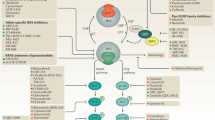
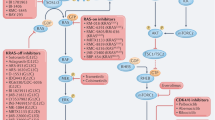
Abstract
Although RAS was formerly considered undruggable, various agents that inhibit RAS or specific RAS oncoproteins have now been developed. Indeed, the importance of directly targeting RAS has recently been illustrated by the clinical success of mutant-selective KRAS inhibitors. Nevertheless, responses to these agents are typically incomplete and restricted to a subset of patients, highlighting the need to develop more effective treatments, which will likely require a combinatorial approach. Vertical strategies that target multiple nodes within the RAS pathway to achieve deeper suppression are being investigated and have precedence in other contexts. However, alternative strategies that co-target RAS and other therapeutic vulnerabilities have been identified, which may mitigate the requirement for profound pathway suppression. Regardless, the efficacy of any given approach will likely be dictated by genetic, epigenetic and tumour-specific variables. Here we discuss various combinatorial strategies to treat KRAS-driven cancers, highlighting mechanistic concepts that may extend to tumours harbouring other RAS mutations. Although many promising combinations have been identified, clinical responses will ultimately depend on whether a therapeutic window can be achieved and our ability to prospectively select responsive patients. Therefore, we must continue to develop and understand biologically diverse strategies to maximize our likelihood of success.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Simanshu, D. K., Nissley, D. V. & McCormick, F. RAS proteins and their regulators in human disease. Cell 170, 17–33 (2017).
The AACR Project GENIE Consortium AACR project GENIE: powering precision medicine through an international consortium. Cancer Discov. 7, 818–831 (2017).
Zehir, A. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 23, 703–713 (2017).
Tate, J. G. et al. COSMIC: the catalogue of somatic mutations in cancer. Nucleic Acids Res. 47, D941–D947 (2019).
Prior, I. A., Hood, F. E. & Hartley, J. L. The frequency of Ras mutations in cancer. Cancer Res. 80, 2969–2974 (2020).
Moore, A. R., Rosenberg, S. C., McCormick, F. & Malek, S. RAS-targeted therapies: is the undruggable drugged? Nat. Rev. Drug. Discov. 19, 533–552 (2020).
Punekar, S. R., Velcheti, V., Neel, B. G. & Wong, K.-K. The current state of the art and future trends in RAS-targeted cancer therapies. Nat. Rev. Clin. Oncol. 19, 637–655 (2022).
Peters, S., Mok, T., Passaro, A. & Jänne, P. A. The promising evolution of targeted therapeutic strategies in cancer. Cancer Discov. 11, 810–814 (2021).
Konieczkowski, D. J., Johannessen, C. M. & Garraway, L. A. A convergence-based framework for cancer drug resistance. Cancer Cell 33, 801–815 (2018).
Ryan, M. B. & Corcoran, R. B. Therapeutic strategies to target RAS-mutant cancers. Nat. Rev. Clin. Oncol. 15, 709–720 (2018).
Gysin, S., Salt, M., Young, A. & McCormick, F. Therapeutic strategies for targeting Ras proteins. Genes. Cancer 2, 359–372 (2011).
Karoulia, Z., Gavathiotis, E. & Poulikakos, P. I. New perspectives for targeting RAF kinase in human cancer. Nat. Rev. Cancer 17, 676–691 (2017).
Zhang, C. et al. RAF inhibitors that evade paradoxical MAPK pathway activation. Nature 526, 583–586 (2015).
Yao, Z. et al. RAF inhibitor PLX8394 selectively disrupts BRAF dimers and RAS-independent BRAF-mutant-driven signaling. Nat. Med. 25, 284–291 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04916236 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05375994 (2023).
Sullivan, R. J. et al. First-in-class ERK1/2 inhibitor ulixertinib (BVD-523) in patients with MAPK mutant advanced solid tumors: results of a phase I dose-escalation and expansion study. Cancer Discov. 8, 184–195 (2018).
Bhagwat, S. V. et al. ERK inhibitor LY3214996 targets ERK pathway-driven cancers: a therapeutic approach toward precision medicine. Mol. Cancer Ther. 19, 325–336 (2020).
Dombi, E. et al. Activity of selumetinib in neurofibromatosis type 1-related plexiform neurofibromas. N. Engl. J. Med. 375, 2550–2560 (2016).
Kun, E., Tsang, Y. T. M., Ng, C. W., Gershenson, D. M. & Wong, K. K. MEK inhibitor resistance mechanisms and recent developments in combination trials. Cancer Treat. Rev. 92, 102137 (2021).
Han, J. et al. MEK inhibitors for the treatment of non-small cell lung cancer. J. Hematol. Oncol. 14, 1 (2021).
Ostrem, J. M., Peters, U., Sos, M. L., Wells, J. A. & Shokat, K. M. K-RasG12C inhibitors allosterically control GTP affinity and effector interactions. Nature 503, 548–551 (2013). This work is the first report of a direct KRAS-G12C small molecule inhibitor.
Lim, S. M. et al. Therapeutic targeting of oncogenic K-Ras by a covalent catalytic site inhibitor. Angew. Chem. Int. Ed. Engl. 53, 199–204 (2014).
Skoulidis, F. et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N. Engl. J. Med. 384, 2371–2381 (2021).
Jänne, P. A. et al. Adagrasib in non-small-cell lung cancer harboring a KRASG12C mutation. N. Engl. J. Med. 387, 120–131 (2022).
Canon, J. et al. The clinical KRASG12C inhibitor AMG 510 drives anti-tumour immunity. Nature 575, 217–223 (2019).
Hallin, J. et al. The KRASG12C inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 10, 54–71 (2020).
Hofmann, M. H., Gerlach, D., Misale, S., Petronczki, M. & Kraut, N. Expanding the reach of precision oncology by drugging all KRAS mutants. Cancer Discov. 12, 924–937 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05379985 (2024).
Wang, X. et al. Identification of MRTX1133, a noncovalent, potent, and selective KRASG12D inhibitor. J. Med. Chem. 65, 3123–3133 (2022). This study describes the development of the first reported small molecule inhibitor with selective activity against KRAS-G12D and represents ongoing efforts to expand the druggable RAS repertoire beyond KRAS-G12C.
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05737706 (2023).
Zhou, C. et al. Design, synthesis, and biological evaluation of potent and selective PROTAC degraders of oncogenic KRASG12D. J. Med. Chem. 67, 1147–1167 (2024).
Tanaka, N. et al. Clinical acquired resistance to KRASG12C inhibition through a novel KRAS switch-II pocket mutation and polyclonal alterations converging on RAS–MAPK reactivation. Cancer Discov. 11, 1913–1922 (2021).
Schulze, C. J. et al. Chemical remodeling of a cellular chaperone to target the active state of mutant KRAS. Science 381, 794–799 (2023).
Molina-Arcas, M., Samani, A. & Downward, J. Drugging the undruggable: advances on RAS targeting in cancer. Genes 12, 899 (2021).
Sacher, A. et al. Single-agent divarasib (GDC-6036) in solid tumors with a KRAS G12C mutation. N. Engl. J. Med. 389, 710–721 (2023).
Awad, M. M. et al. Acquired resistance to KRASG12C inhibition in cancer. N. Engl. J. Med. 384, 2382–2393 (2021). This study describes genetic alterations that mediate resistance in post-treatment tumours in patients treated with adagrasib.
Zhao, Y. et al. Diverse alterations associated with resistance to KRASG12C inhibition. Nature 599, 679–683 (2021). This study describes genetic alterations that mediate resistance in post-treatment tumours in patients treated with sotorasib.
Van Allen, E. M. et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov. 4, 94–109 (2014).
Emery, C. M. et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc. Natl Acad. Sci. USA 106, 20411–20416 (2009).
Ahronian, L. G. et al. Clinical acquired resistance to RAF inhibitor combinations in BRAF-mutant colorectal cancer through MAPK pathway alterations. Cancer Discov. 5, 358–367 (2015).
Negrao, M. V. et al. Comutations and KRASG12C inhibitor efficacy in advanced NSCLC. Cancer Discov. 13, 1556–1571 (2023).
Robert, C. et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 372, 30–39 (2015).
Weekes, C. et al. A phase Ib study to evaluate the MEK inhibitor cobimetinib in combination with the ERK1/2 inhibitor GDC-0994 in patients with advanced solid tumors. Oncologist 25, 833-e1438 (2020).
Stathis, A. et al. Results of an open-label phase 1b study of the ERK inhibitor MK-8353 plus the MEK inhibitor selumetinib in patients with advanced or metastatic solid tumors. Invest. N. Drugs 41, 380–390 (2023).
Kim, D. et al. Pan-KRAS inhibitor disables oncogenic signalling and tumour growth. Nature 619, 160–166 (2023).
Nazarian, R. et al. Melanomas acquire resistance to B-RAFV600E inhibition by RTK or N-RAS upregulation. Nature 468, 973–977 (2010).
Prahallad, A. et al. Unresponsiveness of colon cancer to BRAFV600E inhibition through feedback activation of EGFR. Nature 483, 100–103 (2012).
Corcoran, R. B. et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2, 227–235 (2012).
Sun, C. et al. Intrinsic resistance to MEK inhibition in KRAS mutant lung and colon cancer through transcriptional induction of ERBB3. Cell Rep. 7, 86–93 (2014).
Lito, P. et al. Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell 22, 668–682 (2012).
Douville, E. & Downward, J. EGF induced SOS phosphorylation in PC12 cells involves P90 RSK-2. Oncogene 15, 373–383 (1997).
Hanafusa, H., Torii, S., Yasunaga, T. & Nishida, E. Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat. Cell Biol. 4, 850–858 (2002).
Eblaghie, M. C. et al. Negative feedback regulation of FGF signaling levels by Pyst1/MKP3 in chick embryos. Curr. Biol. CB 13, 1009–1018 (2003).
Dougherty, M. K. et al. Regulation of Raf-1 by direct feedback phosphorylation. Mol. Cell 17, 215–224 (2005).
Macrae, M. et al. A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell 8, 111–118 (2005).
Courtois-Cox, S. et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell 10, 459–472 (2006).
Avraham, R. & Yarden, Y. Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat. Rev. Mol. Cell Biol. 12, 104–117 (2011).
Chandarlapaty, S. et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 19, 58–71 (2011).
Rodrik-Outmezguine, V. S. et al. mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discov. 1, 248–259 (2011).
Duncan, J. S. et al. Dynamic reprogramming of the kinome in response to targeted MEK inhibition in triple-negative breast cancer. Cell 149, 307–321 (2012).
Long, G. V. et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 371, 1877–1888 (2014).
Corcoran, R. B. et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J. Clin. Oncol. 33, 4023–4031 (2015).
Manchado, E. et al. A combinatorial strategy for treating KRAS-mutant lung cancer. Nature 534, 647–651 (2016). This study, through analysis of a trametinib-anchored short hairpin RNA knockdown screen, establishes FGFR1-mediated reactivation of the RAS pathway as resistance mechanism to MEK inhibition in KRAS-mutant lung cancer and highlights the tissue specificity of RTK-mediated resistance.
Kitai, H. et al. Epithelial-to-mesenchymal transition defines feedback activation of receptor tyrosine kinase signaling induced by MEK inhibition in KRAS-mutant lung cancer. Cancer Discov. 6, 754–769 (2016). This paper, beyond the tissue specificity of RTK-mediated resistance, further divides KRAS-mutant lung cancer into epithelial and mesenchymal subtypes and highlights the unique susceptibility of each subtype to MEK inhibition combined with HER3 or FGFR1 suppression, respectively.
Arbour, K. C. et al. Phase 1 clinical trial of trametinib and ponatinib in patients with NSCLC harboring KRAS mutations. JTO Clin. Res. Rep. 3, 100256 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03704688 (2023).
Hong, D. S. et al. KRASG12C inhibition with sotorasib in advanced solid tumors. N. Engl. J. Med. 383, 1207–1217 (2020).
Amodio, V. et al. EGFR blockade reverts resistance to KRASG12C inhibition in colorectal cancer. Cancer Discov. 10, 1129–1139 (2020). This study identifies high basal EGFR activation as a mechanism of resistance to KRAS-G12C inhibition in CRC and provides the first preclinical evidence of the therapeutic efficacy of KRAS-G12C inhibition in combination with EGFR inhibition in CRC, leading to the development of a therapeutic strategy that has been recently granted breakthrough therapy designation by the FDA.
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03785249 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05198934 (2024).
Fakih, M. G. et al. Sotorasib plus panitumumab in refractory colorectal cancer with mutated KRAS G12C. N. Engl. J. Med. 389, 2125–2139 (2023).
Kuboki, Y. et al. Sotorasib with panitumumab in chemotherapy-refractory KRASG12C-mutated colorectal cancer: a phase 1b trial. Nat. Med. 30, 265–270 (2024).
Kopetz, S. et al. Encorafenib, binimetinib, and cetuximab in BRAFV600E-mutated colorectal cancer. N. Engl. J. Med. 381, 1632–1643 (2019).
Misale, S. et al. KRAS G12C NSCLC models are sensitive to direct targeting of KRAS in combination with PI3K inhibition. Clin. Cancer Res. 25, 796–807 (2019).
Fedele, C. et al. SHP2 inhibition prevents adaptive resistance to MEK inhibitors in multiple cancer models. Cancer Discov. 8, 1237–1249 (2018).
Zawistowski, J. S. et al. Enhancer remodeling during adaptive bypass to MEK inhibition is attenuated by pharmacologic targeting of the P-TEFb complex. Cancer Discov. 7, 302–321 (2017).
Ryan, M. B. et al. Vertical pathway inhibition overcomes adaptive feedback resistance to KRASG12C inhibition. Clin. Cancer Res. 26, 1633–1643 (2020).
Kerr, D. L., Haderk, F. & Bivona, T. G. Allosteric SHP2 inhibitors in cancer: targeting the intersection of RAS, resistance, and the immune microenvironment. Curr. Opin. Chem. Biol. 62, 1–12 (2021).
Nichols, R. J. et al. RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers. Nat. Cell Biol. 20, 1064–1073 (2018).
Lito, P., Solomon, M., Li, L.-S., Hansen, R. & Rosen, N. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science 351, 604–608 (2016).
Patricelli, M. P. et al. Selective inhibition of oncogenic KRAS output with small molecules targeting the inactive state. Cancer Discov. 6, 316–329 (2016).
Mainardi, S. et al. SHP2 is required for growth of KRAS-mutant non-small-cell lung cancer in vivo. Nat. Med. 24, 961–967 (2018).
Ruess, D. A. et al. Mutant KRAS-driven cancers depend on PTPN11/SHP2 phosphatase. Nat. Med. 24, 954–960 (2018).
Drilon, A. et al. SHP2 inhibition sensitizes diverse oncogene-addicted solid tumors to re-treatment with targeted therapy. Cancer Discov. 13, 1789–1801 (2023).
Fedele, C. et al. SHP2 inhibition diminishes KRASG12C cycling and promotes tumor microenvironment remodeling. J. Exp. Med. 218, e20201414 (2021).
Liu, C. et al. Combinations with allosteric SHP2 inhibitor TNO155 to block receptor tyrosine kinase signaling. Clin. Cancer Res. 27, 342–354 (2021).
Lv, Y. et al. A potent SOS1 PROTAC degrader with synergistic efficacy in combination with KRASG12C inhibitor. J. Med. Chem. https://doi.org/10.1021/acs.jmedchem.3c01598 (2024).
Hofmann, M. H. et al. BI-3406, a potent and selective SOS1–KRAS interaction inhibitor, is effective in KRAS-driven cancers through combined MEK inhibition. Cancer Discov. 11, 142–157 (2021).
Ketcham, J. M. et al. Design and discovery of MRTX0902, a potent, selective, brain-penetrant, and orally bioavailable inhibitor of the SOS1:KRAS protein–protein interaction. J. Med. Chem. 65, 9678–9690 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04975256 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05578092 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04111458 (2024).
Bian, Y. et al. Development of SOS1 inhibitor-based degraders to target KRAS -mutant colorectal cancer. J. Med. Chem. 65, 16432–16450 (2022).
Zhou, Z. et al. Discovery of a potent, cooperative, and selective SOS1 PROTAC ZZ151 with in vivo antitumor efficacy in KRAS-mutant cancers. J. Med. Chem. 66, 4197–4214 (2023).
Lavoie, H. & Therrien, M. Regulation of RAF protein kinases in ERK signalling. Nat. Rev. Mol. Cell Biol. 16, 281–298 (2015).
Cordeddu, V. et al. Mutation of SHOC2 promotes aberrant protein N-myristoylation and causes Noonan-like syndrome with loose anagen hair. Nat. Genet. 41, 1022–1026 (2009).
Sulahian, R. et al. Synthetic lethal interaction of SHOC2 depletion with MEK inhibition in RAS-driven cancers. Cell Rep. 29, 118–134.e8 (2019).
Jones, G. G. et al. SHOC2 phosphatase-dependent RAF dimerization mediates resistance to MEK inhibition in RAS-mutant cancers. Nat. Commun. 10, 2532 (2019).
Bonsor, D. A. et al. Structure of the SHOC2–MRAS–PP1C complex provides insights into RAF activation and Noonan syndrome. Nat. Struct. Mol. Biol. 29, 966–977 (2022).
Hauseman, Z. J. et al. Structure of the MRAS–SHOC2–PP1C phosphatase complex. Nature 609, 416–423 (2022).
Kwon, J. J. et al. Structure–function analysis of the SHOC2–MRAS–PP1C holophosphatase complex. Nature 609, 408–415 (2022).
Kimmelman, A. C. & White, E. Autophagy and tumor metabolism. Cell Metab. 25, 1037–1043 (2017).
Lock, R. et al. Autophagy facilitates glycolysis during Ras-mediated oncogenic transformation. Mol. Biol. Cell 22, 165–178 (2011).
Guo, J. Y. et al. Activated Ras requires autophagy to maintain oxidative metabolism and tumorigenesis. Genes. Dev. 25, 460–470 (2011).
Yang, S. et al. Pancreatic cancers require autophagy for tumor growth. Genes. Dev. 25, 717–729 (2011).
Guo, J. Y. et al. Autophagy suppresses progression of K-ras-induced lung tumors to oncocytomas and maintains lipid homeostasis. Genes. Dev. 27, 1447–1461 (2013).
Perera, R. M. et al. Transcriptional control of autophagy–lysosome function drives pancreatic cancer metabolism. Nature 524, 361–365 (2015).
Guo, J. Y. et al. Autophagy provides metabolic substrates to maintain energy charge and nucleotide pools in Ras-driven lung cancer cells. Genes. Dev. 30, 1704–1717 (2016).
Bryant, K. L. et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat. Med. 25, 628–640 (2019).
Kinsey, C. G. et al. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat. Med. 25, 620–627 (2019). This study, together with Bryant et al. (2019), makes the surprising observation that RAS pathway inhibition increases autophagy in RAS-driven cancers, demonstrating that co-targeting this protective increase in autophagy cooperates with RAS pathway inhibition, which is a therapeutic strategy that is being tested in clinical trials.
Lee, C.-S. et al. MAP kinase and autophagy pathways cooperate to maintain RAS mutant cancer cell survival. Proc. Natl Acad. Sci. USA 116, 4508–4517 (2019).
Silvis, M. R. et al. MYC-mediated resistance to trametinib and HCQ in PDAC is overcome by CDK4/6 and lysosomal inhibition. J. Exp. Med. 220, e20221524 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04892017 (2024).
Yamamoto, K. et al. Autophagy promotes immune evasion of pancreatic cancer by degrading MHC-I. Nature 581, 100–105 (2020).
Ravichandran, M. et al. Coordinated transcriptional and catabolic programs support iron-dependent adaptation to RAS–MAPK pathway inhibition in pancreatic cancer. Cancer Discov. 12, 2198–2219 (2022).
Santana-Codina, N. et al. NCOA4-mediated ferritinophagy is a pancreatic cancer dependency via maintenance of iron bioavailability for iron–sulfur cluster proteins. Cancer Discov. 12, 2180–2197 (2022).
Tan, N. et al. Bcl-2/Bcl-xL inhibition increases the efficacy of MEK inhibition alone and in combination with PI3 kinase inhibition in lung and pancreatic tumor models. Mol. Cancer Ther. 12, 853–864 (2013).
Corcoran, R. B. et al. Synthetic lethal interaction of combined BCL-XL and MEK inhibition promotes tumor regressions in KRAS mutant cancer models. Cancer Cell 23, 121–128 (2013). This study is among the first to show that concomitant inhibition of the RAS pathway and anti-apoptotic factors is an effective therapeutic strategy for RAS-driven tumours.
Perurena, N. et al. USP9X mediates an acute adaptive response to MAPK suppression in pancreatic cancer but creates multiple actionable therapeutic vulnerabilities. Cell Rep. Med. 4, 101007 (2023).
Montero, J. et al. Destabilization of NOXA mRNA as a common resistance mechanism to targeted therapies. Nat. Commun. 10, 5157 (2019).
Li, C. et al. LKB1 loss rewires JNK-induced apoptotic protein dynamics through NUAKs and sensitizes KRAS-mutant NSCLC to combined KRASG12C + MCL-1 blockade. Preprint at bioRxiv https://doi.org/10.1101/2022.09.29.510137 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02079740 (2024).
Liu, J. et al. Activity of combination trametinib/navitoclax in patients with RAS-mutated gynecologic (GYN) cancers in a phase 1/2 study (LBA 12). Gynecol. Oncol. 166, S67 (2022).
Nangia, V. et al. Exploiting MCL1 dependency with combination MEK + MCL1 inhibitors leads to induction of apoptosis and tumor regression in KRAS-mutant non-small cell lung cancer. Cancer Discov. 8, 1598–1613 (2018).
Hogdal, L. J. & Letai, A. BCL-2 inhibition: stemming the tide of myeloid malignancies. Cell Stem Cell 12, 269–270 (2013).
Puyol, M. et al. A synthetic lethal interaction between K-Ras oncogenes and Cdk4 unveils a therapeutic strategy for non-small cell lung carcinoma. Cancer Cell 18, 63–73 (2010).
Ruscetti, M. et al. NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science 362, 1416–1422 (2018). This study shows that the combination of MEKi with CDK4 and CKD6 inhibitors is cytotoxic in immunocompetent models of lung cancer in vivo.
Willobee, B. A. et al. Combined blockade of MEK and CDK4/6 pathways induces senescence to improve survival in pancreatic ductal adenocarcinoma. Mol. Cancer Ther. 20, 1246–1256 (2021).
Knudsen, E. S. et al. Targeting dual signalling pathways in concert with immune checkpoints for the treatment of pancreatic cancer. Gut 70, 127–138 (2021).
Ruscetti, M. et al. Senescence-induced vascular remodeling creates therapeutic vulnerabilities in pancreas cancer. Cell 181, 424–441.e21 (2020). This study highlights tumour type-specific effects of the combination of MEKi with CDK4 and CDK6 inhibitors in vivo.
Ziemke, E. K. et al. Sensitivity of KRAS-mutant colorectal cancers to combination therapy that cotargets MEK and CDK4/6. Clin. Cancer Res. 22, 405–414 (2016).
Lee, M. S. et al. Efficacy of the combination of MEK and CDK4/6 inhibitors in vitro and in vivo in KRAS mutant colorectal cancer models. Oncotarget 7, 39595–39608 (2016).
Sorokin, A. V. et al. Targeting RAS mutant colorectal cancer with dual inhibition of MEK and CDK4/6. Cancer Res. 82, 3335–3344 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03981614 (2024).
Castellano, E. & Downward, J. Role of RAS in the regulation of PI3-kinase. Curr. Top. Microbiol. Immunol. 346, 143–169 (2010).
Malone, C. F. et al. Defining key signaling nodes and therapeutic biomarkers in NF1-mutant cancers. Cancer Discov. 4, 1062–1073 (2014).
Britten, C. D. PI3K and MEK inhibitor combinations: examining the evidence in selected tumor types. Cancer Chemother. Pharmacol. 71, 1395–1409 (2013).
Brown, W. S. et al. Overcoming adaptive resistance to KRAS and MEK inhibitors by co-targeting mTORC1/2 complexes in pancreatic cancer. Cell Rep. Med. 1, 100131 (2020).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05840510 (2024).
Hagiwara, A. et al. Hepatic mTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab. 15, 725–738 (2012).
Schreiber, K. H. et al. A novel rapamycin analog is highly selective for mTORC1 in vivo. Nat. Commun. 10, 3194 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04774952 (2024).
Truitt, M. L. et al. Differential requirements for eIF4E dose in normal development and cancer. Cell 162, 59–71 (2015).
Nardi, F. et al. Cotargeting a MYC/eIF4A-survival axis improves the efficacy of KRAS inhibitors in lung cancer. J. Clin. Invest. 133, e167651 (2023). This study shows that eIF4A inhibitors substantially improve the effects of KRAS inhibitors in NSCLC by suppressing the translation of multiple BCL-2 family members and further demonstrates that MYC overexpression creates this dependency and that MYC amplification or overexpression can be used as a biomarker.
Rosen, E. et al. Phase 1/2 dose expansion study evaluating first-in-class eIF4A inhibitor zotatifin in patients with ER+ metastatic breast cancer. J. Clin. Oncol. 41, 1080–1080 (2023).
Dhanasekaran, R. et al. The MYC oncogene—the grand orchestrator of cancer growth and immune evasion. Nat. Rev. Clin. Oncol. 19, 23–36 (2022).
Sears, R., Leone, G., DeGregori, J. & Nevins, J. R. Ras enhances Myc protein stability. Mol. Cell 3, 169–179 (1999).
Sears, R. et al. Multiple Ras-dependent phosphorylation pathways regulate Myc protein stability. Genes. Dev. 14, 2501–2514 (2000).
Soucek, L. et al. Modelling Myc inhibition as a cancer therapy. Nature 455, 679–683 (2008).
Kortlever, R. M. et al. Myc cooperates with Ras by programming inflammation and immune suppression. Cell 171, 1301–1315.e14 (2017).
Garralda, E. et al. MYC targeting by OMO-103 in solid tumors: a phase 1 trial. Nat. Med. https://doi.org/10.1038/s41591-024-02805-1 (2024).
Nishida, Y. et al. C-MYC targeting by degradation: novel dual c-Myc/GSPT1 degrader GT19715 induces TP53-independent cell death in acute myeloid leukemia and lymphomas. Blood 140, 483–484 (2022).
Madden, S. K., de Araujo, A. D., Gerhardt, M., Fairlie, D. P. & Mason, J. M. Taking the Myc out of cancer: toward therapeutic strategies to directly inhibit c-Myc. Mol. Cancer 20, 3 (2021).
Vaseva, A. V. et al. KRAS suppression-induced degradation of MYC Is antagonized by a MEK5–ERK5 compensatory mechanism. Cancer Cell 34, 807–822.e7 (2018).
Blake, D. R. et al. Application of a MYC degradation screen identifies sensitivity to CDK9 inhibitors in KRAS-mutant pancreatic cancer. Sci. Signal. 12, eaav7259 (2019).
Luo, J., Solimini, N. L. & Elledge, S. J. Principles of cancer therapy: oncogene and non-oncogene addiction. Cell 136, 823–837 (2009).
Sun, C. et al. Rational combination therapy with PARP and MEK inhibitors capitalizes on therapeutic liabilities in RAS mutant cancers. Sci. Transl. Med. 9, eaal5148 (2017).
Yang, B. et al. MEK inhibition remodels the immune landscape of mutant KRAS tumors to overcome resistance to PARP and immune checkpoint inhibitors. Cancer Res. 81, 2714–2729 (2021).
Maertens, O. et al. MAPK pathway suppression unmasks latent DNA repair defects and confers a chemical synthetic vulnerability in BRAF-, NRAS-, and NF1-mutant melanomas. Cancer Discov. 9, 526–545 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03162627 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05554328 (2024).
Chan, W. Y., Brown, L. J., Reid, L. & Joshua, A. M. PARP inhibitors in melanoma — an expanding therapeutic option? Cancers 13, 4520 (2021).
Rodon Ahnert, J. et al. Avelumab or talazoparib in combination with binimetinib in metastatic pancreatic ductal adenocarcinoma: dose-finding results from phase Ib of the JAVELIN PARP MEKi trial. ESMO Open. 8, 101584 (2023).
Diehl, J. N. et al. The KRAS-regulated kinome identifies WEE1 and ERK coinhibition as a potential therapeutic strategy in KRAS-mutant pancreatic cancer. J. Biol. Chem. 297, 101335 (2021).
Klomp, J. E. et al. CHK1 protects oncogenic KRAS-expressing cells from DNA damage and is a target for pancreatic cancer treatment. Cell Rep. 37, 110060 (2021).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Xiao, Y. et al. Emerging therapies in cancer metabolism. Cell Metab. 35, 1283–1303 (2023).
Stine, Z. E., Schug, Z. T., Salvino, J. M. & Dang, C. V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug. Discov. 21, 141–162 (2022).
Mukhopadhyay, S., Vander Heiden, M. G. & McCormick, F. The metabolic landscape of RAS-driven cancers from biology to therapy. Nat. Cancer 2, 271–283 (2021).
Kimmelman, A. C. Metabolic dependencies in RAS-driven cancers. Clin. Cancer Res. 21, 1828–1834 (2015).
Heuer, T. S. et al. FASN inhibition and taxane treatment combine to enhance anti-tumor efficacy in diverse xenograft tumor models through disruption of tubulin palmitoylation and microtubule organization and FASN inhibition-mediated effects on oncogenic signaling and gene expression. EBioMedicine 16, 51–62 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03808558 (2023).
Ying, H. et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149, 656–670 (2012).
Le, A. et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc. Natl Acad. Sci. USA 107, 2037–2042 (2010).
Commisso, C. et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature 497, 633–637 (2013).
Ramirez, C., Hauser, A. D., Vucic, E. A. & Bar-Sagi, D. Plasma membrane V-ATPase controls oncogenic RAS-induced macropinocytosis. Nature 576, 477–481 (2019).
Yan, L. et al. Targeting glucose metabolism sensitizes pancreatic cancer to MEK inhibition. Cancer Res. 81, 4054–4065 (2021).
Xia, M., Li, X., Diao, Y., Du, B. & Li, Y. Targeted inhibition of glutamine metabolism enhances the antitumor effect of selumetinib in KRAS-mutant NSCLC. Transl. Oncol. 14, 100920 (2021).
Encarnación-Rosado, J. et al. Targeting pancreatic cancer metabolic dependencies through glutamine antagonism. Nat. Cancer 5, 85–99 (2024).
Lemberg, K. M., Gori, S. S., Tsukamoto, T., Rais, R. & Slusher, B. S. Clinical development of metabolic inhibitors for oncology. J. Clin. Invest. 132, e148550 (2022).
Lim, J. K. M. & Leprivier, G. The impact of oncogenic RAS on redox balance and implications for cancer development. Cell Death Dis. 10, 955 (2019).
Redding, A., Aplin, A. E. & Grabocka, E. RAS-mediated tumor stress adaptation and the targeting opportunities it presents. Dis. Model. Mech. 15, dmm049280 (2022).
DeNicola, G. M. et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475, 106–109 (2011).
Badgley, M. A. et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 368, 85–89 (2020).
Cullis, J., Das, S. & Bar-Sagi, D. Kras and tumor immunity: friend or foe? Cold Spring Harb. Perspect. Med. 8, a031849 (2018).
Skoulidis, F. et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 8, 822–835 (2018).
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Overman, M. J. et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 18, 1182–1191 (2017).
Brahmer, J. et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 373, 123–135 (2015).
Royal, R. E. et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. Hagerstown Md. 1997 33, 828–833 (2010).
Coelho, M. A. et al. Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA. Immunity 47, 1083–1099.e6 (2017).
Zhang, Y. et al. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut 66, 124–136 (2017).
Briere, D. M. et al. The KRASG12C nhibitor MRTX849 reconditions the tumor immune microenvironment and sensitizes tumors to checkpoint inhibitor therapy. Mol. Cancer Ther. 20, 975–985 (2021).
Tang, K. H. et al. Combined inhibition of SHP2 and CXCR1/2 promotes antitumor T-cell response in NSCLC. Cancer Discov. 12, 47–61 (2022).
van Maldegem, F. et al. Characterisation of tumour microenvironment remodelling following oncogene inhibition in preclinical studies with imaging mass cytometry. Nat. Commun. 12, 5906 (2021).
Poon, E. et al. The MEK inhibitor selumetinib complements CTLA-4 blockade by reprogramming the tumor immune microenvironment. J. Immunother. Cancer 5, 63 (2017).
Kemp, S. B. et al. Efficacy of a small-molecule inhibitor of KrasG12D in immunocompetent models of pancreatic cancer. Cancer Discov. 13, 298–311 (2023). This study shows that the anti-tumoural activity of the KRAS-G12D inhibitor MRTX1133 is, in part, mediated by the adaptive immune system.
Mahadevan, K. K. et al. KRASG12D inhibition reprograms the microenvironment of early and advanced pancreatic cancer to promote FAS-mediated killing by CD8+ T cells. Cancer Cell 41, 1606–1620.e8 (2023).
Ebert, P. J. R. et al. MAP kinase inhibition promotes T cell and anti-tumor activity in combination with PD-L1 checkpoint blockade. Immunity 44, 609–621 (2016).
Garassino, M. C. et al. LBA65 KRYSTAL-7: efficacy and safety of adagrasib with pembrolizumab in patients with treatment-naïve, advanced non-small cell lung cancer (NSCLC) harboring a KRASG12C mutation. Ann. Oncol. 34, S1309–S1310 (2023).
Hu, H. et al. Oncogenic KRAS signaling drives evasion of innate immune surveillance in lung adenocarcinoma by activating CD47. J. Clin. Invest 133, e153470 (2023).
Bouwstra, R., van Meerten, T. & Bremer, E. CD47-SIRPα blocking-based immunotherapy: current and prospective therapeutic strategies. Clin. Transl. Med. 12, e943 (2022).
Zhang, Z. et al. A covalent inhibitor of K-RasG12C induces MHC class I presentation of haptenated peptide neoepitopes targetable by immunotherapy. Cancer Cell 40, 1060–1069.e7 (2022).
Hattori, T. et al. Creating MHC-restricted neoantigens with covalent inhibitors that can be targeted by immune therapy. Cancer Discov. 13, 132–145 (2023).
Bear, A. S. et al. Biochemical and functional characterization of mutant KRAS epitopes validates this oncoprotein for immunological targeting. Nat. Commun. 12, 4365 (2021).
Tran, E. et al. T-cell transfer therapy targeting mutant KRAS in cancer. N. Engl. J. Med. 375, 2255–2262 (2016).
Boumelha, J. et al. An immunogenic model of KRAS-mutant lung cancer enables evaluation of targeted therapy and immunotherapy combinations. Cancer Res. 82, 3435–3448 (2022).
Shen, H. & Laird, P. W. Interplay between the cancer genome and epigenome. Cell 153, 38–55 (2013).
You, J. S. & Jones, P. A. Cancer genetics and epigenetics: two sides of the same coin? Cancer Cell 22, 9–20 (2012).
Flavahan, W. A., Gaskell, E. & Bernstein, B. E. Epigenetic plasticity and the hallmarks of cancer. Science 357, eaal2380 (2017).
Garraway, L. A. & Lander, E. S. Lessons from the cancer genome. Cell 153, 17–37 (2013).
Dawson, M. A. & Kouzarides, T. Cancer epigenetics: from mechanism to therapy. Cell 150, 12–27 (2012).
Plass, C. et al. Mutations in regulators of the epigenome and their connections to global chromatin patterns in cancer. Nat. Rev. Genet. 14, 765–780 (2013).
Eichner, L. J. et al. HDAC3 is critical in tumor development and therapeutic resistance in Kras-mutant non-small cell lung cancer. Sci. Adv. 9, eadd3243 (2023).
Malone, C. F. et al. mTOR and HDAC inhibitors converge on the TXNIP/thioredoxin pathway to cause catastrophic oxidative stress and regression of RAS-driven tumors. Cancer Discov. 7, 1450–1463 (2017).
Wang, L. et al. An acquired vulnerability of drug-resistant melanoma with therapeutic potential. Cell 173, 1413–1425.e14 (2018).
Delmore, J. E. et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 146, 904–917 (2011).
Singleton, K. R. et al. Melanoma therapeutic strategies that select against resistance by exploiting MYC-driven evolutionary convergence. Cell Rep. 21, 2796–2812 (2017).
De Raedt, T. et al. PRC2 loss amplifies Ras-driven transcription and confers sensitivity to BRD4-based therapies. Nature 514, 247–251 (2014).
Guerra, S. L. et al. A deregulated HOX gene axis confers an epigenetic vulnerability in KRAS-mutant lung cancers. Cancer Cell 37, 705–719.e6 (2020). This study is a relevant example of a therapeutic strategy co-targeting epigenetic and oncogenic pathways, in which the combination of MEK and BET inhibitors induces stalled DNA replication and DNA damage to cause cell death.
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05253131 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05111561 (2023).
Andricovich, J. et al. Loss of KDM6A activates super-enhancers to induce gender-specific squamous-like pancreatic cancer and confers sensitivity to BET inhibitors. Cancer Cell 33, 512–526.e8 (2018).
Plana, D., Palmer, A. C. & Sorger, P. K. Independent drug action in combination therapy: implications for precision oncology. Cancer Discov. 12, 606–624 (2022).
Hwangbo, H., Patterson, S. C., Dai, A., Plana, D. & Palmer, A. C. Additivity predicts the efficacy of most approved combination therapies for advanced cancer. Nat. Cancer 4, 1693–1704 (2023).
Misale, S. et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 486, 532–536 (2012).
Diaz, L. A. Jr et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 486, 537–540 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05074810 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05358249 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04959981 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03087071 (2023).
Reissig, T. M. et al. Lasting response by vertical inhibition with cetuximab and trametinib in KRAS-mutated colorectal cancer patient-derived xenografts. Mol. Oncol. 17, 2396–2414 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT01927341 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT01287130 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04793958 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05002270 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05194995 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04956640 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04449874 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04330664 (2024).
Ho, C. S. L. et al. HER2 mediates clinical resistance to the KRASG12C inhibitor sotorasib, which is overcome by co-targeting SHP2. Eur. J. Cancer Oxf. Engl. 1990 159, 16–23 (2021).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05054725 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04699188 (2024).
Frank, K. J. et al. Extensive preclinical validation of combined RMC-4550 and LY3214996 supports clinical investigation for KRAS mutant pancreatic cancer. Cell Rep. Med. 3, 100815 (2022).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04973163 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03825289 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04132505 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04735068 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04386057 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04145297 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05691504 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02065063 (2018).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03170206 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02022982 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05178888 (2022).
Lou, K. et al. KRASG12C inhibition produces a driver-limited state revealing collateral dependencies. Sci. Signal. 12, eaaw9450 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04185883 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05039177 (2024).
Goodwin, C. M. et al. Combination therapies with CDK4/6 inhibitors to treat KRAS-mutant pancreatic cancer. Cancer Res. 83, 141–157 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03454035 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03299088 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03225664 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT02900664 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03833427 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03991819 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03374254 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03475004 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04348045 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03600701 (2023).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT03337698 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT04613596 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/study/NCT05340621 (2023).
Acknowledgements
This work was supported by R01CA111754 and the Ludwig Center at Harvard (K.C.). L.S. was supported by a Landry Cancer Biology Research Fellowship.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article, contributed substantially to discussion of the content and wrote the article. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
K.C. is an adviser at Genentech and serves on the scientific advisory board of Erasca, Inc. N.P. and L.S. declare no competing interests.
Peer review
Peer review information
Nature Reviews Cancer thanks Julian Downward, Rene Bernards and the other, anonymous, reviewer for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Adaptive responses
-
Cell-intrinsic protective mechanisms that are triggered in response to a given drug exposure and allow tumour cells to survive.
- Allele-specific inhibitors
-
A class of inhibitors that selectively target specific mutant alleles of KRAS, NRAS or HRAS oncoproteins and do not have activity against wild-type isoforms.
- Anchor agent
-
In the context of this Review, an agent that suppresses a core component of the RAS pathway and is co-administered with a second drug against a different target as part of a combination therapy.
- Class I HDAC inhibitors
-
Drugs that target class I histone deacetylases (HDAC1, HDAC2, HDAC3, HDAC8), enzymes that are located in the nucleus and remove acetyl groups from the chromatin.
- Converging oncogenic targets
-
Gene products that function downstream of multiple oncogenic pathways and whose inhibition may allow simultaneous suppression of several oncogenic processes.
- Cytostasis
-
A lack of change over time in the number of tumour cells, which can reflect a balance between cell proliferation and cell death.
- Degraders
-
A class of small molecule inhibitors that bring a target into close proximity with an E3 ubiquitin ligase to promote polyubiquitination and subsequent proteasomal degradation of the target.
- Ferritinophagy
-
A selective form of autophagy that involves the degradation of ferritin and subsequent mobilization of iron.
- Hydroxychloroquine
-
(HCQ). A drug that inhibits the recycling of cellular components through autophagy by preventing lysosomal acidification and hindering autophagosome–lysosome fusion.
- Macropinocytosis
-
A form of endocytosis in which the plasma membrane non-selectively engulfs extracellular fluid to internalize and degrade macromolecules.
- Metabolic stress
-
A state of stress in which cellular bioenergetic and biosynthetic demands impact metabolic homeostasis and result in the underproduction or overproduction of critical metabolites.
- Mitotic stress
-
A state of stress in which dysregulated mitosis leads to the mis-segregation of chromosomes during uncontrolled cell division.
- Molecular glues
-
A class of small molecules that induce or stabilize the interaction between two given proteins.
- Noonan-like syndrome
-
A disorder caused by germ-line mutations in SHOC2 that is characterized by phenotypes similar to those observed in the RASopathy Noonan syndrome that include loose anagen hair, hyperpigmentation, and craniofacial and cardiac anomalies.
- On-target mechanisms of resistance
-
Resistance that occurs through mutations in genes or pathways directly targeted by a drug.
- Oxidative stress
-
An imbalance between the production of reactive chemical species and the antioxidant response that promotes various signalling pathways but can lead to intracellular damage.
- Proteotoxic stress
-
An imbalance of protein levels that can result from dysregulated protein translation, protein folding or protein degradation and apply strain on the unfolded protein response or proteasomal degradation pathway.
- RASopathy
-
A group of medical syndromes characterized by germ-line mutations in genes of the RAS pathway that lead to deregulated pathway activity.
- Replication stress
-
A state of genomic dysregulation in which triggers such as DNA damage lead to the stalling of replication forks during DNA replication.
- Synthetic lethality
-
An interaction between two genes wherein perturbation of each single gene is not lethal on its own whereas concurrent perturbations of both genes induce cell death.
- Therapeutic window
-
The range of drug concentrations in which the therapeutic effect is achieved with minimal toxicity.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Perurena, N., Situ, L. & Cichowski, K. Combinatorial strategies to target RAS-driven cancers. Nat Rev Cancer 24, 316–337 (2024). https://doi.org/10.1038/s41568-024-00679-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41568-024-00679-6