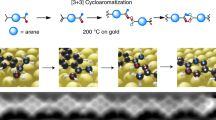
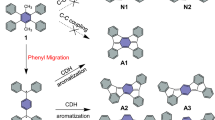
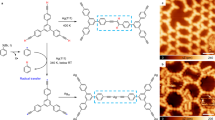
Abstract
Aromaticity is an established and widely used concept for the prediction of the reactivity of organic molecules. However, its role remains largely unexplored in on-surface chemistry, where the interaction with the substrate can alter the electronic and geometric structure of the adsorbates. Here we investigate how aromaticity affects the reactivity of alkyne-substituted porphyrin molecules in cyclization and coupling reactions on a Au(111) surface. We examine and quantify the regioselectivity in the reactions by scanning tunnelling microscopy and bond-resolved atomic force microscopy at the single-molecule level. Our experiments show a substantially lower reactivity of carbon atoms that are stabilized by the aromatic diaza[18]annulene pathway of free-base porphyrins. The results are corroborated by density functional theory calculations, which show a direct correlation between aromaticity and thermodynamic stability of the reaction products. These insights are helpful to understand, and in turn design, reactions with aromatic species in on-surface chemistry and heterogeneous catalysis.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings are included in this published article (and its supplementary information files). Source data are provided with this paper. Source data for the graphs (Figs. 5 and 6a,b and Supplementary Figs. 9–17), DFT-optimized geometries (used in Figs. 4 and 5, Extended Data Figs. 3 and 4, and Supplementary Figs. 8–17, 20 and 22), and Bader charge analysis data (used in Supplementary Fig. 19) are made available in a zip file. Any additional datasets (STM and AFM data) generated during and/or analysed during the current study are available from the corresponding authors on reasonable request.
Code availability
Apps and libraries used to analyse the data and to generate images were made available in open-source repositories (https://github.com/alexriss/SpmImageTycoon.jl and https://github.com/alexriss/ChemfilesViewer.jl). Any other code generated during the current study is available from the corresponding authors on reasonable request.
References
Krygowski, T. M., Cyrañski, M. K., Czarnocki, Z., Häfelinger, G. & Katritzky, A. R. Aromaticity: a theoretical concept of immense practical importance. Tetrahedron 56, 1783–1796 (2000).
Ragué Schleyer, P. V. Introduction: aromaticity. Chem. Rev. 101, 1115–1117 (2001).
Minkin, V. I. Glossary of terms used in theoretical organic chemistry. Pure Appl. Chem. 71, 1919–1981 (1999).
Hückel, E. Quantentheoretische Beiträge zum Benzolproblem. Z. Phys. 70, 204–286 (1931).
Vogel, E., Köcher, M., Schmickler, H. & Lex, J. Porphycene—a novel porphin isomer. Angew. Chem. Int. Ed. Engl. 25, 257–259 (1986).
Vogel, E. The porphyrins from the ‘annulene chemist’s’ perspective. Pure Appl. Chem. 65, 143–152 (1993).
Wu, J. I., Fernández, I. & Schleyer, P. V. R. Description of aromaticity in porphyrinoids. J. Am. Chem. Soc. 135, 315–321 (2013).
Vogel, E. From small carbocyclic rings to porphyrins: a personal account of 50 years of research. Angew. Chem. Int. Ed. 50, 4278–4287 (2011).
Solov’ev, K. N., Mashenkov, V. A., Gradyushko, A. T., Turkova, A. E. & Lezina, V. P. High resolution NMR spectra of porphin and of porphin derivatives. J. Appl. Spectrosc. 13, 1106–1111 (1970).
Scheer, H. & Katz, J. J. in Porphyrins and Metalloporphyrins (ed Smith, K. M.) 399–524 (Elsevier, 1975).
Brückner, C. The breaking and mending of meso-tetraarylporphyrins: transmuting the pyrrolic building blocks. Acc. Chem. Res. 49, 1080–1092 (2016).
Senge, M. O., Sergeeva, N. N. & Hale, K. J. Classic highlights in porphyrin and porphyrinoid total synthesis and biosynthesis. Chem. Soc. Rev. 50, 4730–4789 (2021).
Pavliček, N. et al. Synthesis and characterization of triangulene. Nat. Nanotechnol. 12, 308–311 (2017).
Wang, X. Y. et al. Exploration of pyrazine-embedded antiaromatic polycyclic hydrocarbons generated by solution and on-surface azomethine ylide homocoupling. Nat. Commun. 8, 1–7 (2017).
Majzik, Z. et al. Studying an antiaromatic polycyclic hydrocarbon adsorbed on different surfaces. Nat. Commun. 9, 1–6 (2018).
Di Giovannantonio, M. et al. On-surface synthesis of antiaromatic and open-shell indeno[2,1-b]fluorene polymers and their lateral fusion into porous ribbons. J. Am. Chem. Soc. 141, 12346–12354 (2019).
Kawai, S. et al. Competing annulene and radialene structures in a single anti-aromatic molecule studied by high-resolution atomic force microscopy. ACS Nano 11, 8122–8130 (2017).
Zuzak, R., Stoica, O., Blieck, R., Echavarren, A. M. & Godlewski, S. On-surface synthesis and intermolecular cycloadditions of indacenoditetracenes, antiaromatic analogues of undecacene. ACS Nano 15, 1548–1554 (2021).
Fatayer, S. et al. Molecular structure elucidation with charge-state control. Science 365, 142–145 (2019).
Cai, J. et al. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 466, 470–473 (2010).
Clair, S. & De Oteyza, D. G. Controlling a chemical coupling reaction on a surface: tools and strategies for on-surface synthesis. Chem. Rev. 119, 4717–4776 (2019).
Riss, A. et al. Imaging single-molecule reaction intermediates stabilized by surface dissipation and entropy. Nat. Chem. 8, 678–683 (2016).
Grill, L. et al. Nano-architectures by covalent assembly of molecular building blocks. Nat. Nanotechnol. 2, 687–691 (2007).
Mallada, B. et al. On-surface synthesis of one-dimensional coordination polymers with tailored magnetic anisotropy. ACS Appl. Mater. Interf. 13, 32393–32401 (2021).
Saywell, A., Browning, A. S., Rahe, P., Anderson, H. L. & Beton, P. H. Organisation and ordering of 1D porphyrin polymers synthesised by on-surface Glaser coupling. Chem. Commun. 52, 10342–10345 (2016).
Seufert, K. et al. Porphine homocoupling on Au(111). J. Phys. Chem. C 123, 16690–16698 (2019).
Wiengarten, A. et al. Surface-assisted dehydrogenative homocoupling of porphine molecules. J. Am. Chem. Soc. 136, 9346–9354 (2014).
Sun, Q. et al. Bottom-up fabrication and atomic-scale characterization of triply linked, laterally π-extended porphyrin nanotapes. Angew. Chem. Int. Ed. 60, 16208–16214 (2021).
Auwärter, W., Écija, D., Klappenberger, F. & Barth, J. V. Porphyrins at interfaces. Nat. Chem. 7, 105–120 (2015).
Bischoff, F. et al. Exploration of interfacial porphine coupling schemes and hybrid systems by bond-resolved scanning probe microscopy. Angew. Chem. Int. Ed. 57, 16030–16035 (2018).
Chen, S. et al. On-surface synthesis of 2D porphyrin-based covalent organic frameworks using terminal alkynes. Chem. Mater. 33, 8677–8684 (2021).
Yue, J. Y., Liu, X. H., Sun, B. & Wang, D. The on-surface synthesis of imine-based covalent organic frameworks with non-aromatic linkage. Chem. Commun. 51, 14318–14321 (2015).
Meng, X. et al. Effect of central π-system in silylated-tetraynes on σ-bond metathesis on surfaces. J. Phys. Chem. C 122, 6230–6235 (2018).
Riss, A. et al. Polycyclic aromatic chains on metals and insulating layers by repetitive [3+2] cycloadditions. Nat. Commun. 11, 1–8 (2020).
Kawai, S. et al. Diacetylene linked anthracene oligomers synthesized by one-shot homocoupling of trimethylsilyl on Cu(111). ACS Nano 12, 8791–8797 (2018).
Zhang, L. et al. On-surface activation of trimethylsilyl-terminated alkynes on coinage metal surfaces. ChemPhysChem 20, 2382–2393 (2019).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Riss, A. et al. Local electronic and chemical structure of oligo-acetylene derivatives formed through radical cyclizations at a surface. Nano Lett. 14, 2251–2255 (2014).
Schuler, B. et al. Adsorption geometry determination of single molecules by atomic force microscopy. Phys. Rev. Lett. 111, 106103 (2013).
Albrecht, F., Bischoff, F., Auwärter, W., Barth, J. V. & Repp, J. Direct identification and determination of conformational response in adsorbed individual nonplanar molecular species using noncontact atomic force microscopy. Nano Lett. 16, 7703–7709 (2016).
Schwarz, M. et al. Corrugation in the weakly interacting hexagonal-BN/Cu(111) system: Structure determination by combining noncontact atomic force microscopy and X-ray standing waves. ACS Nano 11, 9151–9161 (2017).
Braun, J. et al. NMR study of the tautomerism of porphyrin including the kinetic HH/HD/DD isotope effects in the liquid and the solid state. J. Am. Chem. Soc. 116, 6593–6604 (1994).
Schlabach, M., Rumpel, H. & Limbach, H.-H. Investigation of the tautomerism of15N-labeled hydroporphyrins by dynamic NMR spectroscopy. Angew. Chemie Int. Ed. Engl. 28, 76–79 (1989).
Liljeroth, P., Repp, J. & Meyer, G. Current-induced hydrogen tautomerization and conductance switching of naphthalocyanine molecules. Science 317, 1203–1206 (2007).
Auwärter, W. et al. A surface-anchored molecular four-level conductance switch based on single proton transfer. Nat. Nanotechnol. 7, 41–46 (2011).
Bischoff, F. et al. How surface bonding and repulsive interactions cause phase transformations: ordering of a prototype macrocyclic compound on Ag(111). ACS Nano 7, 3139–3149 (2013).
Schlabach, M. et al. NMR and NIR studies of the tautomerism of 5,10,15,20-tetraphenylporphyrin including kinetic HH/HD/DD isotope and solid state effects. Ber. Bunsenges. Phys. Chem. 96, 821–833 (1992).
Storm, C. B. & Teklu, Y. Nitrogen-hydrogen tautomerism in porphyrines and chlorines. J. Am. Chem. Soc. 3939, 1745–1747 (1970).
Claramunt, R. M., Elguero, J. & Katritzky, A. R. Tautomerism involving other than five-and six-membered rings. Adv. Heterocycl. Chem. 77, 1–50 (2000).
Kruszewski, J. & Krygowski, T. M. Definition of aromaticity basing on the harmonic oscillator model. Tetrahedron Lett. 13, 3839–3842 (1972).
Krygowski, T. M., Szatylowicz, H., Stasyuk, O. A., Dominikowska, J. & Palusiak, M. Aromaticity from the viewpoint of molecular geometry: application to planar systems. Chem. Rev. 114, 6383–6422 (2014).
Krygowski, T. M. & Cyrański, M. K. Structural aspects of aromaticity. Chem. Rev. 101, 1385–1419 (2001).
Garcia-Borràs, M., Osuna, S., Luis, J. M., Swart, M. & Solà, M. The role of aromaticity in determining the molecular structure and reactivity of (endohedral metallo) fullerenes. Chem. Soc. Rev. 43, 5089–5105 (2014).
Yu, D. et al. Global and local aromaticity of acenes from the information-theoretic approach in density functional reactivity theory. Phys. Chem. Chem. Phys. 21, 18195–18210 (2019).
Radula-Janik, K., Kopka, K., Kupka, T. & Ejsmont, K. Substituent effect of nitro group on aromaticity of carbazole rings. Chem. Heterocycl. Compd. 50, 1244–1251 (2014).
Frizzo, C. P. & Martins, M. A. P. Aromaticity in heterocycles: new HOMA index parametrization. Struct. Chem. 23, 375–380 (2011).
Gross, L. et al. Bond-order discrimination by atomic force microscopy. Science 337, 1326–1329 (2012).
Hapala, P. et al. Mechanism of high-resolution STM/AFM imaging with functionalized tips. Phys. Rev. B 90, 085421 (2014).
Hapala, P., Temirov, R., Tautz, F. S. & Jelínek, P. Origin of high-resolution IETS-STM images of organic molecules with functionalized tips. Phys. Rev. Lett. 113, 226101 (2014).
Bischoff, F. et al. Surface-mediated ring-opening and porphyrin deconstruction via conformational distortion. J. Am. Chem. Soc. 143, 15131–15138 (2021).
Lu, J. et al. Identification and electronic characterization of four cyclodehydrogenation products of H2TPP molecules on Au(111). Phys. Chem. Chem. Phys. 23, 11784–11788 (2021).
Wiengarten, A. et al. Surface-assisted cyclodehydrogenation; break the symmetry, enhance the selectivity. Chemistry 21, 12285–12290 (2015).
Giessibl, F. J. The qPlus sensor, a powerful core for the atomic force microscope. Rev. Sci. Instrum. 90, 011101 (2019).
Bartels, L., Meyer, G. & Rieder, K. H. Controlled vertical manipulation of single CO molecules with the scanning tunnelling microscope: a route to chemical contrast. Appl. Phys. Lett. 71, 213–215 (1997).
Riss, A. SpmImage Tycoon: organize and analyze scanning probe microscopy data. J. Open Source Softw. 7, 4644 (2022).
Kresse, G. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Hamada, I. Van der Waals density functional made accurate. Phys. Rev. B 89, 121103 (2014).
Henkelman, G. & Jonsson, H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J. Chem. Phys. 111, 7010–7022 (1999).
Henkelman, G. & Jonsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Henkelman, G., Uberuaga, B. P. & Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Henkelman, G., Arnaldsson, A. & Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 36, 354–360 (2006).
Sperl, A., Kröger, J. & Berndt, R. Controlled metalation of a single adsorbed phthalocyanine. Angew. Chem. 123, 5406–5409 (2011).
Pham, V. D. et al. Control of molecule–metal interaction by hydrogen manipulation in an organic molecule. J. Phys. Chem. Lett. 7, 1416–1421 (2016).
Cyrañski, M. K., Krygowski, T. M., Wisiorowski, M., Van Eikema Hommes, N. J. R. & Ragué Schleyer, P. V. Global and local aromaticity in porphyrins: an analysis based on molecular geometries and nucleus-independent chemical shifts. Angew. Chem. Int. Ed. 37, 177–180 (1998).
Islyaikin, M. K., Ferro, V. R. & García De La Vega, J. M. Aromaticity in tautomers of triazoleporphyrazine. J. Chem. Soc. Perkin Trans. 2, 2104–2109 (2002).
Aihara, J. I., Kimura, E. & Krygowski, T. M. Aromatic conjugation pathways in porphyrins. Bull. Chem. Soc. Jpn 81, 826–835 (2008).
Acknowledgements
This work was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—453903355, 326785818, under Germany’s Excellence Strategy—EXC 2089/1–390776260 (e-conversion), and a grant from the Irish Research Council (New Foundations, PorOrgMat), and the Guangdong Basic and Applied Basic Research Foundation (grant no. 2019A1515110819). N.C. acknowledges the support from China Scholarship Council (CSC). M.O.S. was supported by the Technical University of Munich–Institute for Advanced Study through a Hans Fischer Senior Fellowship and by Science Foundation Ireland (21/FFP-A/9469). J.B. acknowledges funding from the Swedish Research Council. The computations were enabled by resources provided by the National Academic Infrastructure for Supercomputing in Sweden (NAISS) and the Swedish National Infrastructure for Computing (SNIC) at the National Supercomputer Centre (NSC), partially funded by the Swedish Research Council through grant agreements no. 2022-06725 and no. 2018-05973. Funding for E.C.-R. was provided by CONACYT-Chihuahua, Mexico (Scholarship 591246). We kindly thank F. Klappenberger for his support during the initial project stage. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
A.R. conceived the analysis. N.C and A.R. designed the experiments. N.C. performed the experiments and the DFT calculations. E.C.-R. helped with the experiments and interpretation. A.R. supervised the experiments and did the HOMA analysis. J.B. supervised the DFT calculations. Z.C. synthesized the precursors, supervised by M.R. M.O.S. provided insights and guidance for the chemical interpretation. J.V.B. provided materials and methods and supervised the project. N.C. and A.R. wrote the manuscript with help from all other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Nan Jiang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Identification of hydrogen tautomers via AFM.
(a-c) AFM images of a chain of three trans-cyclized and one cis-cyclized molecule at different tip-sample distances. The trans-cyclized species show brighter features associated with one pair of nitrogen atoms at opposite sides, as marked by white arrows. (d) The same image as in (c), but with increased contrast. Such bright features connecting one pair of opposing nitrogen atoms were observed in previous work and assigned to iminic nitrogen atoms (Supplementary Fig. 6)19, that is, nitrogen atoms within the pyrrole rings that do not carry internal hydrogens. This assignment corresponds to the 2a-trans2 type. (e) Proposed chemical structure of the chain, consisting of three 2a-trans2 and one 2a-cis molecule. (f),(g) AFM images of an unsubstituted porphyrin molecule adsorbed on Au(111) shows similar features at larger tip-sample heights: a line along the direction of the iminic nitrogen atoms can be seen, marked by the white arrow. (h) Chemical structure of the molecule in (f) and (g). Red arrows mark aminic pyrroles. Scan parameters: Vs = 0 V, constant height. We have investigated 165 trans-cyclized molecules (see also Supplementary Fig. 5), all of which exhibit these particular features, suggesting that (almost) all of the trans-cyclized species are of type 2a-trans2. Further support for this assignment is obtained by tip manipulation experiments (see Extended Data Fig. 2).
Extended Data Fig. 2 Tip-induced cleavage of inner hydrogens.
(a) Application of a voltage pulse (Vs = 3.2 V) close to the center of a trans-cyclized molecule within a chain leads to cleavage of an inner hydrogen atom45,73,74. A substantial contrast change on the lower side can be observed, as marked by filled (before manipulation) and outlined (after manipulation) red arrows. Thus, we deduce that the pyrrole ring at this position was carrying one of the inner hydrogen atoms before the manipulation. This assignment corresponds to a 2a-trans2 type molecule. (b) A similar experiment (with a voltage pulse of Vs = 3.2 V) shows the cleavage of both inner hydrogen atoms, inducing contrast changes at the top and bottom (marked by red arrows). Again, these positions are associated with the pyrroles that carry the inner hydrogen atoms, indicating that this molecule is of type 2a-trans2. We have successfully performed such manipulation experiments on 10 molecules, all of which were confirmed to be of type 2a-trans2. (c) Analogous experiments on unsubstituted porphyrin molecules adsorbed on Au(111). The two inner hydrogens can be cleaved off in two steps, both times associated with contrast changes at the respective hydrogen-carrying pyrrole rings (marked by red arrows). Scan parameters: Vs = 0 V, constant height.
Extended Data Fig. 3 Relaxed geometries of the cyclization products.
Top and side views of relaxed structures of (a) 2a-cis, (b) 2a-trans1 and (c) 2a-trans2 at different adsorption positions, as well as in their free-standing geometry. For each structure, the reaction energies (with respect to the model of the precursor 1a), the adsorption energies, as well as the HOMA indices associated with the aromatic diaza[18]annulene pathway are shown.
Extended Data Fig. 4 Local HOMA indices and relative bond lengths.
The images show the local HOMA indices for the pyrrole rings, as well as the relative bond lengths of each C-C and C-N bond (red for shortened and blue for elongated bond lengths, compared with the ‘optimal’ aromatic bond lengths ‘dopt’). The precursor 1a exhibits an asymmetry, with one pair of two pyrrole rings at opposite ‘corners’ having higher HOMA indices than the other pair. This is in line with the aromatic diaza[18]annulene pathway (see Fig. 1 in the main text) observed in porphyrins75,76,77. The two pairs of carbon atoms with olefinic character outside of the aromatic pathway are marked by gray arrows. The cyclized products also exhibit this twofold symmetry, but there are significant changes in the HOMA indices for the rings. The newly formed five-membered rings lead to a reduction of the HOMA indices of the pyrrole rings that they are attached to. For the structures 2a-cis, 2a-trans1, and 2a-trans2, an additional 22 π-electron circuit exists along all the carbon atoms in the periphery of the molecules. The HOMA indices for these circuits are lower: 0.552 for 2a-cis, 0.464 for 2a-trans1, and 0.540 for 2a-trans2. Furthermore, some of the atoms at the newly formed five-membered rings might be sp3-hybridized (see Supplementary Fig. 7), thus inhibiting conjugation along these 22 π-electron circuits.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–22 (additional experimental and theory data) and References 1–37.
Supplementary Data 1
xyz coordinates of all DFT-optimized structures.
Supplementary Data 2
Source data for Supplementary Fig. 9.
Supplementary Data 3
Source data for Supplementary Fig. 10.
Supplementary Data 4
Source data for Supplementary Fig. 11.
Supplementary Data 5
Source data for Supplementary Fig. 12.
Supplementary Data 6
Source data for Supplementary Fig. 13.
Supplementary Data 7
Source data for Supplementary Fig. 14.
Supplementary Data 8
Source data for Supplementary Fig. 15.
Supplementary Data 9
Source data for Supplementary Fig. 16.
Supplementary Data 10
Source data for Supplementary Fig. 17.
Source data
Source Data Fig. 5
Source data for the graph in Fig. 5.
Source Data Fig. 6
Source data for the graphs in Fig. 6a,b.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, N., Björk, J., Corral-Rascon, E. et al. The role of aromaticity in the cyclization and polymerization of alkyne-substituted porphyrins on Au(111). Nat. Chem. 15, 1765–1772 (2023). https://doi.org/10.1038/s41557-023-01327-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01327-6