Abstract
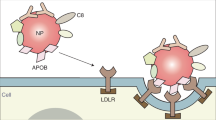
Understanding how cells process nanoparticles is crucial to optimize nanomedicine efficacy. However, characterizing cellular pathways is challenging, especially if non-canonical mechanisms are involved. In this Article a genome-wide forward genetic screening based on insertional mutagenesis is applied to discover receptors and proteins involved in the intracellular accumulation (uptake and intracellular processing) of silica nanoparticles. The nanoparticles are covered by a human serum corona known to target the low-density lipoprotein receptor (LDLR). By sorting cells with reduced nanoparticle accumulation and deep sequencing after each sorting, 80 enriched genes are identified. We find that, as well as LDLR, the scavenger receptor SCARB1 also mediates nanoparticle accumulation. Additionally, heparan sulfate acts as a specific nanoparticle receptor, and its role varies depending on cell and nanoparticle type. Furthermore, some of the identified targets affect nanoparticle trafficking to the lysosomes. These results show the potential of genetic screening to characterize nanoparticle pathways. Additionally, they indicate that corona-coated nanoparticles are internalized via multiple receptors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the article and its Supplementary Information and Supplementary Data. The raw sequencing data are deposited at the European Nucleotide Archive under accession code PRJEB71925. The complete analysis of the sequencing results is included as Supplementary Data 1. The raw imaging and nanoparticle physicochemical characterization data are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
The code to analyse the sequencing data generated in this study is available at https://github.com/Vityay/NanoScreen.
References
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Chou, L. Y. T., Ming, K. & Chan, W. C. W. Strategies for the intracellular delivery of nanoparticles. Chem. Soc. Rev. 40, 233–245 (2011).
Duncan, R. & Richardson, S. C. W. Endocytosis and intracellular trafficking as gateways for nanomedicine delivery: opportunities and challenges. Mol. Pharm. 9, 2380–2402 (2012).
Iversen, T.-G., Skotland, T. & Sandvig, K. Endocytosis and intracellular transport of nanoparticles: present knowledge and need for future studies. Nano Today 6, 176–185 (2011).
Rennick, J. J., Johnston, A. P. R. & Parton, R. G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 16, 266–276 (2021).
Francia, V. et al. Corona composition can affect the mechanisms cells use to internalize nanoparticles. ACS Nano 13, 11107–11121 (2019).
Iversen, T. G., Frerker, N. & Sandvig, K. Uptake of ricinB-quantum dot nanoparticles by a macropinocytosis-like mechanism. J. Nanobiotechnol. 10, 33 (2012).
Sharma, S., Bartholdson, S. J., Couch, A. C. M., Yusa, K. & Wright, G. J. Genome-scale identification of cellular pathways required for cell surface recognition. Genome Res. 28, 1372–1382 (2018).
Collinet, C. et al. Systems survey of endocytosis by multiparametric image analysis. Nature 464, 243–249 (2010).
Carette, J. E. et al. Haploid genetic screens in human cells identify host factors used by pathogens. Science 326, 1231–1235 (2009).
Navarro Negredo, P. et al. Contribution of the clathrin adaptor AP-1 subunit µ1 to acidic cluster protein sorting. J. Cell Biol. 216, 2927–2943 (2017).
Jae, L. T. et al. Deciphering the glycosylome of dystroglycanopathies using haploid screens for lassa virus entry. Science 340, 479–483 (2013).
Duncan, L. M. et al. Fluorescence-based phenotypic selection allows forward genetic screens in haploid human cells. PLoS ONE 7, e39651 (2012).
Davis, E. M. et al. Comparative haploid genetic screens reveal divergent pathways in the biogenesis and trafficking of glycophosphatidylinositol-anchored proteins. Cell Rep. 11, 1727–1736 (2015).
Luteijn, R. D. et al. A genome-wide haploid genetic screen identifies heparan sulfate-associated genes and the macropinocytosis modulator TMED10 as factors supporting vaccinia virus infection. J. Virol. 93, e02160-18 (2019).
Carette, J. E. et al. Ebola virus entry requires the cholesterol transporter Niemann–Pick C1. Nature 477, 340–343 (2011).
Ngo, W. et al. Identifying cell receptors for the nanoparticle protein corona using genome screens. Nat. Chem. Biol. 18, 1023–1031 (2022).
Riblett, A. M. et al. A haploid genetic screen identifies heparan sulfate proteoglycans supporting Rift Valley fever virus infection. J. Virol. 90, 1414–1423 (2016).
Pillay, S. et al. An essential receptor for adeno-associated virus infection. Nature 530, 108–112 (2016).
Lara, S. et al. Identification of receptor binding to the biomolecular corona of nanoparticles. ACS Nano 11, 1884–1893 (2017).
Akinc, A. et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 14, 1084–1087 (2019).
Liu, K. et al. Multiomics analysis of naturally efficacious lipid nanoparticle coronas reveals high-density lipoprotein is necessary for their function. Nat. Commun. 14, 4007 (2023).
Rees, P., Wills, J. W., Brown, M. R., Barnes, C. M. & Summers, H. D. The origin of heterogeneous nanoparticle uptake by cells. Nat. Commun. 10, 2341 (2019).
Panet, E. et al. The interface of nanoparticles with proliferating mammalian cells. Nat. Nanotechnol. 12, 598–600 (2017).
Åberg, C., Piattelli, V., Montizaan, D. & Salvati, A. Sources of variability in nanoparticle uptake by cells. Nanoscale 13, 17530–17546 (2021).
Christianson, H. C., Svensson, K. J., van Kuppevelt, T. H., Li, J. P. & Belting, M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl Acad. Sci. USA 110, 17380–17385 (2013).
Joshi, B. S. & Zuhorn, I. S. Heparan sulfate proteoglycan-mediated dynamin-dependent transport of neural stem cell exosomes in an in vitro blood–brain barrier model. Eur. J. Neurosci. 53, 706–719 (2021).
Panarella, A. et al. A systematic high-content screening microscopy approach reveals key roles for Rab33b, OATL1 and Myo6 in nanoparticle trafficking in HeLa cells. Sci. Rep. 6, 28865 (2016).
Hofmann, D. et al. Mass spectrometry and imaging analysis of nanoparticle-containing vesicles provide a mechanistic insight into cellular trafficking. ACS Nano 8, 10077–10088 (2014).
Shapero, K. et al. Time and space resolved uptake study of silica nanoparticles by human cells. Mol. BioSyst. 7, 371–378 (2011).
Turnbull, J., Powell, A. & Guimond, S. Heparan sulfate: decoding a dynamic multifunctional cell regulator. Trends Cell Biol. 11, 75–82 (2001).
Martinez, P. et al. Macrophage polarization alters the expression and sulfation pattern of glycosaminoglycans. Glycobiology 25, 502–513 (2015).
Thomas, M. & Klibanov, A. M. Non-viral gene therapy: polycation-mediated DNA delivery. Appl. Microbiol. Biotechnol. 62, 27–34 (2003).
Favretto, M. E., Wallbrecher, R., Schmidt, S., van de Putte, R. & Brock, R. Glycosaminoglycans in the cellular uptake of drug delivery vectors—bystanders or active players? J. Control. Release 180, 81–90 (2014).
Olivieri, P. H., Jesus, M. B., Nader, H. B., Justo, G. Z. & Sousa, A. A. Cell-surface glycosaminoglycans regulate the cellular uptake of charged polystyrene nanoparticles. Nanoscale 14, 7350–7363 (2022).
Christianson, H. C. & Belting, M. Heparan sulfate proteoglycan as a cell-surface endocytosis receptor. Matrix Biol. 35, 51–55 (2014).
Zhang, Q. et al. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discov. 6, 80 (2020).
Stanford, K. I. et al. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance of triglyceride-rich lipoproteins in mice. J. Clin. Invest. 119, 3236–3245 (2009).
Williams, K. J. & Fuki, I. V. Cell-surface heparan sulfate proteoglycans: dynamic molecules mediating ligand catabolism. Curr. Opin. Lipidol. 8, 253–262 (1997).
Shen, W. J., Asthana, S., Kraemer, F. B. & Azhar, S. Scavenger receptor B type 1: expression, molecular regulation, and cholesterol transport function. J. Lipid Res. 59, 1114–1131 (2018).
Kolset, S. O. & Salmivirta, M. Cell surface heparan sulfate proteoglycans and lipoprotein metabolism. Cell. Mol. Life Sci. 56, 857–870 (1999).
Lesniak, A. et al. Nanoparticle adhesion to the cell membrane and its effect on nanoparticle uptake efficiency. J. Am. Chem. Soc. 135, 1438–1444 (2013).
Yang, K., Mesquita, B., Horvatovich, P. & Salvati, A. Tuning liposome composition to modulate corona formation in human serum and cellular uptake. Acta Biomater. 106, 314–327 (2020).
Dilliard, S. A., Cheng, Q. & Siegwart, D. J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl Acad. Sci. USA 118, e2109256118 (2021).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Ritz, S. et al. Protein corona of nanoparticles: distinct proteins regulate the cellular uptake. Biomacromolecules 16, 1311–1321 (2015).
Jones, A. L., Hulett, M. D. & Parish, C. R. Histidine-rich glycoprotein binds to cell-surface heparan sulfate via its N-terminal domain following Zn2+ chelation. J. Biol. Chem. 279, 30114–30122 (2004).
Acknowledgements
This study was funded by the European Research Council (ERC) as part of the European Union’s Horizon 2020 research and innovation programme under grant agreement 637614 (A.S.) (NanoPaths). We acknowledge T. Brummelkamp (Netherlands Cancer Institute, Amsterdam, The Netherlands) for providing the plasmids for retroviral mutagenesis, and J. Carette (Stanford University, Stanford, CA, USA) for sharing detailed information on the mutagenesis and LAM-PCR methods. We also thank H. Haisma (GRIP) and J. van den Born for suggestions on retroviral production and heparan sulfate detection respectively, P. Ettema (GRIP) and K. Hoekstra-Wakker (ERIBA) for technical assistance with bacterial culture and preparation of samples for next generation sequencing, respectively, and K. Yang (GRIP) for liposome preparation. E. Frijlink and W. Hinrichs (GRIP) are acknowledged for access to nanoparticle tracking analysis (NTA), H. van der Mei (University Medical Center Groningen) for access to dynamic light scattering (DLS) and Herman Sillje (University Medical Center Groningen) for access to epifluorescence microscopy. FACS was performed at the flow cytometry facility of the University Medical Center Groningen. Confocal fluorescence imaging was performed at the imaging facility of the University Medical Center Groningen.
Author information
Authors and Affiliations
Contributions
D.M. designed and performed all experiments for the forward genetic screening (from the production of the viral particles, the preparation of the mutagenized library and the selection of the cells with reduced nanoparticle intracellular accumulation to the LAM-PCR preparation for sequencing, pathway analysis on the results and all their validation with the exceptions specified below), analysed and interpreted the data and wrote the manuscript. R.B. performed nanoparticle intracellular accumulation and adhesion kinetics in silenced cells, with competitors and after inhibition of SCARB1, as well as the imaging and quantification of nanoparticle colocalization with lysosomes, and tested the panel of targets in A549 cells and with the negatively charged liposomes together with contributions by C.R.-S. and analysed and interpreted these data. In addition, C.R.S. also assisted in cell sorting for forward genetic screening and performed some of the digestion and competition experiments with the panel of nanoparticles. S.d.W. performed nanoparticle tracking analysis experiments and analysis. C.Å. contributed to the analysis of the results. V.G. performed the mapping of the sequenced inserts to the genome and supervised the analysis of the sequencing results. D.C.J.S. supervised the LAM-PCR-based next-generation sequencing library preparation and sequencing. A.S. designed and supervised the whole project, analysed and interpreted the data and wrote the manuscript. All authors have read and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–20, Tables 1–6 and References.
Supplementary Data 1
Number of insertions per gene before and after each selection of mutagenized HAP1 cells with reduced nanoparticle intracellular accumulation (up to six sorts).
Supplementary Data 2
Description of the role of the 80 enriched genes identified by time-resolved forward genetic screening.
Supplementary Data 3
Source data for Supplementary Figures.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Montizaan, D., Bartucci, R., Reker-Smit, C. et al. Genome-wide forward genetic screening to identify receptors and proteins mediating nanoparticle uptake and intracellular processing. Nat. Nanotechnol. (2024). https://doi.org/10.1038/s41565-024-01629-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41565-024-01629-x