2019).
Rueckert, D. & Schnabel, J. A. Model-based and data-driven strategies in medical image computing. Proc. IEEE 108, 110–124 (2020).
Saba, L. et al. The present and future of deep learning in radiology. Eur. J. Radiol. 114, 14–24 (2019).
Litjens, G. et al. A survey on deep learning in medical image analysis. Med. Image Anal. 42, 60–88 (2017).
Ronneberger, O., Fischer, P. & Brox, T. in Medical Image Computing and Computer-Assisted Intervention — MICCAI 2015 (eds Navab, N., Hornegger, J., Wells, W. M. & Frangi, A. F.) 234–241 (Springer, 2015).
Goodfellow, I. et al. in Advances in Neural Information Processing Systems 27 (eds Ghahramani, Z., Welling, M., Cortes, C., Lawrence, N. D. & Weinberger, K. Q.) 2672–2680 (Curran Associates, 2014).
Kingma, D. P. & Welling, M. An introduction to variational autoencoders. Found. Trends Mach. Learn. 12, 307–392 (2019).
Pesapane, F., Codari, M. & Sardanelli, F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur. Radiol. Exp. 2, 35 (2018).
Bhuva, A. et al. A multicenter, scan-rescan, human and machine learning CMR study to test generalizability and precision in imaging biomarker analysis. Circ. Cardiovasc. Imaging 12, e009214 (2019).
Oksuz, I. et al. Automatic CNN-based detection of cardiac MR motion artefacts using k-space data augmentation and curriculum learning. Med. Image Anal. 55, 136–147 (2019).
Oksuz, I. et al. in Medical Image Computing and Computer Assisted Intervention — MICCAI 2019 (eds Shen, D. et al.) 695–703 (Springer, 2019).
Schlemper, J. et al. in Medical Image Computing and Computer Assisted Intervention — MICCAI 2019 (eds Shen, D. et al.) 57–64 (Springer, 2019).
Hyun, C. M., Kim, H. P., Lee, S. M., Lee, S. & Seo, J. K. Deep learning for undersampled MRI reconstruction. Phys. Med. Biol. 63, 135007 (2018).
Qin, C. et al. Convolutional recurrent neural networks for dynamic MR image reconstruction. IEEE Trans. Med. Imaging 38, 280–290 (2019).
Bustin, A., Fuin, N., Botnar, R. M. & Prieto, C. From compressed-sensing to artificial intelligence-based cardiac MRI reconstruction. Front. Cardiovasc. Med. 7, 17 (2020).
Oksuz, I. et al. Magnetic resonance fingerprinting using recurrent neural networks. IEEE Int. Symp. Biomed. Imaging https://doi.org/10.1109/ISBI.2019.8759502 (2019).
Willemink, M. J. & Noël, P. B. The evolution of image reconstruction for CT-from filtered back projection to artificial intelligence. Eur. Radiol. 29, 2185–2195 (2019).
Green, M., Marom, E. M., Konen, E., Kiryati, N. & Mayer, A. 3-D Neural denoising for low-dose Coronary CT Angiography (CCTA). Comput. Med. Imaging Graph. 70, 185–191 (2018).
Lossau, T. et al. Motion artifact recognition and quantification in coronary CT angiography using convolutional neural networks. Med. Image Anal. 52, 68–79 (2019).
Zhang, L. et al. in Simulation and Synthesis in Medical Imaging (eds Tsaftaris, S. A., Gooya, A., Frangi, A. F. & Prince, J. L.) 138–145 (Springer, 2016).
Biasiolli, L. et al. Automated localization and quality control of the aorta in cine CMR can significantly accelerate processing of the UK Biobank population data. PLoS ONE 14, e0212272 (2019).
Tarroni, G. et al. Learning-based quality control for cardiac MR images. IEEE Trans. Med. Imaging 38, 1127–1138 (2019).
Zhang, L. et al. Automatic assessment of full left ventricular coverage in cardiac cine magnetic resonance imaging with fisher discriminative 3D CNN. IEEE Trans. Biomed. Eng. 66, 1975–1986 (2018).
Dong, J. et al. A generic quality control framework for fetal ultrasound cardiac four-chamber planes. IEEE J. Biomed. Health Inform. 24, 931–942 (2019).
Robinson, R. et al. Automated quality control in image segmentation: application to the UK Biobank cardiovascular magnetic resonance imaging study. J. Cardiovasc. Magn. Reson. 21, 18 (2019).
Albà, X. et al. Automatic initialization and quality control of large-scale cardiac MRI segmentations. Med. Image Anal. 43, 129–141 (2018).
Audelan, B. & Delingette, H. in Medical Image Computing and Computer Assisted Intervention — MICCAI 2019 (eds Shen, D. et al.) 21–29 (Springer, 2019).
Vigneault, D. M., Xie, W., Ho, C. Y., Bluemke, D. A. & Noble, J. A. Ω-Net (Omega-Net): Fully automatic, multi-view cardiac MR detection, orientation, and segmentation with deep neural networks. Med. Image Anal. 48, 95–106 (2018).
Zheng, Q., Delingette, H., Duchateau, N. & Ayache, N. 3-D consistent and robust segmentation of cardiac images by deep learning with spatial propagation. IEEE Trans. Med. Imaging 37, 2137–2148 (2018).
Ambrosini, P. et al. in Medical Image Computing and Computer-Assisted Intervention — MICCAI 2017 (eds Descoteaux, M. et al) 577–585 (Springer, 2017).
Ghorbani, A. et al. Deep learning interpretation of echocardiograms. NPJ Digital Med. 3, 10 (2020).
Ghesu, F.-C. et al. Multi-scale deep reinforcement learning for real-time 3D-landmark detection in CT scans. IEEE Trans. Pattern Anal. Mach. Intell. 41, 176–189 (2019).
Noothout, J. M. H. et al. Deep learning-based regression and classification for automatic landmark localization in medical images. IEEE Trans. Med. Imaging 39, 4011–4022 (2020).
Chen, C. et al. Deep learning for cardiac image segmentation: a review. Front. Cardiovasc. Med. 7, 25 (2020).
Isensee, F. et al. in Statistical Atlases and Computational Models of the Heart. ACDC and MMWHS Challenges (eds Pop, M. et al.) 120–129 (Springer, 2018).
Clough, J. R., Oksuz, I., Byrne, N., Schnabel, J. A. & King, A. P. in Information Processing in Medical Imaging (eds Chung, A. C. S., Gee, J. C., Yushkevich, P. A. & Bao, S.) 16–28 (Springer, 2019).
Duan, J. et al. Automatic 3D Bi-ventricular segmentation of cardiac images by a shape-refined multi- task deep learning approach. IEEE Trans. Med. Imaging 38, 2151–2164 (2019).
Albà, X. et al. An algorithm for the segmentation of highly abnormal hearts using a generic statistical shape model. IEEE Trans. Med. Imaging 35, 845–859 (2016).
Liao, F., Chen, X., Hu, X. & Song, S. Estimation of the volume of the left ventricle from MRI images using deep neural networks. IEEE Trans. Cybern. 49, 495–504 (2019).
Margeta, J. et al. in Statistical Atlases and Computational Models of the Heart. Imaging and Modelling Challenges (eds Camara, O. et al.) 49–56 (Springer, 2014).
Bai, W. et al. Automated cardiovascular magnetic resonance image analysis with fully convolutional networks. J. Cardiovasc. Magn. Reson. 20, 65 (2018).
Gilbert, K. et al. Independent left ventricular morphometric atlases show consistent relationships with cardiovascular risk factors: A UK biobank study. Sci. Rep. 9, 1130 (2019).
Lee, M. C. H., Petersen, K., Pawlowski, N., Glocker, B. & Schaap, M. Tetris: template transformer networks for image segmentation with shape priors. IEEE Trans. Med. Imaging 38, 2596–2606 (2019).
Zhuang, X. et al. Evaluation of algorithms for multi-modality whole heart segmentation: an open-access grand challenge. Med. Image Anal. 58, 101537 (2019).
Gilbert, A. et al. in Smart Ultrasound Imaging and Perinatal, Preterm and Paediatric Image Analysis (eds Wang, Q. et al.) 29–37 (Springer, 2019).
Huang, X. et al. Contour tracking in echocardiographic sequences via sparse representation and dictionary learning. Med. Image Anal. 18, 253–271 (2014).
Leclerc, S. et al. Deep learning for segmentation using an open large-scale dataset in 2D echocardiography. IEEE Trans. Med. Imaging 38, 2198–2210 (2019).
Asch, F. M. et al. Automated echocardiographic quantification of left ventricular ejection fraction without volume measurements using a machine learning algorithm mimicking a human expert. Circ. Cardiovasc. Imaging 12, e009303 (2019).
Andreassen, B. S., Veronesi, F., Gerard, O., Solberg, A. H. S. & Samset, E. Mitral annulus segmentation using deep learning in 3-D transesophageal echocardiography. IEEE J. Biomed. Health Inform. 24, 994–1003 (2020).
Wolterink, J. M., Leiner, T. & Išgum, I. in Graph Learning in Medical Imaging (eds Zhang, D., Zhou, L., Jie, B. & Liu, M.) 62–69 (Springer, 2019).
Itu, L. et al. A machine-learning approach for computation of fractional flow reserve from coronary computed tomography. J. Appl. Physiol. 121, 42–52 (2016).
Yang, S. et al. Deep learning segmentation of major vessels in X-ray coronary angiography. Sci. Rep. 9, 16897 (2019).
Duchateau, N., King, A. P. & De Craene, M. Machine learning approaches for myocardial motion and deformation analysis. Front. Cardiovasc. Med. 6, 190 (2019).
Krebs, J., Delingette, H., Mailhe, B., Ayache, N. & Mansi, T. Learning a probabilistic model for diffeomorphic registration. IEEE Trans. Med. Imaging 38, 2165–2176 (2019).
Zheng, Q., Delingette, H. & Ayache, N. Explainable cardiac pathology classification on cine MRI with motion characterization by semi-supervised learning of apparent flow. Med. Image Anal. 56, 80–95 (2019).
Yan, W., Wang, Y., van der Geest, R. J. & Tao, Q. Cine MRI analysis by deep learning of optical flow: adding the temporal dimension. Comput. Biol. Med. 111, 103356 (2019).
Parajuli, N. et al. Flow network tracking for spatiotemporal and periodic point matching: applied to cardiac motion analysis. Med. Image Anal. 55, 116–135 (2019).
Lu, A. et al. in Medical Image Computing and Computer-Assisted Intervention — MICCAI 2017 (eds Descoteaux, M. et al.) 323–331 (Springer, 2017).
Song, S. et al. Deep motion tracking from multiview angiographic image sequences for synchronization of cardiac phases. Phys. Med. Biol. 64, 025018 (2019).
Attar, R. et al. Quantitative CMR population imaging on 20,000 subjects of the UK Biobank imaging study: LV/RV quantification pipeline and its evaluation. Med. Image Anal. 56, 26–42 (2019).
Mantilla, J. J. et al. Discriminative dictionary learning for local LV wall motion classification in cardiac MRI. Expert. Syst. Appl. 129, 286–295 (2019).
Duchateau, N., De Craene, M., Piella, G. & Frangi, A. F. Constrained manifold learning for the characterization of pathological deviations from normality. Med. Image Anal. 16, 1532–1549 (2012).
Sengupta, P. P. et al. Cognitive machine-learning algorithm for cardiac imaging: a pilot study for differentiating constrictive pericarditis from restrictive cardiomyopathy. Circ. Cardiovasc. Imaging 9, e004330 (2016).
Sanchez-Martinez, S. et al. Characterization of myocardial motion patterns by unsupervised multiple kernel learning. Med. Image Anal. 35, 70–82 (2017).
Meyer, H. V. et al. Genetic and functional insights into the fractal structure of the heart. Nature 584, 589–594 (2020).
Zreik, M. et al. Deep learning analysis of coronary arteries in cardiac CT angiography for detection of patients requiring invasive coronary angiography. IEEE Trans. Med. Imaging 36, 1545–1557 (2019).
Martin, S. S. et al. Evaluation of a deep learning-based automated CT coronary artery calcium scoring algorithm. JACC Cardiovasc. Imaging 13, 524–526 (2019).
Cikes, M. et al. Machine learning-based phenogrouping in heart failure to identify responders to cardiac resynchronization therapy. Eur. J. Heart Fail. 21, 74–85 (2019).
Alis, D., Guler, A., Yergin, M. & Asmakutlu, O. Assessment of ventricular tachyarrhythmia in patients with hypertrophic cardiomyopathy with machine learning-based texture analysis of late gadolinium enhancement cardiac MRI. Diagn. Interv. Imaging 101, 137–146 (2019).
Hilbert, A. et al. Data-efficient deep learning of radiological image data for outcome prediction after endovascular treatment of patients with acute ischemic stroke. Comput. Biol. Med. 115, 103516 (2019).
Bruse, J. L. et al. Detecting clinically meaningful shape clusters in medical image data: metrics analysis for hierarchical clustering applied to healthy and pathological aortic arches. IEEE Trans. Biomed. Eng. 64, 2373–2383 (2017).
Hunter, P. The virtual physiological human: the physiome project aims to develop reproducible, multiscale models for clinical practice. IEEE Pulse 7, 36–42 (2016).
Ayache, N. Medical imaging informatics: towards a personalized computational patient. Yearb. Med. Inform. 25 (Suppl. 1), S8–S9 (2016).
Bassingthwaighte, J., Hunter, P. & Noble, D. The cardiac physiome: perspectives for the future. Exp. Physiol. 94, 597–605 (2009).
Chapelle, D., Le Tallec, P., Moireau, P. & Sorine, M. Energy-preserving muscle tissue model: formulation and compatible discretizations. Int. J. Mult. Comp. Eng. 10, 189–211 (2012).
Suinesiaputra, A., McCulloch, A. D., Nash, M. P., Pontre, B. & Young, A. A. Cardiac image modelling: Breadth and depth in heart disease. Med. Image Anal. 33, 38–43 (2016).
Niederer, S. A., Lumens, J. & Trayanova, N. A. Computational models in cardiology. Nat. Rev. Cardiol. 16, 100–111 (2019).
Comaniciu, D., Engel, K., Georgescu, B. & Mansi, T. Shaping the future through innovations: from medical imaging to precision medicine. Med. Image Anal. 33, 19–26 (2016).
Molléro, R. et al. Multifidelity-CMA: a multifidelity approach for efficient personalisation of 3D cardiac electromechanical models. Biomech. Model. Mechanobiol. 17, 285–300 (2018).
Corral-Acero, J. et al. The ‘Digital Twin’ to enable the vision of precision cardiology. Eur. Heart J. 41, 4556–4564 (2020).
Chabiniok, R. et al. Multiphysics and multiscale modelling, data-model fusion and integration of organ physiology in the clinic: ventricular cardiac mechanics. Interface Focus. 6, 20150083 (2016).
Sermesant, M. et al. Toward patient-specific myocardial models of the heart. Heart Fail. Clin. 4, 289–301 (2008).
This, A., Morales, H. G., Bonnefous, O., Fernández, M. A. & Gerbeau, J.-F. A pipeline for image based intracardiac CFD modeling and application to the evaluation of the PISA method. Comput. Methods Appl. Mech. Eng. 358, 112627 (2020).
Vignon-Clementel, I. E., Marsden, A. L. & Feinstein, J. A. A primer on computational simulation in congenital heart disease for the clinician. Prog. Pediatr. Cardiol. 30, 3–13 (2010).
Sermesant, M. et al. Patient-specific electromechanical models of the heart for the prediction of pacing acute effects in CRT: a preliminary clinical validation. Med. Image Anal. 16, 201–215 (2012).
Chen, Z. et al. Biophysical modeling predicts ventricular tachycardia inducibility and circuit morphology: a combined clinical validation and computer modeling approach. J. Cardiovasc. Electrophysiol. 27, 851–860 (2016).
Baillargeon, B., Rebelo, N., Fox, D. D., Taylor, R. L. & Kuhl, E. The living heart project: a robust and integrative simulator for human heart function. Eur. J. Mech. A Solids 48, 38–47 (2014).
Kayvanpour, E. et al. Towards personalized cardiology: multi-scale modeling of the failing heart. PLoS ONE 10, e0134869 (2015).
Zhang, F. et al. Towards patient-specific modeling of mitral valve repair: 3D transesophageal echocardiography-derived parameter estimation. Med. Image Anal. 35, 599–609 (2017).
Lluch, È. et al. Breaking the state of the heart: meshless model for cardiac mechanics. Biomech. Model. Mechanobiol. 18, 1549–1561 (2019).
Garny, A., Noble, D. & Kohl, P. Dimensionality in cardiac modelling. Prog. Biophys. Mol. Biol. 87, 47–66 (2005).
Neumann, D. et al. A self-taught artificial agent for multi-physics computational model personalization. Med. Image Anal. 34, 52–64 (2016).
Lozoya, R. C. et al. Model-based feature augmentation for cardiac ablation target learning from images. IEEE Trans. Biomed. Eng. 66, 30–40 (2018).
Alber, M. et al. Integrating machine learning and multiscale modeling-perspectives, challenges, and opportunities in the biological, biomedical, and behavioral sciences. NPJ Digital Med. 2, 115 (2019).
Prakosa, A. et al. Generation of synthetic but visually realistic time series of cardiac images combining a biophysical model and clinical images. IEEE Trans. Med. Imaging 32, 99–109 (2013).
Duchateau, N., Sermesant, M., Delingette, H. & Ayache, N. Model-based generation of large databases of cardiac images: synthesis of pathological cine MR sequences from real healthy cases. IEEE Trans. Med. Imaging 37, 755–766 (2018).
Heimann, T., Mountney, P., John, M. & Ionasec, R. Real-time ultrasound transducer localization in fluoroscopy images by transfer learning from synthetic training data. Med. Image Anal. 18, 1320–1328 (2014).
Kissas, G. et al. Machine learning in cardiovascular flows modeling: predicting arterial blood pressure from non-invasive 4D flow MRI data using physics-informed neural networks. Comput. Methods Appl. Mech. Eng. 358, 112623 (2020).
Ayed, I., Cedilnik, N., Gallinari, P. & Sermesant, M. in Functional Imaging and Modeling of the Heart (eds Coudière, Y., Ozenne, V., Vigmond, E. & Zemzemi, N.) 55–63 (Springer, 2019).
Coenen, A. et al. Diagnostic accuracy of a machine-learning approach to coronary computed tomographic angiography-based fractional flow reserve: result from the MACHINE consortium. Circ. Cardiovasc. Imaging 11, e007217 (2018).
Papademetris, X., Sinusas, A. J., Dione, D. P. & Duncan, J. S. Estimation of 3D left ventricular deformation from echocardiography. Med. Image Anal. 5, 17–28 (2001).
Finsberg, H. et al. Computational quantification of patient-specific changes in ventricular dynamics associated with pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 317, H1363–H1375 (2019).
Giffard-Roisin, S. et al. Transfer learning from simulations on a reference anatomy for ECGI in personalized cardiac resynchronization therapy. IEEE Trans. Biomed. Eng. 66, 343–353 (2019).
Meister, F. et al. Deep learning acceleration of total Lagrangian explicit dynamics for soft tissue mechanics. Comput. Methods Appl. Mech. Eng. 358, 112628 (2020).
Konukoglu, E. et al. Efficient probabilistic model personalization integrating uncertainty on data and parameters: application to eikonal-diffusion models in cardiac electrophysiology. Prog. Biophys. Mol. Biol. 107, 134–146 (2011).
The Medical Futurist. FDA approvals for smart algorithms in medicine in one giant infographic. Medical Futurist https://medicalfuturist.com/fda-approvals-for-algorithms-in-medicine (2019).
Saltybaeva, N., Schmidt, B., Wimmer, A., Flohr, T. & Alkadhi, H. Precise and automatic patient positioning in computed tomography: avatar modeling of the patient surface using a 3-dimensional camera. Invest. Radiol. 53, 641–646 (2018).
Taylor, C. A., Fonte, T. A. & Min, J. K. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J. Am. Coll. Cardiol. 61, 2233–2241 (2013).
Lu, M. T. et al. Noninvasive FFR derived from coronary CT angiography: management and outcomes in the PROMISE trial. JACC Cardiovasc. Imaging 10, 1350–1358 (2017).
Bluemke, D. A. Radiology in 2018: are you working with AI or being replaced by AI? Radiology 287, 365–366 (2018).
Willemink, M. J. et al. Photon-counting CT: technical principles and clinical prospects. Radiology 289, 293–312 (2018).
Weese, J. & Lorenz, C. Four challenges in medical image analysis from an industrial perspective. Med. Image Anal. 33, 44–49 (2016).
Hutter, F., Kotthoff, L. & Vanschoren, J. (eds) Automated Machine Learning: Methods, Systems, Challenges (Springer, 2019).
Minter, S. et al. Crowdsourcing consensus: proposal of a novel method for assessing accuracy in echocardiography interpretation. Int. J. Cardiovasc. Imaging 34, 1725–1730 (2018).
Pace, D. F. et al. Interactive whole-heart segmentation in congenital heart disease. Med. Image Comput. Comput. Assist. Interv. 9351, 80–88 (2015).
Chen, L. et al. Self-supervised learning for medical image analysis using image context restoration. Med. Image Anal. 58, 101539 (2019).
Arafati, A. et al. Artificial intelligence in pediatric and adult congenital cardiac MRI: an unmet clinical need. Cardiovasc. Diagn. Ther. 9 (Suppl. 2), S310–S325 (2019).
Chartsias, A. et al. Disentangled representation learning in cardiac image analysis. Med. Image Anal. 58, 101535 (2019).
Sengupta, P. P. et al. Proposed requirements for cardiovascular imaging-related machine learning evaluation (PRIME): A checklist: reviewed by the American College of Cardiology Healthcare Innovation Council. JACC Cardiovasc. Imaging 13, 2017–2035 (2020).
Barredo Arrieta, A. et al. Explainable artificial intelligence (XAI): concepts, taxonomies, opportunities and challenges toward responsible AI. Inf. Fusion. 58, 82–115 (2020).
Cabitza, F., Rasoini, R. & Gensini, G. F. Unintended consequences of machine learning in medicine. JAMA 318, 517–518 (2017).
European Commission. Ethics guidelines for trustworthy AI. European Commission https://ec.europa.eu/digital-single-market/en/news/ethics-guidelines-trustworthy-ai (2019).
Recht, M. P. et al. Integrating artificial intelligence into the clinical practice of radiology: challenges and recommendations. Eur. Radiol. 30, 3576–3584 (2020).
Acknowledgements
Part of the authors’ work has been supported by the French Government, through the National Research Agency (ANR): 3IA Côte d’Azur (ANR-19-P3IA-0002), IHU Liryc (ANR-10-IAHU-04) and Equipex MUSIC (ANR-11-EQPX-0030). The research leading to these results has also received European funding from the ERC starting grant ECSTATIC (715093).
Author information
Authors and Affiliations
Contributions
All the authors contributed to researching data for the article, discussing its content, writing the manuscript, and reviewing and editing it before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Cardiology thanks T. Leiner, P. Sengupta and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
CAMUS: https://www.creatis.insa-lyon.fr/Challenge/camus/
Kaggle: https://www.kaggle.com/datasets
PyTorch: https://pytorch.org/
STACOM: http://stacom.cardiacatlas.org/
TensorFlow: https://www.tensorflow.org/
UK Biobank: https://www.ukbiobank.ac.uk/
Glossary
- Artificial intelligence
-
(AI). In general, algorithms that mimic human intelligence; in this article, algorithms that interpret medical images and data to assist the diagnosis, prognosis and therapy of cardiovascular diseases.
- Deep learning
-
Machine learning with artificial neural networks that have a large number of hidden layers.
- Features
-
Distinctive attributes of an image or a signal.
- Convolutional neural networks
-
Artificial neural network using convolution operations to compute features within its layers.
- Convolution operations
-
The weighted sum of neighbouring pixel values in an image.
- Generalizability
-
The ability of a machine learning algorithm to perform sufficiently well on a new data set unseen during the training stage; also known as robustness.
- Biophysical modelling
-
Mathematical representation of biological phenomena using methods from physics.
- Machine learning
-
The capacity of an algorithm to solve a task by exploiting training examples, instead of following predefined explicit instructions.
- Accuracy
-
Measurement of agreement between the algorithm prediction and the expected result.
- Supervised learning
-
Learning process using user-defined annotations on a training data set.
- Ground truth
-
Data corresponding to the expected result of an algorithm.
- Image segmentation
-
Specifying regions with labels in a medical image.
- Image registration
-
Geometric transformation of an image to align it on another image.
- Motion analysis
-
Computation and analysis of apparent displacements from time series of images.
- Overfitting
-
When an algorithm is adjusted too closely to the training data during learning at the expense of generalizability to new data.
- Image annotations
-
User-defined information associated with the input data.
- Unsupervised learning
-
Learning process without user-defined annotations.
- Transfer learning
-
Adjustment of a machine learning algorithm from one task to another.
- Artificial neural networks
-
Algorithm mapping input to output data, involving multiple layers of non-linear computations.
- Cost function
-
Criterion to be minimized during the training phase of machine learning algorithms.
- MRI fingerprinting
-
Acquisition of quantitative information from MRI scans that enables clinical decision-making on the basis of digital data rather than visual impressions.
- Multi-scale
-
Involving several spatial or temporal resolutions of observation.
- Multi-physics
-
Involving several different physical phenomena (such as electrophysiology and solid or fluid mechanics).
- Digital twin
-
Patient-specific computational model (of the heart) to visualize and simulate anatomy and physiology.
- Causal
-
In which one event (cause) contributes to the occurrence of another event (effect).
- Deterministic
-
A system in which a given input always produces the same output (as opposed to probabilistic systems).
- Mechanistic
-
Providing explicit information about the underlying biological or physical processes.
- Uncertainty quantification
-
Determination of how the output of an algorithm varies if some of its parameters or input are not exactly known.
- Explainability
-
An explainable algorithm must produce details that make its process easy to understand.
Rights and permissions
About this article
Cite this article
Sermesant, M., Delingette, H., Cochet, H. et al. Applications of artificial intelligence in cardiovascular imaging. Nat Rev Cardiol 18, 600–609 (2021). https://doi.org/10.1038/s41569-021-00527-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41569-021-00527-2
This article is cited by
-
Seeing is believing: pathway strategies for a personalised non-invasive diagnosis of coronary artery disease
Internal and Emergency Medicine (2024)
-
Roadmap on the use of artificial intelligence for imaging of vulnerable atherosclerotic plaque in coronary arteries
Nature Reviews Cardiology (2024)
-
Medical image captioning via generative pretrained transformers
Scientific Reports (2023)
-
Real-time speech MRI datasets with corresponding articulator ground-truth segmentations
Scientific Data (2023)
-
Assisting schizophrenia diagnosis using clinical electroencephalography and interpretable graph neural networks: a real-world and cross-site study
Neuropsychopharmacology (2023)