Abstract
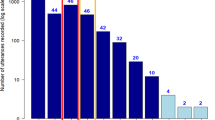
Why did our ancestors combine the first consonant- and vowel-like utterances to produce the first syllable or word? To answer this question, it is essential to know what constituted the communicative function of proto-consonants and of proto-vowels before their combined use became universal. Almost nothing is known, however, about consonant-like calls in the primate order1,2. Here, we investigate a large collection of voiceless consonant-like calls in nonhuman great apes (our closest relatives), namely orangutans (Pongo spp.). We analysed 4,486 kiss-squeaks collected across 48 individuals in four wild populations. Despite idiosyncratic production mechanics, consonant-like calls displayed information-dense content and the same acoustic signatures found in voiced vowel-like calls by nonhuman primates, implying similar biological functions. Selection regimes between proto-consonants and proto-vowels were thus probably indistinguishable at the dawn of spoken language evolution. Our findings suggest that the first proto-syllables or proto-words in our lineage probably constituted message reiterations, instead of messages of increasing intricacy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Lameira, A. R., Maddieson, I. & Zuberbuhler, K. Primate feedstock for the evolution of consonants. Trends Cogn. Sci. 18, 60–62 (2014).
Lameira, A. R. The forgotten role of consonant-like calls in theories of speech evolution. Behav. Brain Sci. 37, 559–560 (2014).
Fitch, W. T. The evolution of speech: a comparative review. Trends Cogn. Sci. 4, 258–267 (2000).
Fitch, W. T. & Zuberbühler, K. in The Evolution of Emotional Communication: From Sounds in Nonhuman Mammals to Speech and Music in Man (eds Altenmüller, E., Schmidt, S. & Zimmermann, E. ) 26–48 (Oxford Univ. Press, 2013).
Lemasson, A., Ouattara, K. & Zuberbühler, K. in The Evolutionary Emergence of Language (eds Botha, R. & Everaert, M. ) 181–203 (Oxford Univ. Press, 2013).
Taylor, A. M. & Reby, D. The contribution of source–filter theory to mammal vocal communication research. J. Zool. 280, 221–236 (2010).
Luo, Z.-X., Ji, Q., Wible, J. R. & Yuan, C.-X. An Early Cretaceous tribosphenic mammal and metatherian evolution. Science 302, 1934–1940 (2003).
Ni, X. et al. The oldest known primate skeleton and early haplorhine evolution. Nature 498, 60–64 (2013).
Hardus, M. E., Lameira, A. R., van Schaik, C. P. & Wich, S. A. Tool use in wild orang-utans modifies sound production: a functionally deceptive innovation? Proc. R. Soc. B 276, 3689–3694 (2009).
Lameira, A. R. et al. Population-specific use of the same tool-assisted alarm call between two wild orangutan populations (Pongopygmaeus wurmbii) indicates functional arbitrariness. PLoS ONE 8, e69749 (2013).
Hobolth, A., Dutheil, J. Y., Hawks, J., Schierup, M. H. & Mailund, T. Incomplete lineage sorting patterns among human, chimpanzee, and orangutan suggest recent orangutan speciation and widespread selection. Genome Res. 21, 349–356 (2011).
White, T. D., Lovejoy, C. O., Asfaw, B., Carlson, J. P. & Suwa, G. Neither chimpanzee nor human, Ardipithecus reveals the surprising ancestry of both. Proc. Natl Acad. Sci. USA 112, 4877–4884 (2015).
Wich, S. A., Schel, A. M. & de Vries, H. Geographic variation in Thomas langur (Presbytis thomasi) loud calls. Am. J. Primatol. 70, 566–574 (2008).
Fitch, W. T. Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J. Acoust. Soc. Am. 102, 1213–1222 (1997).
Lameira, A. R. & Wich, S. Orangutan long call degradation and individuality over distance: a playback approach. Int. J. Primatol. 29, 615–625 (2008).
Spillmann, B. et al. Acoustic properties of long calls given by flanged male orang-utans (Pongo pygmaeus wurmbii) reflect both individual identity and context. Ethology 116, 385–395 (2010).
Hardus, M. E. et al. in Orangutans: Geographic Variation in Behavioral Ecology and Conservation (eds Wich, S., Mitra Setia, T., Utami, S. S. & Schaik, C. P. ) 49–60 (Oxford Univ. Press, 2009).
Salmi, R., Hammerschmidt, K. & Doran-Sheehy, D. M. Western gorilla vocal repertoire and contextual use of vocalizations. Ethology 119, 831–847 (2013).
Lameira, A. R. et al. Orangutan (Pongo spp.) whistling and implications for the emergence of an open-ended call repertoire: a replication and extension. J. Acoust. Soc. Am. 134, 1–11 (2013).
Lameira, A. R. et al. Speech-like rhythm in a voiced and voiceless orangutan call. 10, e116136 (2015).
De Boer, B., Wich, S. A., Hardus, M. E. & Lameira, A. R. Acoustic models of orangutan hand-assisted alarm calls. J. Exp. Biol. 218, 907–914 (2015).
MacNeilage, P. F. The frame/content theory of evolution of speech production. Behav. Brain Sci. 21, 499–511 (1998).
Ghazanfar, A. A., Takahashi, D. Y., Mathur, N. & Fitch, W. T. Cineradiography of monkey lip-smacking reveals putative precursors of speech dynamics. Curr. Biol. 22, 1176–1182 (2012).
Waser, P. M. & Brown, C. H. Habitat acoustics and primate communication. Am. J. Primatol. 10, 135–154 (1986).
Delgado, R. A. et al. in Orangutans: Geographic Variation in Behavioral Ecology and Conservation (eds Wich, S., Mitra Setia, T., Utami, S. S. & Schaik, C. P. ) 215–224 (Oxford Univ. Press, 2009).
R Development CoreTeam. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2010); http://www.gbif.org/resource/81287
Bates, D., Maechler, M. & Dai, B. lme4: Linear-Mixed Effects Models Using S4 Classes (2008); http://cran.r-project.org/web/packages/lme4/lme4.pdf
Puts, D. A. et al. Sexual selection on male vocal fundamental frequency in humans and other anthropoids. Proc. R. Soc. B 283, 20152830 (2016).
Acknowledgements
We thank the Indonesian Institute of Science (LIPI), the Indonesian Ministry of Research and Technology (RISTEK), the Indonesian Directorate General of Forest Protection and Nature Conservation (PHKA), Gunung Palung National Park Bureau (BTNGP), Gunung Leuser National Park (TNGL) and Leuser Ecosystem Management Authority (BPKEL) for authorization to carry out research in Indonesia. We thank Universitas National (UNAS), Tanjungpura University (UNTAN) and Universitas Sumatera Utara (USU) for supporting the project and acting as counter-partner. Bornean Orangutan Survival (BOS, Palangka Raya, Central Kalimantan), Sumatran Orangutan Conservation Programme (SOCP, Medan, North Sumatra) and Gunung Palung Orangutan Project (GPOCP, Ketapang, West Kalimantan) acted as sponsors. We thank M.-C. Pagano for technical support. R. Mundry and J. Kendal provided input on the design of the generalized linear mixed models, as did H. Colleran and S. Roberts at the First Quantitative Methods Spring School 2016 at the Max Plank Institute for the Science of Human History, Jena, Germany. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
A.R.L. conceived and designed the study. A.R.L., R.V., A.A. and M.E.H. collected data. A.R.L., R.V., A.A. and M.E.H. analysed data. A.R.L., G.C.-S., C.K. and S.W. contributed collection of materials and data, and analysis tools. A.R.L., G.C.-S., C.K., S.W. and M.E.H. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Supplementary Information
Supplementary Model (PDF 103 kb)
Rights and permissions
About this article
Cite this article
Lameira, A., Vicente, R., Alexandre, A. et al. Proto-consonants were information-dense via identical bioacoustic tags to proto-vowels. Nat Hum Behav 1, 0044 (2017). https://doi.org/10.1038/s41562-017-0044
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41562-017-0044
This article is cited by
-
Open plains are not a level playing field for hominid consonant-like versus vowel-like calls
Scientific Reports (2023)
-
Sociality predicts orangutan vocal phenotype
Nature Ecology & Evolution (2022)
-
Is the consonant bias specifically human? Long–Evans rats encode vowels better than consonants in words
Animal Cognition (2019)