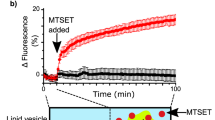
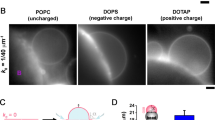
Abstract
Model membranes can be used to elucidate the intricacies of the chemical processes that occur in cell membranes, but the perfectly biomimetic, yet bespoke, model membrane has yet to be built. Droplet interface bilayers are a new type of model membrane able to mimic some features of real cell membranes better than traditional models, such as liposomes and black lipid membranes. In this Perspective, we discuss recent work in the field that is starting to showcase the potential of these model membranes to enable the quantification of membrane processes, such as the behaviour of protein transporters and the prediction of in vivo drug movement, and their use as scaffolds for electrophysiological measurements. We also highlight the challenges that remain to enable droplet interface bilayers to achieve their full potential as artificial cells, and as biological analytical platforms to quantify molecular transport.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hwang, W. L., Holden, M. A., White, S. & Bayley, H. Electrical behavior of droplet interface bilayer networks: experimental analysis and modeling. J. Am. Chem. Soc. 129, 11854–11864 (2007).
Ginzberg, M. B., Kafri, R. & Kirschner, M. On being the right (cell) size. Science 348, 1245075 (2015).
Korner, J. L., Stephenson, E. B. & Elvira, K. S. A bespoke microfluidic pharmacokinetic compartment model for drug absorption using artificial cell membranes. Lab Chip 20, 1898–1906 (2020).
Hwang, W. L., Chen, M., Cronin, B., Holden, M. A. & Bayley, H. Asymmetric droplet interface bilayers. J. Am. Chem. Soc. 130, 5878–5879 (2008).
Stephenson, E. B. & Elvira, K. S. Biomimetic artificial cells to model the effect of membrane asymmetry on chemoresistance. Chem. Commun. 57, 6534–6537 (2021).
Barlow, N. E. et al. Measuring bilayer surface energy and curvature in asymmetric droplet interface bilayers. J. R. Soc. Interface 15, 20180610 (2018).
Malmstadt, N., Nash, M. A., Purnell, R. F. & Schmidt, J. J. Automated formation of lipid-bilayer membranes in a microfluidic device. Nano Lett. 6, 1961–1965 (2006).
Sarles, S. A. & Leo, D. J. Tailored current–voltage relationships of droplet-interface bilayers using biomolecules and external feedback control. J. Intell. Mater. Syst. Struct. 20, 1233–1247 (2009).
Aghdaei, S., Sandison, M. E., Zagnoni, M., Green, N. G. & Morgan, H. Formation of artificial lipid bilayers using droplet dielectrophoresis. Lab Chip 8, 1617 (2008).
Holden, M. A., Needham, D. & Bayley, H. Functional bionetworks from nanoliter water droplets. J. Am. Chem. Soc. 129, 8650–8655 (2007).
Renner, S., Geltinger, S. & Simmel, F. C. Nanopore translocation and force spectroscopy experiments in microemulsion droplets. Small 6, 190–194 (2010).
Poulos, J. L., Nelson, W. C., Jeon, T.-J., Kim, C.-J. & Schmidt, J. J. Electrowetting on dielectric-based microfluidics for integrated lipid bilayer formation and measurement. Appl. Phys. Lett. 95, 013706 (2009).
Creasy, M. A. & Leo, D. J. Non-invasive measurement techniques for measuring properties of droplet interface bilayers. Smart Mater. Struct. 19, 094016 (2010).
Sarles, S. A. & Leo, D. J. Physical encapsulation of droplet interface bilayers for durable, portable biomolecular networks. Lab Chip 10, 710–717 (2010).
Sarles, S. A. & Leo, D. J. Regulated attachment method for reconstituting lipid bilayers of prescribed size within flexible substrates. Anal. Chem. 82, 959–966 (2010).
Punnamaraju, S. & Steckl, A. J. Voltage control of droplet interface bilayer lipid membrane dimensions. Langmuir 27, 618–626 (2011).
Syeda, R., Holden, M. A., Hwang, W. L. & Bayley, H. Screening blockers against a potassium channel with a droplet interface bilayer array. J. Am. Chem. Soc. 130, 15543–15548 (2008).
Bayley, H. et al. Droplet interface bilayers. Mol. Biosyst. 4, 1191–1208 (2008).
Huang, J., Lein, M., Gunderson, C. & Holden, M. A. Direct quantitation of peptide-mediated protein transport across a droplet-interface bilayer. J. Am. Chem. Soc. 133, 15818–15821 (2011).
Nisisako, T., Portonovo, S. A. & Schmidt, J. J. Microfluidic passive permeability assay using nanoliter droplet interface lipid bilayers. Analyst 138, 6793–6800 (2013).
Findlay, H. E., Harris, N. J. & Booth, P. J. In vitro synthesis of a major facilitator transporter for specific active transport across droplet interface bilayers. Sci. Rep. 6, 39349 (2016).
Barriga, H. M. G. et al. Droplet interface bilayer reconstitution and activity measurement of the mechanosensitive channel of large conductance from Escherichia coli. J. R. Soc. Interface 11, 20140404 (2014).
Najem, J. S. et al. Multifunctional, micropipette-based method for incorporation and stimulation of bacterial mechanosensitive ion channels in droplet interface bilayers. J. Vis. Exp. 105, e53362 (2015).
El-Beyrouthy, J., Makhoul-Mansour, M. M., Taylor, G., Sarles, S. A. & Freeman, E. C. A new approach for investigating the response of lipid membranes to electrocompression by coupling droplet mechanics and membrane biophysics. J. R. Soc. Interface 16, 20190652 (2019).
Zhang, Y., Bracken, H., Woolhead, C. & Zagnoni, M. Functionalisation of human chloride intracellular ion channels in microfluidic droplet-interface-bilayers. Biosens. Bioelectron. 150, 111920 (2020).
Stanley, C. E. et al. A microfluidic approach for high-throughput droplet interface bilayer (DIB) formation. Chem. Commun. 46, 1620–1622 (2010).
Carreras, P., Law, R. V., Brooks, N., Seddon, J. M. & Ces, O. Microfluidic generation of droplet interface bilayer networks incorporating real-time size sorting in linear and non-linear configurations. Biomicrofluidics 8, 054113 (2014).
Elani, Y., Casadevall i Solvas, X., Edel, J. B., Law, R. V. & Ces, O. Microfluidic generation of encapsulated droplet interface bilayer networks (multisomes) and their use as cell-like reactors. Chem. Commun. 52, 5961–5964 (2016).
Mueller, P., Rudin, D. O., Ti Tien, H. & Wescott, W. C. Reconstitution of cell membrane structure in vitro and its transformation into an excitable system. Nature 194, 979–980 (1962).
White, S. H. in Ion Channel Reconstitution (ed. Miller, C.) 3–35 (Springer US, 1986).
Oiki, S. in Patch Clamp Techniques: From Beginning to Advanced Protocols (ed. Okada, Y.) 229–275 (Springer Japan, 2012).
Peetla, C., Stine, A. & Labhasetwar, V. Biophysical interactions with model lipid membranes: applications in drug discovery and drug delivery. Mol. Pharm. 6, 1264–1276 (2009).
Elani, Y., deMello, A. J., Niu, X. & Ces, O. Novel technologies for the formation of 2-D and 3-D droplet interface bilayer networks. Lab Chip 12, 3514–3520 (2012).
Carreras, P. et al. A microfluidic platform for size-dependent generation of droplet interface bilayer networks on rails. Biomicrofluidics 9, 064121 (2015).
Nguyen, M.-A., Srijanto, B., Collier, C. P., Retterer, S. T. & Sarles, S. A. Hydrodynamic trapping for rapid assembly and in situ electrical characterization of droplet interface bilayer arrays. Lab Chip 16, 3576–3588 (2016).
Czekalska, M. A. et al. A droplet microfluidic system for sequential generation of lipid bilayers and transmembrane electrical recordings. Lab Chip 15, 541–548 (2015).
Schlicht, B. & Zagnoni, M. Droplet-interface-bilayer assays in microfluidic passive networks. Sci. Rep. 5, 9951 (2015).
Taylor, G. et al. Electrophysiological interrogation of asymmetric droplet interface bilayers reveals surface-bound alamethicin induces lipid flip-flop. Biochim. Biophys. Acta Biomembr. 1861, 335–343 (2019).
Tamaddoni, N., Taylor, G., Hepburn, T., Kilbey, S. M. & Sarles, S. A. Reversible, voltage-activated formation of biomimetic membranes between triblock copolymer-coated aqueous droplets in good solvents. Soft Matter 12, 5096–5109 (2016).
Bai, Y. et al. A double droplet trap system for studying mass transport across a droplet–droplet interface. Lab Chip 10, 1281–1285 (2010).
Taylor, G. J. et al. Capacitive detection of low-enthalpy, higher-order phase transitions in synthetic and natural composition lipid membranes. Langmuir 33, 10016–10026 (2017).
Taylor, G. J. & Sarles, S. A. Heating-enabled formation of droplet interface bilayers using Escherichia coli total lipid extract. Langmuir 31, 325–337 (2015).
Barlow, N. E. et al. Engineering plant membranes using droplet interface bilayers. Biomicrofluidics 11, 024107 (2017).
Najem, J. S. et al. Memristive ion channel-doped biomembranes as synaptic mimics. ACS Nano 12, 4702–4711 (2018).
Michalak, Z., Muzzio, M., Milianta, P. J., Giacomini, R. & Lee, S. Effect of monoglyceride structure and cholesterol content on water permeability of the droplet bilayer. Langmuir 29, 15919–15925 (2013).
Lopez, M., Evangelista, S. E., Morales, M. & Lee, S. Enthalpic effects of chain length and unsaturation on water permeability across droplet bilayers of homologous monoglycerides. Langmuir 33, 900–912 (2017).
Lopez, M. et al. Effects of acyl chain unsaturation on activation energy of water permeability across droplet bilayers of homologous monoglycerides: role of cholesterol. Langmuir 34, 2147–2157 (2018).
Taylor, G. J., Venkatesan, G. A., Collier, C. P. & Sarles, S. A. Direct in situ measurement of specific capacitance, monolayer tension, and bilayer tension in a droplet interface bilayer. Soft Matter 11, 7592–7605 (2015).
de Wit, G., Danial, J. S. H., Kukura, P. & Wallace, M. I. Dynamic label-free imaging of lipid nanodomains. Proc. Natl Acad. Sci. USA 112, 12299–12303 (2015).
Faugeras, V., Duclos, O., Bazile, D. & Thiam, A. R. Membrane determinants for the passive translocation of analytes through droplet interface bilayers. Soft Matter 16, 5970–5980 (2020).
Korner, J. L. & Elvira, K. S. The role of temperature in the formation of human–mimetic artificial cell membranes using droplet interface bilayers (DIBs). Soft Matter 17, 8891–8901 (2021).
van Meer, G., Voelker, D. R. & Feigenson, G. W. Membrane lipids: where they are and how they behave. Nat. Rev. Mol. Cell Biol. 9, 112–124 (2008).
McMahon, H. T. & Boucrot, E. Membrane curvature at a glance. J. Cell Sci. 128, 1065–1070 (2015).
Venkatesan, G. A. et al. Adsorption kinetics dictate monolayer self-assembly for both lipid-in and lipid-out approaches to droplet interface bilayer formation. Langmuir 31, 12883–12893 (2015).
Yanagisawa, M., Yoshida, T.-A., Furuta, M., Nakata, S. & Tokita, M. Adhesive force between paired microdroplets coated with lipid monolayers. Soft Matter 9, 5891–5897 (2013).
Gross, L. C. M., Heron, A. J., Baca, S. C. & Wallace, M. I. Determining membrane capacitance by dynamic control of droplet interface bilayer area. Langmuir 27, 14335–14342 (2011).
Beltramo, P. J., Scheidegger, L. & Vermant, J. Toward realistic large-area cell membrane mimics: excluding oil, controlling composition, and including ion channels. Langmuir 34, 5880–5888 (2018).
Milianta, P. J., Muzzio, M., Denver, J., Cawley, G. & Lee, S. Water permeability across symmetric and asymmetric droplet interface bilayers: interaction of cholesterol sulfate with DPhPC. Langmuir 31, 12187–12196 (2015).
Braziel, S., Sullivan, K. & Lee, S. Quantitative Raman microspectroscopy for water permeability parameters at a droplet interface bilayer. Analyst 143, 747–755 (2018).
Valet, M., Pontani, L.-L., Voituriez, R., Wandersman, E. & Prevost, A. Diffusion through nanopores in connected lipid bilayer networks. Phys. Rev. Lett. 123, 088101 (2019).
Barlow, N. E. et al. Rheological droplet interface bilayers (rheo-DIBs): probing the unstirred water layer effect on membrane permeability via spinning disk induced shear stress. Sci. Rep. 7, 1–12 (2017).
Funakoshi, K., Suzuki, H. & Takeuchi, S. Lipid bilayer formation by contacting monolayers in a microfluidic device for membrane protein analysis. Anal. Chem. 78, 8169–8174 (2006).
Booth, M. J., Cazimoglu, I. & Bayley, H. Controlled deprotection and release of a small molecule from a compartmented synthetic tissue module. Commun. Chem. 2, 1–8 (2019).
Cazimoglu, I., Booth, M. J. & Bayley, H. A lipid-based droplet processor for parallel chemical signals. ACS Nano 15, 20214–20224 (2021).
Li, X., Huang, J., Holden, M. A. & Chen, M. Peptide-mediated membrane transport of macromolecular cargo driven by membrane asymmetry. Anal. Chem. 89, 12369–12374 (2017).
Wood, M. et al. Ibuprofen and the phosphatidylcholine bilayer: membrane water permeability in the presence and absence of cholesterol. Langmuir 37, 4468–4480 (2021).
Allen-Benton, M., Findlay, H. E. & Booth, P. J. Probing membrane protein properties using droplet interface bilayers. Exp. Biol. Med. 244, 709–720 (2019).
Leptihn, S., Thompson, J. R., Ellory, J. C., Tucker, S. J. & Wallace, M. I. In vitro reconstitution of eukaryotic ion channels using droplet interface bilayers. J. Am. Chem. Soc. 133, 9370–9375 (2011).
Smith, S. M. in Protein Chromatography: Methods and Protocols (eds Walls, D. & Loughran, S. T.) 485–496 (Humana, 2011).
Najem, J. S. et al. Activation of bacterial channel MscL in mechanically stimulated droplet interface bilayers. Sci. Rep. 5, 13726 (2015).
Strutt, R. et al. Activating mechanosensitive channels embedded in droplet interface bilayers using membrane asymmetry. Chem. Sci. 12, 2138–2145 (2021).
Restrepo Schild, V. et al. Light-patterned current generation in a droplet bilayer array. Sci. Rep. 7, 46585 (2017).
Alcinesio, A. et al. Controlled packing and single-droplet resolution of 3D-printed functional synthetic tissues. Nat. Commun. 11, 2105 (2020).
Graham, A. D. et al. High-resolution patterned cellular constructs by droplet-based 3D printing. Sci. Rep. 7, 7004 (2017).
Challita, E. J., Makhoul-Mansour, M. M. & Freeman, E. C. Reconfiguring droplet interface bilayer networks through sacrificial membranes. Biomicrofluidics 12, 034112 (2018).
Villar, G., Heron, A. J. & Bayley, H. Formation of droplet networks that function in aqueous environments. Nat. Nanotechnol. 6, 803–808 (2011).
Maglia, G. et al. Droplet networks with incorporated protein diodes show collective properties. Nat. Nanotechnol. 4, 437–440 (2009).
Bayoumi, M., Bayley, H., Maglia, G. & Sapra, K. T. Multi-compartment encapsulation of communicating droplets and droplet networks in hydrogel as a model for artificial cells. Sci. Rep. 7, 45167 (2017).
Challita, E. J., Najem, J. S., Monroe, R., Leo, D. J. & Freeman, E. C. Encapsulating networks of droplet interface bilayers in a thermoreversible organogel. Sci. Rep. 8, 1–11 (2018).
Shen, H.-H., Lithgow, T. & Martin, L. L. Reconstitution of membrane proteins into model membranes: seeking better ways to retain protein activities. Int. J. Mol. Sci. 14, 1589–1607 (2013).
Giacomini, K. M. et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 9, 215–236 (2010).
Rofeh, J. & Theogarajan, L. Instantaneous tension measurements in droplet interface bilayers using an inexpensive, integrated pendant drop camera. Soft Matter 16, 4484–4493 (2020).
Liu, P., Zabala-Ferrera, O. & Beltramo, P. J. Fabrication and electromechanical characterization of freestanding asymmetric membranes. Biophys. J. 120, 1755–1764 (2021).
Okuno, D. et al. A simple method for ion channel recordings using fine gold electrode. Anal. Sci. 32, 1353–1357 (2016).
Shoji, K., Kawano, R. & White, R. J. Recessed Ag/AgCl microelectrode-supported lipid bilayer for nanopore sensing. Anal. Chem. 92, 10856–10862 (2020).
Challita, E. J. & Freeman, E. C. Hydrogel microelectrodes for the rapid, reliable, and repeatable characterization of lipid membranes. Langmuir 34, 15166–15173 (2018).
Makhoul-Mansour, M. M. & Freeman, E. C. Droplet-based membranous soft materials. Langmuir 37, 3231–3247 (2021).
Sarles, S. A., Madden, J. D. W. & Leo, D. J. Hair cell inspired mechanotransduction with a gel-supported, artificial lipid membrane. Soft Matter 7, 4644 (2011).
Tamaddoni, N., Freeman, E. C. & Sarles, S. A. Sensitivity and directionality of lipid bilayer mechanotransduction studied using a revised, highly durable membrane-based hair cell sensor. Smart Mater. Struct. 24, 065014 (2015).
Venkatesan, G. A. et al. Evaporation-induced monolayer compression improves droplet interface bilayer formation using unsaturated lipids. Biomicrofluidics 12, 024101 (2018).
Dupin, A. & Simmel, F. C. Signalling and differentiation in emulsion-based multi-compartmentalized in vitro gene circuits. Nat. Chem. 11, 32–39 (2019).
Farley, S., Ramsay, K. & Elvira, K. S. A plug-and-play modular microcapillary platform for the generation of multicompartmental double emulsions using glass or fluorocarbon capillaries. Lab Chip 21, 2781–2790 (2021).
Bachler, S., Ort, M., Krämer, S. D. & Dittrich, P. S. Permeation studies across symmetric and asymmetric membranes in microdroplet arrays. Anal. Chem. 93, 5137–5144 (2021).
Czekalska, M. A., Kaminski, T. S., Horka, M., Jakiela, S. & Garstecki, P. An automated microfluidic system for the generation of droplet interface bilayer networks. Micromachines 8, 93 (2017).
Barlow, N. E. et al. Multiplexed droplet interface bilayer formation. Lab Chip 16, 4653–4657 (2016).
Acknowledgements
K.S.E. is a Canada Research Chair and a Michael Smith Foundation for Health Research Scholar in partnership with the Pacific Alzheimer Research Foundation and receives funding from both. E.B.S. and J.L.K. are funded through K.S.E.’s Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grant.
Author information
Authors and Affiliations
Contributions
E.B.S. and J.L.K. conducted the literature review and contributed to the writing. K.S.E. conceptualized and supervised the project, and wrote, reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Stephen Sarles and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stephenson, E.B., Korner, J.L. & Elvira, K.S. Challenges and opportunities in achieving the full potential of droplet interface bilayers. Nat. Chem. 14, 862–870 (2022). https://doi.org/10.1038/s41557-022-00989-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-00989-y
This article is cited by
-
A simple method to make, trap and deform a vesicle in a gel
Scientific Reports (2023)