Abstract
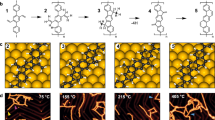
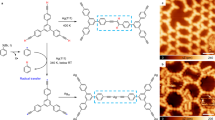
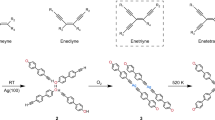
Immobilization of organic molecules on metal surfaces and their coupling via thermally induced C–C bond formation is an important technique in organic and polymer synthesis. Using this approach, insoluble and reactive carbon nanostructures can be synthesized and the reactions monitored in situ using scanning probe microscopy methods. The diversity of conceivable products, however, is limited by the number and variety of known on-surface reactions. Here, we introduce the on-surface synthesis of polyarylenes by intermolecular oxidative coupling of isopropyl substituents of arenes. This [3+3] dimerization reaction forms a new phenylene ring and can be regarded as a formal cycloaromatization. The synthetic value of this reaction is proved by the synthesis of polyarylenes and co-polyarylenes, which we demonstrate by synthesizing poly(2,7-pyrenylene-1,4-phenylene). Scanning tunnelling microscopy and non-contact atomic force microscopy studies, complemented by density functional theory calculations, offer mechanistic insight into the on-surface cycloaromatization reaction.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the STM, nc-AFM and computational datasets shown in the paper and Supplementary Information are available from Materials Cloud at https://doi.org/10.24435/materialscloud:yy-sc (ref. 58).
Code availability
The CP2K software package is available at https://www.cp2k.org/ and the AiiDAlab software package can be downloaded at https://www.materialscloud.org/work/aiidalab.
References
Qiu, Z., Hammer, B. A. G. & Müllen, K. Conjugated polymers – problems and promises. Prog. Polym. Sci. 100, 101179 (2020).
Cheng, Y.-J., Yang, S.-H. & Hsu, C.-S. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 109, 5868–5923 (2009).
Kawano, S. et al. Blue-emitting poly(2,7-pyrenylene)s: synthesis and optical properties. Macromolecules 41, 7933–7937 (2008).
Morin, P.-O., Bura, T. & Leclerc, M. Realizing the full potential of conjugated polymers: innovation in polymer synthesis. Mater. Horiz. 3, 11–20 (2016).
Sakamoto, J., Rehahn, M., Wegner, G. & Schlüter, A. D. Suzuki polycondensation: polyarylenes à la carte. Macromol. Rapid Commun. 30, 653–687 (2009).
Yamamoto, T. Electrically conducting and thermally stable π-conjugated poly(arylene)s prepared by organometallic processes. Prog. Polym. Sci. 17, 1153–1205 (1992).
Li, Y. et al. Energy level and molecular structure engineering of conjugated donor–acceptor copolymers for photovoltaic applications. Macromolecules 42, 4491–4499 (2009).
Wu, P.-T., Kim, F. S., Champion, R. D. & Jenekhe, S. A. Conjugated donor–acceptor copolymer semiconductors. Synthesis, optical properties, electrochemistry, and field-effect carrier mobility of pyridopyrazine-based copolymers. Macromolecules 41, 7021–7028 (2008).
Wu, J.-S., Cheng, S.-W., Cheng, Y.-J. & Hsu, C.-S. Donor–acceptor conjugated polymers based on multifused ladder-type arenes for organic solar cells. Chem. Soc. Rev. 44, 1113–1154 (2015).
Müllen, K. & Pisula, W. Donor–acceptor polymers. J. Am. Chem. Soc. 137, 9503–9505 (2015).
Suraru, S.-L., Lee, J. A. & Luscombe, C. K. C–H arylation in the synthesis of π-conjugated polymers. ACS Macro Lett. 5, 724–729 (2016).
Pouliot, J.-R., Grenier, F., Blaskovits, J. T., Beaupré, S. & Leclerc, M. Direct (hetero)arylation polymerization: simplicity for conjugated polymer synthesis. Chem. Rev. 116, 14225–14274 (2016).
Leclerc, M., Brassard, S. & Beaupré, S. Direct (hetero)arylation polymerization: toward defect-free conjugated polymers. Polym. J. 52, 13–20 (2020).
Blaskovits, J. T. & Leclerc, M. C–H activation as a shortcut to conjugated polymer synthesis. Macromol. Rapid Commun. 40, 1800512 (2019).
Grill, L. et al. Nano-architectures by covalent assembly of molecular building blocks. Nat. Nanotechnol. 2, 687–691 (2007).
Grill, L. & Hecht, S. Covalent on-surface polymerization. Nat. Chem. 12, 115–130 (2020).
Clair, S. & de Oteyza, D. G. Controlling a chemical coupling reaction on a surface: tools and strategies for on-surface synthesis. Chem. Rev. 119, 4717–4776 (2019).
Shen, Q., Gao, H.-Y. & Fuchs, H. Frontiers of on-surface synthesis: from principles to applications. Nano Today 13, 77–96 (2017).
Hla, S.-W., Bartels, L., Meyer, G. & Rieder, K.-H. Inducing all steps of a chemical reaction with the scanning tunneling microscope tip: towards single molecule engineering. Phys. Rev. Lett. 85, 2777–2780 (2000).
Mistry, A. et al. The synthesis and STM/AFM imaging of ‘olympicene’ benzo[cd]pyrenes. Chem. Eur. J. 21, 2011–2018 (2015).
Pavlicek, N. et al. On-surface generation and imaging of arynes by atomic force microscopy. Nat. Chem. 7, 623–628 (2015).
De Oteyza, D. G. et al. Direct imaging of covalent bond structure in single-molecule chemical reactions. Science 340, 1434–1437 (2013).
Schuler, B. et al. Reversible Bergman cyclization by atomic manipulation. Nat. Chem. 8, 220–224 (2016).
Zhong, D. et al. Linear alkane polymerization on a gold surface. Science 334, 213–216 (2011).
Volger, H. C. Oxidative dimerization of β-substituted α-olefins by palladium acetate. Recl. Trav. Chim. Pays Bas 86, 677–686 (1967).
Chen, S. & Hu, A. Recent advances of the Bergman cyclization in polymer science. Sci. China Chem. 58, 1710–1723 (2015).
Sun, Q. et al. On-surface formation of one-dimensional polyphenylene through Bergman cyclization. J. Am. Chem. Soc. 135, 8448–8451 (2013).
Merino-Díez, N. et al. Width-dependent band gap in armchair graphene nanoribbons reveals Fermi level pinning on Au(111). ACS Nano 11, 11661–11668 (2017).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Gross, L. et al. Bond-order discrimination by atomic force microscopy. Science 337, 1326–1329 (2012).
Hapala, P. et al. Mapping the electrostatic force field of single molecules from high-resolution scanning probe images. Nat. Commun. 7, 11560 (2016).
Björk, J., Stafström, S. & Hanke, F. Zipping up: cooperativity drives the synthesis of graphene nanoribbons. J. Am. Chem. Soc. 133, 14884–14887 (2011).
Fan, Q. et al. Precise monoselective aromatic C–H bond activation by chemisorption of meta-aryne on a metal surface. J. Am. Chem. Soc. 140, 7526–7532 (2018).
Floris, A. et al. Driving forces for covalent assembly of porphyrins by selective C–H bond activation and intermolecular coupling on a copper surface. J. Am. Chem. Soc. 138, 5837–5847 (2016).
Flory, P. J. The mechanism of vinyl polymerizations. J. Am. Chem. Soc. 59, 241–253 (1937).
Bjork, J. Thermodynamics of an electrocyclic ring-closure reaction on Au(111). J. Phys. Chem. C 120, 21716–21721 (2016).
Pavliček, N. et al. Polyyne formation via skeletal rearrangement induced by atomic manipulation. Nat. Chem. 10, 853–858 (2018).
Senkovskiy, B. V. et al. Making graphene nanoribbons photoluminescent. Nano Lett. 17, 4029–4037 (2017).
Borin Barin, G. et al. Optimized graphene transfer: influence of polymethylmethacrylate (PMMA) layer concentration and baking time on graphene final performance. Carbon 84, 82–90 (2015).
Xu, X. et al. On-surface synthesis of dibenzohexacenohexacene and dibenzopentaphenoheptaphene. Bull. Chem. Soc. Jpn 94, 997–999 (2021).
Weiss, C. et al. Imaging Pauli repulsion in scanning tunneling microscopy. Phys. Rev. Lett. 105, 086103 (2010).
Kichin, G., Weiss, C., Wagner, C., Tautz, F. S. & Temirov, R. Single molecule and single atom sensors for atomic resolution imaging of chemically complex surfaces. J. Am. Chem. Soc. 133, 16847–16851 (2011).
Fan, Q. et al. On-surface pseudo-high-dilution synthesis of macrocycles: principle and mechanism. ACS Nano 11, 5070–5079 (2017).
Giessibl, F. J. Atomic resolution on Si(111)-(7×7) by noncontact atomic force microscopy with a force sensor based on a quartz tuning fork. Appl. Phys. Lett. 76, 1470–1472 (2000).
Bartels, L. et al. Dynamics of electron-induced manipulation of individual CO molecules on Cu(111). Phys. Rev. Lett. 80, 2004–2007 (1998).
Yakutovich, A. V. et al. AiiDAlab – an ecosystem for developing, executing, and sharing scientific workflows. Comput. Mater. Sci. 188, 110165 (2021).
Pizzi, G., Cepellotti, A., Sabatini, R., Marzari, N. & Kozinsky, B. AiiDA: automated interactive infrastructure and database for computational science. Comput. Mater. Sci. 111, 218–230 (2016).
Hutter, J., Iannuzzi, M., Schiffmann, F. & VandeVondele, J. CP2K: atomistic simulations of condensed matter systems. WIREs Comput. Mol. Sci. 4, 15–25 (2014).
VandeVondele, J. & Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 127, 114105 (2007).
Goedecker, S., Teter, M. & Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 54, 1703–1710 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Hanke, F. & Björk, J. Structure and local reactivity of the Au(111) surface reconstruction. Phys. Rev. B 87, 235422 (2013).
Hapala, P. et al. Mechanism of high-resolution STM/AFM imaging with functionalized tips. Phys. Rev. B 90, 085421 (2014).
Laio, A. & Parrinello, M. Escaping free-energy minima. Proc. Natl Acad. Sci. USA 99, 12562–12566 (2002).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
E, W., Ren, W. & Vanden-Eijnden, E. String method for the study of rare events. Phys. Rev. B 66, 052301 (2002).
Kinikar, A. et al. On-surface polyarylene synthesis by cycloaromatization of isopropyl substituents. Materials Cloud Archive https://doi.org/10.24435/materialscloud:yy-sc (2022).
Cai, Z., She, L., Wu, L. & Zhong, D. On-surface synthesis of linear polyphenyl wires guided by surface steric effect. J. Phys. Chem. C 120, 6619–6624 (2016).
Acknowledgements
This work was supported by the Swiss National Science Foundation (grant no. 200020_182015), the NCCR MARVEL funded by the Swiss National Science Foundation (grant no. 51NF40-182892), the Johannes Gutenberg-Universität Mainz (JGU) through Gutenberg Forschungskolleg Fellowship (GFK) and the Max Planck Society. J.I.U. acknowledges the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie (grant agreement no. 886314). E.J., Y.G. and Z.Q. would like to acknowledge support from the Alexander von Humboldt Foundation. K.M. thanks the Gutenberg Research College for a scholarship. Computational support from the Swiss Supercomputing Center (CSCS) under project ID s904 is gratefully acknowledged. A.K., M.D.G., J.I.U., P.R. and R.F. thank L. Rotach (Empa, Dübendorf) for technical support.
Author information
Authors and Affiliations
Contributions
A.K., K.M., P.R. and R.F. conceived the project. Z.Q., Y.G., E.J. and X.-Y.W. synthesized and characterized the precursor molecules under the supervision of A.N. and K.M. The on-surface synthesis and the STM/nc-AFM measurements were performed by A.K., M.D.G. and J.I.U. under the supervision of P.R. and R.F. The DFT nc-AFM simulations were carried out by K.E., and C.P. did the DFT constrained geometry replica chain and NEB calculations. A.K. and K.M. wrote the manuscript with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks Leonhard Grill, Leo Gross and Sabine Maier for their contribution to the peer review of this work. Peter Seavill was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–31.
Rights and permissions
About this article
Cite this article
Kinikar, A., Di Giovannantonio, M., Urgel, J.I. et al. On-surface polyarylene synthesis by cycloaromatization of isopropyl substituents. Nat. Synth 1, 289–296 (2022). https://doi.org/10.1038/s44160-022-00032-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-022-00032-5
This article is cited by
-
Universal inter-molecular radical transfer reactions on metal surfaces
Nature Communications (2024)
-
On-surface cyclization of vinyl groups on poly-para-phenylene involving an unusual pentagon to hexagon transformation
Nature Communications (2024)
-
Extending on-surface synthesis from 2D to 3D by cycloaddition with C60
Nature Communications (2023)
-
On-surface synthesis of enetriynes
Nature Communications (2023)
-
Generating antiaromaticity in polycyclic conjugated hydrocarbons by thermally selective skeletal rearrangements at interfaces
Nature Synthesis (2023)