Abstract
In the framework of regenerative circular economy and Sustainable Development Goals #6, water demand and wastewater production are a crucial environmental issue. To cope with this, a system called Zero Mile has been conceived to reuse/upcycle the dishwasher wastewater in both fertilisation of a vertical garden (for home plant production at zero mile) and the subsequent dishwasher rinsing cycle. The core of the Zero Mile system is a biofilter constituted by an ad hoc engineered microbial consortium, based on the mutualistic relationship between autotrophic and heterotrophic microbial partners, that makes the biodegradative process sustainable over time. Two semi-batch tests were performed to evaluate the capability and the efficiency of the microbial consortium to operate in conditions mimicking the Zero Mile system: consortia were challenged in two dishwasher wastewater volumes (50 and 500 mL), with a replenishing rate of 4 days for ¼ of the dishwasher wastewater volume. The results of these tests showed that the consortium thrives well in dishwasher wastewater over time and can survive with the volume and rates of replenishments. Next Generation Sequencing showed that the microbial consortium maintains a stable taxonomic composition, and chemical analyses demonstrated a very high bioremediation efficiency (total nitrogen and phosphorus removal). Aiming to contribute to the circular economy approach, the consortium ‘treated’ dishwasher wastewater was useful for plant fertilisation, as demonstrated by pigment content and quality indices (total phenols and flavonoids, soluble solids) measured in the lettuce plants grown in the Zero Mile demonstrator and watered with consortium reclaimed dishwasher wastewater.
Similar content being viewed by others
Introduction
The circular economy represents the best approach to answer to the most pressing cross-cutting sustainable development challenges and has emerged as the almost obligate alternative to the unsustainable linear take-make-waste approach. By following this principle, resources are not consumed but partially or fully recovered, with the aim of exploiting their highest potential1. This approach is particularly relevant, among others, to the Agenda 2030 targets of the Sustainable Development Goals #6 (Clean water and Sanitation)2. In the framework of Sustainable Goal 6, particularly point 6.3, water demand and amount of wastewater produced are crucial social and environmental issues; in particular, freshwater resources that are fundamental for human health, agricultural production, economic activity, as well as critical ecosystem functions3. In the light of a growing water demand, wastewater is gaining attention as a reliable alternative source of water and nutrients (nitrogen, phosphorus, etc.) for plant cultivation, shifting the paradigm of wastewater management from treatment and disposal to reuse, recycle and resource recovery. In the context of a circular economy, where economic development is balanced with the protection of natural resources and environmental sustainability, wastewater represents a widely available and valuable resource4.
Wastewater treatment has been regulated in Europe and USA since last century (Water Framework Directive, 2000/60/EC5; EPA/810/R-17/0026), aiming to reach the goal of discharging water with only a good ecological status. However, wastewater crop irrigation has been recognised as a valid solution to face the high-water demand in agrotechnics7, and in some cases, the use of wastewater may also reduce the need of fertilisation8. Considering the widespread use of chemicals and the microbial contamination, it has been made clear that the use of untreated greywater for irrigation is highly not recommended9.
In addition to the standard treating procedures, biological filtration and bioremediation strategies based on the synergistic relationship between photosynthetic and heterotrophic microorganisms—the so-called microbial consortia, that can be considered as biofilters – has recently gained recognition as a sustainable procedure, in terms of treatment and cost efficiency10,11,12. In these systems, the wastewater nutrient load is utilised and removed by the consortium’ microorganisms. The simultaneous combination of the phototrophic and heterotrophic metabolisms triggers a robust biological system, structurally/spatially organised as suspended or attached microbial biomass and operating under various conditions and on different nutrient and pollutant loads13,14. Furthermore, the wastewater fed biomass can ultimately be used as a fertiliser, crop bio-stimulant, and energy carrier feedstock (biorefinery approach)11,15, adding value to the sustainability of this bio-based wastewater treatment12,16.
According to the 2030 Agenda definition17, dishwasher wastewater (DWW) is classified as greywater. DWW represents an underestimated water resource although in the EU-28 the water consumption (and consequently wastewater production) was estimated at about 400 million m3 in 2023 and has been predicted to continue increasing at least until 203018. While the overall amount of this water consumption is far from being neglectable, surprisingly, it is rarely taken into account as a recoverable resource because of the small amount produced by point sources. However, dishwasher producers, as other white goods industries, are interested in the reduction of water consumption. Indeed, they are obtaining incremental reduction of water consumption increasing efficiency of washing technologies. Several systems and methods based on reverse osmosis or partial water reuse have been patented, but they all present some shortcomings. Some industrial solutions envisage saving water by wastewater purification using reverse osmosis; however, solutions in this direction are faced with a problem, since each filtering operation produces not only osmotised water but also a concentrate that must be eliminated. Solutions that envisage water recycling without a purification system imply the necessity to drain the detergent-containing wastewater into the sewer system or to wash without detergents19. The Zero Mile system promises to be a groundbreaking innovation in this context, since it allows for wider wastewater reuse. According to Venkatesh (2022)20 ‘engineered’ microbial consortia cultivated for bio-remediation of wastewater are extremely useful agents in a circular bio-economy approach.
Historically, the reuse of kitchen wastewaters has already been practised to irrigate vegetables21,22 due to the high content of nutrients coming from food residues. The DWW reuse is facilitated by the low levels of contaminants23, and the biological treatment operated by a microbial consortium can be a feasible solution to its upcycling24,25,26. In contrast to wastewaters from other sources, toxic compounds such as heavy metals, pathogens or pharmaceutical traces are not expected in dishwasher effluents that only contain tap water, eco-labelled detergents and food leftovers, mineralized by the biofilter to be up-taken by plants. The only harmful compounds for plants could be salt from the de-alkalinisation process, which is discharged by the Zero Mile system19. Taking these issues into account, a specific monitoring campaign is planned, before the product’s engineering for the market, to prove this hypothesis.
In the framework of sustainable development, on-site food production is gaining popularity because of the environmental benefits it can provide in this time of fast-growing population and increasing urbanization27. On-site food production supports healthier diets and reduces the environmental impact of food transport28; the so-called Urban Agriculture and Building-Integrated Agriculture promotes a transition towards a new and healthy urban lifestyle29.
The Jetsons’ Kitchen Project has been designed to face these issues, i.e., to reuse and up-cycle all the kitchen wastewater. The first output of this project has been the Zero Mile system25, devoted to the reuse of dishwasher wastewater. Zero Mile (Fig. 1) is an innovative system, aimed to reuse and upcycle DWW in both the fertilisation of a vertical garden—for plant production at home, i.e., at zero mile—and the subsequent dishwasher rinsing cycle.
The system is composed essentially of the dishwasher, the wastewater collection, decontamination (including a biofilter), distribution and return system, the operation control system, the home garden, the lighting system and the user interface (Fig. 1). The core of the Zero Mile system is a biofilter constituted by an ad hoc engineered microbial consortium, composed of a filamentous photosynthetic and nitrogen-fixing cyanobacterium and three heterotrophic bacteria isolated from DWW26.
The aim of this study was to test the capability of this ad hoc engineered microbial consortium used in the previous batch test to operate in conditions, more similar to operating ones, in order to step forward in the Technology Readiness Level (TRL) from the experimental proof of concept (TRL3) towards the technology validation (TRL4-5)30. Although we used the same microbes as in ref. 26 (2020), this study applied a newly reconstituted consortium, along with a completely different experimental set up (from a batch static test to a semi-continuous one, with a different DWW/consortia contact time), at different volumes (from 50 to 500 mL) and operating conditions (different tap water, dishwasher machines and detergents; see Supplementary Table 1), aiming to evaluate its compliance with these changing parameters.
After testing a small volume (30 mL) of a single DWW over a very short time scale (48 h)26, in this study the robustness of the consortium was evaluated while facing different DWWs (same tap water and detergent, different content of food residues), in two test volumes (50 and 500 mL), over a long-time scale (up to 52 days), and with a different experimental setting, i.e., under partial DWW replenishment (see also Supplementary Table 1). Additionally, the robustness of the consortium was evaluated under a regular partial DWW replenishment (¼ volume, every 4 days), thus simulating an operational condition/cycle of the Zero Mile system. The consortium survival, its growth and taxonomic structure, as well as the efficiency in nutrient removal were evaluated in the two experimental testing volumes (50 and 500 mL). Furthermore, the consortium treated wastewater was used to irrigate lettuce plants in the vertical indoor garden of a Zero Mile demonstrator, specifically built to test the vegetable growth with the reclaimed wastewater. The growth performance and the organoleptic characteristics of lettuce plants were analysed at the end of the productive cycle, to evaluate the whole system capability to produce reclaimed water useful for plant indoor growth.
Results and discussion
The efficiency in wastewater reclamation of the ad hoc engineered consortium already proposed26, was tested in a new approach to assess the degradative capability over time and in different experimental conditions.
Consortium features
Microbial consortia were reassembled using the cyanobacterium T. variabilis and the three heterotrophic bacterial strains, isolated from a dishwasher wastewater (from a Milan Polytechnic dishwasher, in 2019, as in ref. 26). The consortia were assembled at least in triplicates, in two volumes (50 and 500 mL), grown in semi-batch conditions using DWW collected in Rome as the growth medium, and were maintained for 28 and 52 days, respectively.
Soon after the bacterial consortia assemblage, even with the naked eye, the consortia displayed the important emergent property already observed by ref. 26: a three-dimensional (3D) organisation, as a blue green suspended aggregates (biofilms), not adhering to the container surfaces. Consortia aggregates displayed different shapes and sizes depending on growth vessels and consortium age (Fig. 2).
The aggregates exhibited tightly entangled filaments in the centre, giving a more compact aspect to the biofilms, while more diffluent trichomes were present at the edges. This 3D structure was reversible, and it disaggregated under strong manual shaking (producing a homogeneous green suspension; Supplementary Video 1), and quickly reconstituted in a few hours when the flask was left to rest (Supplementary Fig. 1 and Supplementary Video 2). Hence, the consortia 3D structure must be advantageous for all the four consortium partners, probably linked to the cooperative interactions between cyanobacteria and heterotrophic bacteria. Furthermore, although T. variabilis is known to produce biofilms on the exposed surfaces31,32, in both the 50 and the 500 mL of DWW it did not stick to the container, whatever the material it is made of (plastic or glass). Moreover, its ‘shape’ depended/adapted to the shape of the container in which it thrived (Fig. 2). This response agrees with the diffusion-sensing hypothesis proposed by Redfield33. She hypothesised that individual microbial cells synthesise and emit molecules to identify the physical properties of their environment, with the aim of calibrating regulatory and behavioural responses for their own benefit, in this case, fitting with the container shape.
The dark green appearance of the consortia indicated the good cyanobacterial growth in the aggregate. To delve deeper into the consortium structure, samples of the aggregates thriving in DWW were collected from the semi-batch tests and analysed by Confocal Laser Scanning Microscopy (CLSM). CLSM observations allowed us to visualise the different biofilm members’ distribution and viability over the experimental time (Fig. 3). Figure 3a shows a 16-day old community at low magnification (20x) where the DAPI (4′,6-diamidino-2-phenylindole) fluorescence signal, in blue, appeared uniformly distributed and interspersed in the biofilm samples and within the T. variabilis filaments. Chl a autofluorescence (Fig. 3b), demonstrated the healthy and viable state of the cyanobacterial consortium partner, as also visible in Fig. 3c, where the DAPI and Chl a fluorescences were merged to visualise the mutual localization of both consortium partners, the photosynthetic and the heterotrophic ones. DAPI and Chl a merged signals (Fig. 3d) also showed, at higher magnification, the healthy status of T. variabilis within the consortia; indeed, most cells appeared in active vegetative division (forming cross walls and cell constrictions) along the filaments while nucleosomes were regular in shape and localization.
Thus, also the microscopy analyses confirmed the consortium capability to thrive in DWW, maintaining its peculiar characteristics of 3D-aggregation. This capability was retained whatever the DWW, that is, at different types of tap water, detergent and food residues (depending on food habits; see Supplementary Table 1).
Mimicking the Zero Mile operational conditions
The Zero Mile system collects wastewater from each dishwasher cleaning cycle. On average, the dishwasher is used every second day (ISTAT data for Italy34); hence, by rule, the consortium would recycle food leftovers in semi-batch conditions, i.e., with a periodic and almost complete replenishment of dishwasher wastewaters, on average every second day.
The first tests showing the consortium capability to thrive in DWW were performed as batch tests26; hence, to evaluate the capability and the efficiency of the consortium to operate in conditions mimicking the Zero Mile System—that is, a semi-continuous supply of freshly produced DWW—semi-batch tests were necessary.
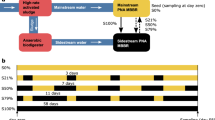
As an intermediate step toward the fully operative conditions, consortia were challenged in two tests based on (i) DWW volumes of 50 and 500 mL, (ii) DWW replenishing pace of 4 days, and (iii) replacement of ¼ DWW volume. Two tests were designed and performed: Small size - Replenish test (S-Rep test; 50 mL volume, lasting 28 days), to evaluate the consortium characteristics while exposed to a periodic replenishment of dishwasher wastewater, and Medium size - Replenish test (M-Rep test; 500 mL volume, lasting 52 days) to evaluate the efficacy of the consortium ‘treated’ dishwasher wastewater as plant fertiliser.
S-Rep test: Testing the consortium with periodic replenishment of dishwasher wastewater
Growth curves clearly indicated how the consortia thrive well in 100% DWW and are able to face the replenishment rate of ¼ medium culture, every 4 days (Fig. 4). The results even suggested that the microbial components of the consortium were fostered by the nutrients supplied with the replenishment of DWW. This was clearly evident after the 12th day of culture, with a better growth performance of the consortia grown in DWW than the control cultures in BG110; from day 12th on, chlorophyll a and turbidity values were significantly higher than in control batches (Two-Way ANOVA Sidak’s multiple comparisons test; p < 0.0001).
This result was further confirmed by the final data about the biomass reached by the 3D consortia thriving in the two types of culture media, after 28 days. Starting from a common dry weight of 10.2 ± 2.9 mg at the beginning of the experiment, in DWW the consortia reached a mean dry weight value of 26.4 ± 1.5 mg, and 24.6 ± 1.8 mg in BG110; hence, even in DWW the biomass more than doubled, without significant differences with the control batches (Student-t test, ns).
The thriving growth depends on the mutualistic relationship between the consortium partners, making the biodegradative process sustainable over time. The photosynthesis of autotrophic bacteria provides oxygen for the catalytic activities of the heterotrophic bacteria, which consume the organic matter present in the dishwasher wastewater, mineralize the nutrients and release CO2. The availability of mineralized nutrients (including CO2) is mandatory for the survival and growth of the cyanobacterial partner, while building an aerobic environment for the heterotrophs degradative activity12,26,35.
The consortium composition during the entire test was evaluated by 16 S rDNA amplicon sequencing by an NGS approach. Globally, the taxonomic composition of both the 3D aggregate and the suspended component in the culture media were maintained over time, notwithstanding the DWW periodic refill (Fig. 5).
The OTUs frequency, from T4 to T20, is evaluated separately for the 3D aggregate and the planktonic component. The experimentally associated bacteria in the consortium belong to the following families: Aeromonadaceae (Aeromonas sp., in purple), Exiguobacteraceae (Exiguobacterium sp., in red), Moraxellaceae (Acinetobacter sp., in azure) and Nostocaceae (Trichormus variabilis, in bright green), these families are in bold in the legend.
Analysis of Amplicon Sequence Variants (ASVs; i.e., each inferred unique DNA sequence recovered from a high-throughput analysis of 16 S rDNA genes) included both the 3D aggregate and the suspended bacteria, sampled at five different time points over a period of 3 weeks (from T4 to T20, every 4 days). This sampling resulted in a total number of 127,637 sequences, representing 243 ASVs that were then clustered in 105 OTUs (97% similarity) (Supplementary Table 2); of these, 71,485 sequences (82 OTUs) were found in the 3D structure, while 56,152 sequences (90 OTUs) belonged to the bacteria present in the culture medium where the consortium thrived (67 OTUs were found in both samples). The rarefaction curves of each sample in each experimental batch, built to evaluate differences and efficiency in the sampling effort, confirmed that the sequencing depth was good (Supplementary Fig. 2).
A stable community, based on the autotrophic T. variabilis (Nostocaceae, in bright green) was found in the 3D aggregate (4th to 8th bar; Fig. 5). The cyanobacterium (present only in the aggregate), was abundant from day 4th to day 12th, followed by small decreases, possibly due to the actual procedure of sampling, (i.e., sampling took place only on the edge of the aggregate). The dark green appearance of the aggregates supported this explanation. The stability of the 3D aggregate depended on the affinity of the most abundant bacterial families of the aggregate: those associated with the not-axenic culture of T. variabilis (Fig. 5; 1st bar) were found only in the 3D aggregate structure, except for Comamonadaceae and Flavobacteriaceae families, which were also found as suspended component; furthermore, the three heterotrophic consortium components remained bound to the 3D aggregate over time, Aeromonas sp. (Aeromonadaceae, in purple) constantly present, Exiguobacterium sp. (Exiguobacteriaceae, in red) increased with time, while Acinetobacter sp. (Moraxellaceae, in azure) decreased with time, possible due to the same topological reason of T. variabilis. Acinetobacter sp. was also found as a suspended component, but this was shown to decrease with time.
It is worth noting that the constancy of the aggregate bacterial components was maintained over time (ANOVA One-Way Tukey’s multiple comparison p > 0.05; Supplementary Table 3) notwithstanding the periodic DWW refill which included several bacterial families (Fig. 5; 2nd and 3rd bar), that were not included in the consortium. These families (e.g., Dysgonomonadaceae, Enterobacteriaceae, Pseudomonadaceae and Stappiaceae) were maintained at relatively low percentages in both the 3D aggregate structures and the suspended components in the medium, while the Enterococcaceae family remained only as suspended component. The periodic refills actually determined significant changes in the suspended component (Supplementary Table 3), and the different response to the refill was highlighted by a significant difference between the 3D consortium aggregate vs the suspended component (beta-diversity PERMANOVA test, p < 0.001). There is no other information on comparable consortia or DWW studies, nevertheless, the composition of the bacterial community found in our DWW is similar—at genus level—to the bacteria found in dishwasher rubber seals biofilms, with a dominance of Proteobacteria and Firmicutes phylum36,37. This similarity in the dominant component can be probably attributed to the to the extremely selective dishwasher environment, while the variation of the less represented bacterial components can be associated with the differences in eating habits (hence, to the characteristics of the food leftovers) and with the different growth conditions in wastewater or on rubber seals environments.
The bioremediation efficiency of the consortium, i.e., the effect of the microbial consortium on the DWW characteristics, was also evaluated. Both pH and solubilized nutrients (total nitrogen and total phosphorus content) were quantified in the medium every 4 days. The first effect of the presence and metabolic activity of the consortium was the increase of pH in DWW, due to the cyanobacterial photosynthetic activity, which acted as a CO2 sink: already at T4, we found pH changes having shifted from neutrality (7.1 in the untreated DWW, at T0) to 8.56 ± 0.12 in the treated DWW at T12; this was followed by slow continuous increases in pH up to T28, where it reached values of 9.28 ± 0.12, as a result of the consortium continuous growth. Analogous changes were found in BG110 pH (the control batches), that increased from 7.0 at T0, to 7.31 ± 0.13 at T4, up to 8.89 ± 0.23 at T28. Photosynthesis-driven increases of pH are very well known38, as the capability of autotroph-heterotroph interactions in microbial consortia to ameliorate wastewater characteristics, reducing the nutrient load12,26,35,39. This was also found in our system, where the activity of the bacterial consortia significantly reduced the content of solubilized total nitrogen and phosphorus in the DWW (treated; Table 1): in 28 days the concentration of both nitrogen and phosphorous were reduced by at least 50% in the consortium treated batches (comparison T4–T28; Two-Way ANOVA Tukey’s multiple comparisons test, p < 0.001) compared to the untreated DWW.
Actually, the consortium efficiency to mineralize and make available nutrients for the growth of the consortium itself is much higher than the nutrient reduction in the growth medium may suggest: it does not take into account the amount of nutrients present in the solid fraction of the wastewater, which has been supplied every 4 days to the consortium with the periodic refill. The total amount of nutrients present in the DWW (Supplementary Table 4) was measured thanks to a technical expedient, i.e., wastewater was vigorously shaken before the collection of samples for analyses; the same procedure was been used before every refill. On the base of the total nutrient concentration in DWW, during the entire experiment a total of 2.88 mg N and 112.50 μg P were supplied to each consortium as both solubilized and solid leftover fraction, and took into account the nutrients in the DWW at the start of the experiment (50 mL, containing 1.15 mg N and 45 μg P) and those supplied with the 6 replenishments (as a total, 1.73 mg N and 67.50 μg P). Moreover, at each replenishment some nutrients were removed with the discarded 12.5 mL that was to be changed. These discarded nutrients were quantified as soluble fraction in 0.713 mg N and 0.109 μg P. Solubilized nutrients in growth media were obtained by centrifuging and filtering (0.22 µm) samples. Therefore, as a total, 2.16 mg N and 112.39 μg P were supplied to the system. At the end of the experiment (T28) the residual amounts in the ‘treated’ DWW as soluble nutrients were only 0.25 mg N and 0.02 μg P, with a very high removal rate: 88.6% for N and almost 100% for P, respectively. This removal depended on the combined activity of photosynthetic and heterotrophic microorganisms, representing a robust biological filtering system, as known from the very many studies on the nutrient removal by phototrophic/heterotrophic consortia utilised for wastewater remediation40. The nutrient load in the DWW can be metabolised and removed by heterotrophic bacteria via different pathways for nitrogen41,42 and phosphorus43,44; then, photosynthetic microorganisms assimilate this “new” inorganic nitrogen and phosphorus and convert them into biomass45.
The capability of this T. variabilis based consortium to remove nutrients was already demonstrated in our previous study26 and, notwithstanding the different experimental set up used here, results presented here are in agreement with those reported in that study26; they confirmed the bioremediation capability of this consortium, demonstrating its capability to thrive in dishwasher wastewater by utilising the available nitrogen and phosphorous even under different conditions (different water, microbial content, detergent, food habits). The high efficiency was maintained over time thanks to the consortium growth and the persistence of its taxonomic structure and composition, even though it was subjected to DWW periodic refill.
In DWW, the heterotrophic partners mineralize and make available to the phototrophs the nutrients present in food leftovers, which contribute to the growth of both consortium partners. The superimposition of the growth curve of the phototrophic component of the consortium with the amount of mineralized nitrogen and phosphorous (Fig. 6) showed the relationship between available nutrients and consortium growth, depending on the autotrophic bacteria capability to fix CO2 and convert it into biomass and other valuable products46,47,48.
Nutrients are reported as bar plot: total nitrogen (mg/L) or total phosphorous (µg/L); growth curve of the consortium in the S-Rep test as a blue line (absorbance at λ = 665 nm). The light blue lines, tangent to the curve at the beginning and at the end of the curve, shows the different slope/growth speed at different growth times.
Figure 6 shows how nitrogen and phosphorus availability in the consortium treated DWW fostered the growth of the consortium itself: the high availability of nutrients (mainly nitrogen) until day 12th triggered the consortium growth; from T16 on, the growth flattened with the reduced nutrient availability. This can be inferred also by the different slope of tangents (in light blue) that clings to the growth curve at the beginning and at the end of the growth curve.
Overall, the S-Rep test data demonstrated the capability of the consortium to thrive in periodically refilled DWW and its capability/efficiency in mineralizing the nutrients (almost the nitrogen and phosphorus) present in the food leftovers of the dishwasher wastewater. Furthermore, the efficiency of the consortium lowers the residual amounts of total nitrogen and phosphorus (as soluble fractions in the treated DWW) into the range of values indicated by the urban wastewater dumping Directive 91/271/EEC (Urban Waste Water Treatment Directive), amended by the Directive 98/15/EC49 (with respect to certain requirements of Annex I). Therefore, the treated DWW could be re-introduced into the environment.
Last but not least, this consortium biomass not only represents a tool for wastewater bioremediation but consists of a useful amount of biomass, which can be used as organic fertiliser, the so called microbial fertiliser50.
Although these results refer to a limited refill (¼ of the medium, every 4 days), if compared to the fully operational conditions of the Zero Mile system, which on the average will require a complete change of the dishwasher wastewater every 2 days, the above reported results let us suppose that the consortium is able to persist, maintaining its taxonomic and functional characteristics, even at the fully operational conditions forecast for the Zero Mile system.
To deepen the results obtained in small volumes (S-Rep test), as an intermediate step towards the final operational scale (volumes of litres and complete refill of DWW every 2 days), a halfway experimental setting was performed, the M-Rep test.
M-Rep test: Testing consortium ‘treated’ dishwasher wastewater to fertilise plants
The M-Rep cultures were maintained for 52 days under the same conditions as for the S-Rep test (mentioned above), although upscaled to a 500 mL volume. This test was designed to produce consortium treated wastewater, that was collected from each replicate every 4 days (125 mL, i.e., ¼ of the total volume) and concomitantly utilised for lettuce plant watering and fertilisation.
The evaluation of the consortia thriving on DWW in upscaled conditions showed that the 3D aggregates remained dark green and well structured for the entire duration of the experiment (Fig. 4b), and the culture turbidity in the growth media (as absorbance values at λ = 730 nm), ranged between 0.665 ± 0.001 at T4 to 0.653 ± 0.001 at T52 OD, with a maximum value of 0.784 ± 0.064 at T20 (see Supplementary Table 5), demonstrating the stability of the entire system and confirming the viability of the consortium over time.
The viability of the consortia was further confirmed by the nutrient analyses, which demonstrated the consortium’s efficacy in mineralizing the DWW organic load, including food leftovers. The consortium efficacy was retained even in a 10 times larger volume and over an almost doubled time scale, when compared to the S-Rep test (Table 2): a high and progressive reduction of the nutrient content was found also in upsized dimension, leading to a significant difference of both nitrogen (one-way ANOVA Tukey’s test, F = 23.8, p < 0.001) and phosphorous (one-way ANOVA Tukey’s test, F = 186.4, p < 0.001) from T4 and T52.
The high and significant total nitrogen removal was clearly evident from day 8th on, it remained constant with time (about 41%), indicating a constant consortium removal efficiency over time (Table 2). The delayed onset of the N reduction was probably linked with the time necessary for the attack and degradation of food leftovers by heterotrophic bacteria, as T. variabilis was grown on a N-deployed medium (BG110), so it should not have stocks of the nutrient; furthermore, in the presence of quite high amounts of N, as in the DWW, it should not have activated the resource-consuming N fixation51. Conversely, the high and significant total phosphorus removal showed a different timing: a very low and not significant removal until day 8th (3.5%, p > 0.05), followed by a progressively increasing and significant removal rate at day 12th (56.41% p < 0.001), and a stable and continuous P removal of about 90% from day 16th to 52nd; taken together these results indicated the consortium’s constant removal efficiency overtime (Table 2). A different process was hypothesised for P removal: at the beginning of the experiment, the consortium’s demand for phosphorus is low, probably because the cyanobacterium has been previously grown in a P rich culture medium (BG110, containing K2HPO4), so it should still have reserves of phosphorus, as it is well known that cyanobacteria accumulate phosphorus in their biomass47,52,53,54. It is possible to hypothesise that the P in the cyanobacterial biomass may be utilised for the metabolic needs of both the phototrophic and the heterotrophic bacterial partners, explaining the low phosphorus removal in the first 12 days. In fact, the interactions among consortium partners remain hitherto yet unknown, although several extra- and intra-cellular processes of P removal from the environment in multi-species consortia are known31,44.
Also in this medium-size experiment, the bioremediation efficiency of the consortium in mineralizing and making available nutrients for the consortium growth was much higher than suggested by the nutrient reduction in the growth medium. Again, these data did not take into account the amount of nutrients present in the solid fraction of the wastewater, which was supplied every 4 days to the consortium. As already discussed in the section on the S-Rep test results, in this test, the total amounts of nitrogen and phosphorous supplied during the entire experiment to each consortium was 46 mg N and 1.80 mg P. This included both the solubilized or solid leftover fractions of the nutrients in the DWW at the start of the experiment (500 mL, containing 11.50 mg N and 0.45 mg P) and the nutrients supplied with the 12 replenishments (as a total, 34.50 mg N and 1.35 mg P). From these nutrient figures, the amount removed at each replenishment must be subtracted, i.e., the amount of nutrients removed with the 125 mL of treated DWW to be used for plant watering. They were quantified as soluble fraction and, as a total, they accounted for 20.34 mg N and 0.44 mg P. Hence, a total of 25.66 mg N and 1.36 mg P were supplied to the system during the entire test. At the end of the experiment (T52), the residual amounts in the treated DWW as soluble nutrients were only 4.61 mg N and 0.11 mg P. Hence, the removal rate in this experiment was estimated to be 82.0% for N and 91.7% for P, respectively. Again, also in this M-Rep test, the efficiency of the consortium in lowering total nitrogen and phosphorus was high enough to allow the treated DWW to be re-introduced into the environment according to the Directives 91/271/EEC and 98/15/EC49.
The efficiency of the ad hoc engineered consortium is a pivotal point for the Zero Mile system, being the consortium treated wastewater planned to irrigate plants in the vertical indoor garden of the Zero Mile demonstrator. The growth performance and the organoleptic characteristics of lettuce plants were analysed at the end of the productive cycle, to test the whole system capability to produce reclaimed water useful for plant indoor growth.
Plants growing in the Zero Mile demonstrator and their descriptors
The seedlings of L. sativa planted in the Zero Mile demonstrator were watered with t-DWW or u-DWW, TW or TW + NP. At the end of the growing cycle (52 days) plants treated differently showed different biomass, as both fresh and dry weight (Table 3). Plants watered with t-DWW showed the best growth performance as fresh weight (Two-Way ANOVA, p < 0.05), and both t-DWW and u-DWW showed significantly higher growth as dry weight, if compared to tap water even added with fertiliser. However, the dry/fresh weight ratio accounted for a good plant condition in all the experimental batches, as already shown by Mañas et al. 55 in lettuce plants watered with treated wastewater.
The plants’ good conditions, particularly those under t-DWW treatment, were confirmed by all the descriptors. Photosynthetic pigments content analyses (Table 4) showed that Chl a was found at comparable concentrations in all batches, while Chl b was reduced in t- or u-DWW watered batches (ANOVA, p < 0.01); conversely, Carotenoid content was found significantly higher in t- or u-DWW watered plants (ANOVA, p < 0.01).
In both t-DWW and u-DWW treated plants an increased production of secondary metabolites was found (Table 4): for example, carotenoids were found four times higher than in plants watered with TW and TW + NP. Probably, this large accumulation of carotenoids was linked to the decrease of the Chl b concentration, as they both are accessory pigments that help plants to collect light energy for photosystem II56.
The pigment content variations provide information on the good physiological status of plants57: plants under stress or during leaf senescence show reduced content of individual pigments (chlorophyll and carotenoid), and their relative proportion changes, with chlorophylls tending to decline more rapidly than carotenoids58,59.
Also, phenolic and flavonoid compounds are widely used to evaluate stress or beneficial effects in cultivated plants, even in innovative agriculture (innovative technologies or new substances, as innovative fertilisers60,61): their use makes possible to identify the effect of the application of DWW.
In the lettuce leaves, the mean total phenol and the soluble solid content were the highest in both t- and u-DWW watered plants, while the mean total flavonoids content was the highest in the t-DWW watered plants, with significant differences with tap water watered plants with or without fertiliser (ANOVA, p < 0.01). The increase in soluble solids, a reliable descriptor to evaluate the overall crop quality, indicated that under DWW treatment, the plants increased their concentrations in sugars and other important compounds (i.e., vitamins, fructans, proteins, pigments, phenolics). This may depend either on the increased rate of synthesis of these compounds, or on a decreased turnover rate (or a combination of both62).
From a human standpoint, carotenoids are an important dietary constituent providing health benefits by decreasing disease risks, particularly cancers and eye diseases63; phenolic compounds act as anti-aging, anti-inflammatory, anti-oxidant and anti-proliferative and it is, therefore, beneficial to eat plants having high content of these compounds, which may cut down the incidence of several chronic diseases related to oxidative stress64; finally flavonoids, a specific class of phenolics, are currently of great interest due to their antioxidative and possibly anticarcinogenic activity65.
Crucial hurdles towards human consumption of vegetables produced with the Zero Mile technologies are not expected, since the main requirements for food safety and product quality in UE concern maximum residue levels (MRLs) for pesticides in and on food products, limits for several contaminants (essentially lead, cadmium and nitrate for fresh fruit and vegetables), absence of Salmonella sp. and E. coli. Pesticides haven’t been used in the Zero Mile system and the eventual need of their quantification will be managed according to UE rules in the final application. Lead and cadmium are not expected, since they cannot be present in tap water, eco-labelled detergents and dishwasher components, which are certified against lead, cadmium and other hazardous substances in compliance with the RoHS Directive66. Furthermore, eventual microbial contaminants are removed by the UV treatment present in the Zero Mile system, for the treated wastewater before irrigation.
As a whole, these results confirmed the robustness of the ad hoc engineered consortium, as it effectively bioremediate dishwasher wastewater over a long contact time (from 48 h to 52 days, with refill every 4 days), simulating the operative conditions of the Zero Mile system). Furthermore, they showed the usefulness of consortium reclaimed wastewater for local plant watering and fertilisation at upscaled size of the biofilter, crucial to move forward from a scientific experimentation phase in the laboratory, towards an industrial application.
In conclusion, the efficient biofilter activity of the ad hoc engineered consortium, core of Zero Mile system, on the dishwasher wastewater propels the usefulness of the system, allowing the effective upcycle of reclaimed wastewater in plant watering and fertilisation. This allows the reduction of both the amount of fresh water consumed (by re-using claimed wastewater in both subsequent dishwasher cycle and plant watering), and the amount of wastewater discharged. Furthermore, the characteristics of the reclaimed wastewater foster the quality of the plant food produced, increasing the content of beneficial metabolites. In addition, also the microbial biomass of the biofilter can be up-cycled as a microbial fertiliser, a secondary resource envisaging nutrient profiles fitting the plant’s needs as regard both the direct effects of fertilisation and the added value properties, such as plant disease prevention50.
Hence, besides the reduction of water use and disposal, further benefits of the Zero Mile system in other domains of sustainable development are: the on-site production of vegetables, which allows the users to grow the wanted vegetables and to freshly consume them, along with the gratification of ‘grow your own’ activities67; the improvement of both the quality of indoor environment/airiness68 and the perceived quality of space due to the presence of plant species for food and non-food use. Furthermore, the home-made production of not contaminated edible vegetables may contribute to a sustainable diet, stimulating healthy eating behaviours, and contemporary may reduce the environmental impact due to food production, transport and marketing by standard procedures. Finally, the Zero Mile system encourages a cultural change that, in the long run, can spring from this experience: the acquisition of a different perception about the real value of wastewater and the transition from an abstract concept of recycling to a real one. These changes may trigger a further positive feed-back in consumer lifestyle, pushing citizens to move from a linear economy to a regenerative circular one.
Methods
Consortium assemblage and testing
The ad hoc engineered consortium cyanobacteria/heterotrophic bacteria has been assembled and tested according to ref. 26. The strain of the heterocytous cyanobacterium Trichormus variabilis (Kützing ex Bornet & Flahault) Komárek & Anagnostidis (VRUC168) from the Tor Vergata Rome University Collection (VRUC168; ATCC Strain designation: IUCC 1444; MSU A-37) and three bacterial strains isolated from dishwasher wastewater (Acinetobacter sp., Aeromonas sp. and Exiguobacterium sp.; for strain identification see ref. 26) were assembled as an one-to-three consortium. The consortium has grown in undiluted wastewater, and growth performance compared with the results obtained in the standard culture medium, BG11069. Each growth experiment was carried out in controlled condition in a thermostatic chamber at 25 °C, under 130 µmol photons m−2 s−1 irradiance.
The wastewater was produced by a household dishwasher (Energy Class A+++, Electrolux EES69300L) by using the ‘eco’ programme as washing cycle and an EU Ecolabel certified dishwasher detergent (Everdrop tablets, Germany; for the composition see Supplementary Table 6). The characteristics of both input water (Rome tap water) and dishwasher wastewater were evaluated (Supplementary Table 4); the DWW used throughout the entire experiment was stored under dark at 4 °C, for the entire duration of the tests (untreated; u-DWW).
Semi-batch growth tests were performed by replenishing ¼ of the consortium exposed DWW (treated; t-DWW) with u-DWW, every 4 days. These tests were performed either in ventilated 100 mL polypropylene flasks (Small size-Replenish test, 50 mL medium; S-Rep test) or in 1.0 l glass flasks (Medium size-Replenish test, 500 mL medium; M-Rep test) by using the same amount of bacterial density and ratio. The wastewater was vigorously shaken before collecting DWW for each refill, to collect even the solid fraction of food leftover.
All experiments were set up in at least 3 replicates. During the semi-batch tests consortium viability/growth was measured as in vivo chlorophyll a absorbance and culture turbidity (measured spectrophotometrically at 665 nm and 730 nm, respectively; spectrophotometer ONDA UV-20). At the end of the experiments, the consortium biomass was evaluated as dry weight, measured after 48 hours in oven at 37 °C. In addition, consortium architecture, microorganism spatial distribution and cell viability were evaluated in consortium subsamples collected using a non-destructive and non-invasive method by Confocal Laser Scanning Microscopy (CLSM, Olympus FV1000) after DAPI staining of nucleic acids70 (DAPI, 4′,6-diamidino-2-phenylindole is a fluorescent stain that binds to adenine-thymine-rich regions in DNA), in order to visualise all community members (both autofluorescent and not). This approach has been widely used for different applications and microbial consortia71,72,73.
For DAPI staining 1 mL of consortium culture was centrifuged (2350 g; 5 min; SCILOGEX D30024) and the supernatant discarded. The pellet was fixed with 200 μL of 4% formaldehyde aqueous solution, rinsed with distilled water and treated with 200 μL of 0.3% Triton-X-100 solution for 5 min; after rinsing in distilled water, a 200 μL DAPI stock solution (2 mg/mL in distilled water; stored under dark at −20 °C) was added and the pellet incubated for 30’ under dark. CLSM observations were conducted in a multichannel mode, using 20x UPLSAPO (NA 0.75, WD 0.65 mm) and 60× Plan-Apochromatic (NA 1.42, oil) objectives. The excitation wavelengths were in the UV (405 nm, diode) green (543 nm, Ar/HeNe), and red (636 nm, Ar/HeNe) regions. Data consisted of a set of two dimensional (2D), cross-sectional images in the x-y plane that were captured along the z-axis. The three-dimensional images were obtained through acquisitions in the XY plane, Z step size 60 µm, using the software package IMARIS 6.2.0 software (Bitplane AG, Zurich, Switzerland).
Testing the consortium with periodic replenishment of DWW (S-Rep test)
The S-Rep test has been planned to evaluate the capability of the consortium (i) to face and operate under periodic wastewater refill, as envisaged for the Zero Mile system functioning, and (ii) to modify the concentration of total nitrogen and total phosphorus in the wastewater, by consuming the food leftover. The consortium was assembled and grown in undiluted wastewater under the same operating conditions already reported. Every 4 days, 12.5 mL of t-DWW (25% of a total volume of 50 mL) were removed without touching/damaging the consortium and replaced with the same amount of u-DWW. The test lasted 28 days, for a total of 6 refills. The consortium growth was measured during the entire test as in vivo chlorophyll a absorbance, culture turbidity and final biomass.
Producing consortium ‘treated’ dishwasher wastewater to fertilise plants (M-Rep test)
The M-Rep test has been planned to evaluate the capability of an upsized consortium (respect to the S-Rep test) to face and operate under periodic wastewater refill, and to evaluate the efficiency of ‘treated’ wastewater in fertilising lettuce plants. The consortium was assembled and grown by using the same conditions already reported, but employing a total u-DWW volume of 500 mL. Every 4 days 125 mL of t-DWW were removed without touching/damaging the consortium and the same amount was replaced with u-DWW. The treated dishwasher wastewater collected was utilised to water lettuce plants in the Zero Mile demonstrator (Fig. 7). The test was performed in five replicates, lasted 52 days, the time necessary for lettuce maturity and consisted in a total of 13 refills and the production of 8.125 L of treated wastewater. In this experiment, the consortium growth was evaluated during the entire test only as culture turbidity, to avoid the consortium resuspension necessary to obtain chlorophyll a data).
Nitrogen and phosphorus concentration in consortium exposed/unexposed wastewaters
The concentration of total nitrogen and total phosphorus solubilized in the DWW was assessed in both the consortium treated and untreated dishwasher wastewater. The analyses were performed on centrifuged (3400 g, 15’) and filtered (0.2 µm) DWW and processed according to the Italian official protocol74, as reported in ref. 26 The data were calculated against a calibration curve for each nutrient. As regard nitrogen, the calibration curve was built with a standard solution of NaNO3 in distilled water at 0, 0.625, 1.25, 2.5, 5, 10 and 20 mg/L N; as regard phosphorous, the calibration curve was built with a standard solution of KH2PO4 in distilled water at 0, 6.25, 12.5, 25.0, 50, 100 and 200 µg/L P, subjected to oxidation as indicated by APAT74.
Analysis of consortium structure and composition: DNA extraction and Next Generation Sequencing data processing
A targeted Next Generation Sequencing (NGS) approach was used to analyse the composition of the microbial community associated with samples of: (i) 3D structure of the aggregate cyanobacteria-heterotrophic bacteria, (ii) bacterial suspension from the liquid medium where the 3D aggregate thrives, (iii) T. variabilis culture, and (iv) DWW. Samples were collected every 4 days from T0 to T20 (T = Time; 20 days).
An aliquot of 0.5 mL of each sample was centrifuged (1920g for 10’; SCILOGEX D3024) and metagenomic DNA was extracted from the pellet by using the Power Soil® DNA isolation kit (Mo Bio, Carlsbad, CA, USA), according to the manufacturer’s instructions. The 16 S rDNA has been amplified by PCR with the primers Pro341F (5′-CCTACGGGNBGCASCAG-3′) and Pro805R (5′-GACTACNVGGGTATCTAATCC-3′) encompassing the V3-V4 region of the 16 S rDNA gene and suitable to identifying bacteria and archaea75. Amplicons were obtained by an external facility (BMR Genomics, Padua, Italy) and subjected to NGS by using an Illumina MiSeq platform. The raw paired-end sequences obtained were analysed by using the Quantitative Insights Into Bacterial Ecology software (QIIME 2.1076). Briefly, the demultiplexed raw pair-end sequences were quality filtered, chimera checked and pair matched for merging, by the DADA2 plugin77. Taxonomic identification of the 16 S rRNA gene sequences was performed by using a naïve Bayes classifier trained with the SILVA 138 SSU database78. The obtained Amplicon Sequence Variant (ASV) were clustered into Operational Taxonomic Units (OTUs) by using a 97% identity threshold.
To assess alfa- and beta-diversity the dataset was resampled at 2048 sequences, allowing the analyses of 26 samples through ANOVA One-Way post hoc Tukey’s and PERMANOVA tests, respectively. Raw data of this Targeted Locus Study project has been deposited at NCBI Sequence Read Archive (SRA) BioProject number: PRJNA942250.
Plant testing and descriptors
The plant testing has been conducted using Lactuca sativa L. (Asteraceae), a cool-season crop that grows quickly and is not very demanding, thriving even in small spaces if sufficiently watered.
Twenty-four seedlings (each of 3.44 ± 0.29 g fresh weight and 0.348 ± 0.03 g dry weight; Fig. 8) were planted in the plant dedicated structure (8 pots, 60 × 15 × 10 cm) of the Zero Mile demonstrator, 3 per pot, by using a commercial soil (Dorater, Vigour Plant). Plants were divided in 4 experimental batches (2 pots each), watered every second day with 40 mL of: (i) consortium t-DWW; (ii) u-DWW (containing 23 mg/L N); (iii) tap water (TW; Rome water main); (iv) tap water added with 22.3 mg/L N (TW + NP; NPK 19-19-19 fertiliser). The Zero Mile demonstrator was placed at room temperature (May-June) under continuous light (irradiance: 130 mM photons m−2s−1). After 52 days, all plants were collected and, for each experimental batch, three plants were weighted (fresh and dry weight) to quantify the final plant biomass, while three plants were used to quantify the plant descriptors: total phenols and flavonoids, soluble solid content.
Soluble solid content (SSC)
Total soluble solids content of a solution is referred to as ‘degrees Brix’ and is measured via its specific gravity using a refractometer, by a refraction index. The refraction index varies according to the solids’ content, which is able to modify the light path79. Brix measurements assume that the dissolved solids consist entirely of sucrose. Nevertheless, in most fruits and vegetables dissolved solids also include small amounts of dissolved vitamins, fructans, proteins, pigments, phenolics, and minerals80: for this reason, the descriptor is considered accurate to evaluate the overall crop quality. According to Braglia et al.81, SSC determination was made on 1.5 g fresh weight of each plant replicate to detect sugars and the other soluble solids in the extracts82. Samples were homogenised in liquid nitrogen with mortar and pestle, then centrifuged at 6089 g for 10’. Aliquots of 100 µL of supernatant were collected and analysed by a digital refractometer (Hanna HI96800, Woonsocket, RI, USA).
Photosynthetic pigment content
Pigments were determined on samples of 500 mg fresh weight of each plant replicate by homogenisation in liquid nitrogen with mortar and pestle, followed by extraction in 80% acetone for 24 h, at 4 °C under dark. The extract was then centrifuged (3000 g; 10’) and supernatant collected for pigment determination. Chlorophyll a (Chl a), b (Chl b) and carotenoids (Car) content was measured spectrophotometrically at 663 nm, 644 nm and 452 nm, respectively (spectrophotometer Hanna HI801, Woonsocket, RI, USA). Pigments concentration in each extract (as µg g-1 of fresh weight, FW) were calculated according to Lichtenthaler83.
Total phenols and flavonoids content
Aliquots of 500 mg (fresh weight) of leaves for each replicate were homogenised in liquid nitrogen with mortar and pestle, then extracted overnight at room temperature in 4 mL of absolute methanol, under continuous mixing (orbital shaker; 110 rpm). Each extract was then centrifuged (5.000 g; 20’) and supernatant was utilised for both total phenol and flavonoids quantification.
Total phenol content was quantified in duplicate according to Folin-Ciocalteu procedure84. Aliquots of 200 µL of plant extract were mixed with 1000 µL of Folin-Ciocalteu reagent (1:10 v/v in distilled water) and 800 µL of 1 M Na2CO3 and the mixture incubated (60’) at room temperature. The absorbance was measured spectrophotometrically at 765 nm and results compared to a reference curve of gallic acid (0–250 mg/L−1). Total phenol content was expressed as mg gallic acid equivalent (GAE) g−1 plant fresh weight.
Total Flavonoid content was quantified by the aluminium chloride colorimetric method85. 200 µL of plant extract were mixed with 40 µL of 10% AlCl3, 40 µL of 1 M CH3COOK, 600 µL of absolute methanol and 1120 µL of dH2O and maintained at room temperature for 30’. The absorbance was measured spectrophotometrically at 415 nm and results compared to a reference curve of quercetin (0–250 µg/mL−1). Total flavonoid content was expressed as mg quercetin equivalent (QE) g−1 plant fresh weight.
Statistical analysis
Two-Way ANOVA and Sidak’s multiple comparisons test were performed to compare the growth in DWW and control medium (BG110) of the S-Rep test consortia. Two-way ANOVA and post hoc Tukey’s have been used (i) to compare the solubilized total nitrogen and phosphorus content among each batch of both the S-Rep test and the M-Rep test, (ii) to evaluate differences in plant growth as both fresh and dry weight. One-way ANOVA and post hoc Tukey’s have been used to compare the amount of plant biomass at the end of the experiment among each batch of the M-Rep test. Shapiro–Wilk test was used to confirm the normal distribution of plant descriptors data, and One-way ANOVA test and post hoc Tukey’s test was applied for multiple comparison.
GraphPad Prism 8.0.2 for Windows (GraphPad Software, San Diego, California USA) and PAST software (Øyvind Hammer, Oslo, Norway) have been utilised to analyse both plant descriptors and NGS data.
Data availability
The raw data of the Targeted Locus Study project included in this study has been deposited at NCBI Sequence Read Archive (SRA) BioProject number: PRJNA942250.
References
Van Kruchten, S. & Van Eijk, F. Circular economy and SDGs. How circular economy practices help to achieve the Sustainable Development Goals, 1–77 (Netherland Enterprise Agency, The Netherlands, 2020).
Schroeder, P., Anggraeni, K. & Weber, U. The relevance of Circular economy practices to the sustainable development Goals. J. Ind. Ecol. 23, 77–95 (2019).
Gleick, P. H. & Palaniappan, M. Peak water limits to freshwater withdrawal and use. Proc. Natl Acad. Sci. 107, 11155–11162 (2010).
The United Nations World Water Development Report 2017. Wastewater: The Untapped Resource (Paris, UNESCO, 2017). https://www.unesco.org/en/wwap/wwdr/2017.
Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy (2000). https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32000L0060.
Potable Reuse Compendium. Office of Ground Water and Drinking Water, Office of Water. U.S. Environmental Protection Agency EPA/810/R-17/002 (Washington, DC, 2017). https://www.epa.gov/ground-water-and-drinking-water/2017-potable-reuse-compendium.
Lyu, S., Wu, L., Wen, X., Wang, J. & Chen, W. Effects of reclaimed wastewater irrigation on soil-crop systems in China: a review. Sci. Total Environ. 813, 152531 (2022).
Cano-Ruiz, J., Ruiz Fernández, J., Alonso, J., Mauri, P. V. & Lobo, M. C. Value-added products from wastewater reduce irrigation needs of Arundo donax energy crop. Chemosphere 285, 131485 (2021).
Gross, A. et al. Environmental impact and health risks associated with greywater irrigation: a case study. Water Sci. Technol. 52, 161–169 (2005).
Muñoz, R. & Guieysse, B. Algal-bacterial processes for the treatment of hazardous contaminants: a review. Water Res. 40, 2799–2815 (2006).
Liu, J. et al. Advanced nutrient removal from surface water by a consortium of attached microalgae and bacteria: a review. Bioresour. Technol. 241, 1127–1137 (2017).
Posadas, E. et al. Microalgae cultivation in wastewaters. in Microalgae-Based Biofuels and Bioproducts (eds. Muñoz, R. & González, C.) 67–91 (Elsevier, 2017).
Fouilland, E. Biodiversity as a tool for waste phycoremediation and biomass production. Rev. Environ. Sci. Biotechnol. 11, 1–4 (2012).
Johnson, D. R., Goldschmidt, F., Lilja, E. E. & Ackermann, M. Metabolic specialization and the assembly of microbial communities. ISME J. 6, 1985–1991 (2012).
Muñoz, R., Köllner, C. & Guieysse, B. Biofilm photobioreactors for the treatment of industrial wastewaters. J. Hazard. Mater. 161, 29–34 (2009).
Ferreira, A. et al. Combining wastewater treatment with biofuel/bioproduct production through microalgae in the frame of biorefinery. in Grand Challenges in Biology and Biotechnology (eds. Hallmann, A. & Rampelotto, P. H.) 319–369 (Springer Nature Switzerland, 2019).
Raschid-Sally, L. & Jayakody, P. Drivers and characteristics of wastewater agriculture in developing countries: results from a global assessment. International Water Management Institute, Research Report 127 (2008).
Boyano, A. & Moons, H. Analysing of the impacts of the revision of Ecodesign and Energy Label Regulations for household dishwashers by using a stock model. Energy Effic. 13, 1147–1162 (2020).
Migliore L. et al. Patent: system for recycling wastewater resulting from the washing and rinsing process of a dishwasher, especially for reuse in said process and for irrigation of plants, and process for the use of said system. WIPO/PCT Int. Publ. Number WO 2020/194224 A1 (2020)
Venkatesh, G. Dishwashers: literature review to summarise the multi-dimensionality of sustainable production and consumption. Sustainability 14, 10302 (2022).
Cisneros, B. J. Water reclamation and sustainability in Water reclamation and sustainability (ed Ahuja, S.) 431–454 (Elsevier, 2014).
Lazarova, V. & Bahri, A. Water reuse practices for agriculture in Water reuse: an international survey of current practice, issues and needs (eds Jimenez, B. & Asano, T.) IWA Scientific and Technical Report 20, 199–227 (IWA Publishing, 2008).
Sardar, S. W., Choi, Y., Park, N. & Jeon, J. Occurrence and concentration of chemical additives in consumer products in Korea. Int. J. Environ. Res. Public Health 16, 5075 (2019).
Posadas, E. et al. Carbon and nutrient removal from centrates and domestic wastewater using algal–bacterial biofilm bioreactors. Bioresour. Technol. 139, 50–58 (2013).
Costa, F. et al. Designing the future: an intelligent system for zero-mile food production by upcycling wastewater. Proceedings 2, 1367 (2018).
Congestri, R. et al. Developing a microbial consortium for removing nutrients in dishwasher wastewater: towards a biofilter for its up-cycling. Water Sci. Technol. 82, 1142–1154 (2020).
Dubois, O. The state of the world’s land and water resources for food and agriculture (SOLAW)—Managing systems at risk (Earthscan, 2011).
D’Ostuni, M., Zaffi, L., Appolloni, E. & Orsini, F. Understanding the complexities of Building-Integrated Agriculture. Can food shape the future built environment? Futures 144, 103061 (2022).
Zaffi, L. & D’Ostuni, M. Metabolic cities of the future. Between agriculture and architecture. AGATHÓN. Int. J. Archit. Art. Des. 8, 82–93 (2020).
Technology readiness levels (TRL); Extract from Part 19 - Commission Decision C(2014)4995 (PDF).ec.europa.eu. 2014. Retrieved 11 November 2019.
Di Pippo, F. et al. Effect of light and temperature on biomass, photosynthesis and capsular polysaccharides in cultured phototrophic biofilms. J. Appl. Phycol. 24, 211–220 (2012).
Bellini, M. I., Kumaresan, D., Tarlera, S., Murrell, J. C. & Fernandez-Scavino, A. Identification of active denitrifiers by DNA-stable isotope probing and amplicon sequencing reveals Betaproteobacteria as responsible for attenuation of nitrate contamination in a low impacted aquifer. FEMS Microbiol. Ecol. 94, fix181 (2018).
Redfield, R. J. Is quorum sensing a side effect of diffusion sensing? Trends Microbiol. 10, 365–370 (2002).
Ricci, R., Polidoro, F. Consumer prices (Provisional data) June 2022. ISTAT Press release (2022). https://www.istat.it/it/files//2022/07/Consumer-prices_Prov_June2022.pdf.
Gonçalves, A. L., Pires, J. C. & Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 24, 403–415 (2017).
Raghupathi, P. K. et al. Microbial diversity and putative opportunistic pathogens in dishwasher biofilm communities. Appl. Environ. Microbiol. 84, e02755–17 (2018).
Zupančič, J., Turk, M., Črnigoj, M., Avguštin, J. A. & Gunde-Cimerman, N. The dishwasher rubber seal acts as a reservoir of bacteria in the home environment. BMC Microbiol. 19, 1–15 (2019).
Tucker, C. S. & D’Abramo, L. R. Managing high pH in freshwater ponds. SRAC Publ. 4604, 1–5 (2008). 2008.
Guzzon, A., Di Pippo, F. & Congestri, R. Wastewater biofilm photosynthesis in photobioreactors. Microorganisms 7, 252 (2019).
Wang, X. & Hong, Y. Microalgae biofilm and bacteria symbiosis in nutrient removal and carbon fixation from wastewater: a review. Curr. Pollut. Rep. 8, 128–146 (2022).
Yao, S., Ni, J., Ma, T. & Li, C. Heterotrophic nitrification and aerobic denitrification at low temperature by a newly isolated bacterium, Acinetobacter sp. HA2. Bioresour. Technol. 139, 80–86 (2013).
Fitzgerald, C. M., Camejo, P., Oshlag, J. Z. & Noguera, D. R. Ammonia-oxidizing microbial communities in reactors with efficient nitrification at low-dissolved oxygen. Water Res. 70, 38–51 (2015).
Schmidt, J. J., Gagnon, G. A. & Jamieson, R. C. Microalgae growth and phosphorus uptake in wastewater under simulated cold region conditions. Ecol. Eng. 95, 588–593 (2016).
Xu, Y., Wu, Y., Esquivel-Elizondo, S., Dolfing, J. & Rittmann, B. E. Using microbial aggregates to entrap aqueous phosphorus. Trends Biotechnol. 38, 1292–1303 (2020).
Fallahi, A., Hajinajaf, N., Tavakoli, O. & Sarrafzadeh, M. H. Cultivation of mixed microalgae using municipal wastewater: biomass productivity, nutrient removal, and biochemical content. Iran. J. Biotechnol. 18, 88–97 (2020).
Di Pippo, F., Bohn, A., Congestri, R., De Philippis, R. & Albertano, P. Capsular polysaccharides of cultured phototrophic biofilms. Biofouling 25, 495–504 (2009).
Gismondi, A., Pippo, F. D., Bruno, L., Antonaroli, S. & Congestri, R. Phosphorus removal coupled to bioenergy production by three cyanobacterial isolates in a biofilm dynamic growth system. Int. J. Phytoremediat. 18, 869–876 (2016).
Srisawat, P., Higuchi-Takeuchi, M. & Numata, K. Microbial autotrophic biorefineries: perspectives for biopolymer production. Polym. J. 54, 1139–1151 (2022).
European Parliament and Council of the European Union, Directive 98/15/EC amending certain requirements of Annex I Directive 91/271/EEC (1998). https://op.europa.eu/en/publication-detail/-/publication/ff7ec087-8cc3-4619-bffc-b08ea4883d2c.
Wambacq, E. et al. Aerobes and phototrophs as microbial organic fertilizers: exploring mineralization, fertilization and plant protection features. PLoS One 17, e0262497 (2022).
Zhang, W., Chen, Y., Huang, K., Wang, F. & Mei, Z. Molecular mechanism and agricultural application of the NifA–NifL system for nitrogen fixation. Int. J. Mol. Sci. 24, 907 (2023).
Guzzon, A., Bohn, A., Diociaiuti, M. & Albertano, P. Cultured phototrophic biofilms for phosphorus removal in wastewater treatment. Water Res. 42, 4357–4367 (2008).
Voronkov, A. & Sinetova, M. Polyphosphate accumulation dynamics in a population of Synechocystis sp. PCC 6803 cells under phosphate overplus. Protoplasma 256, 1153–1164 (2019).
Wang, X. et al. Highly effective polyphosphate synthesis, phosphate removal, and concentration using engineered environmental bacteria based on a simple solo medium-copy plasmid strategy. Environ. Sci. Technol. 52, 214–222 (2018).
Mañas, P., Castro, E. & de Las Heras, J. Irrigation with treated wastewater: effects on soil, lettuce (Lactuca sativa L.) crop and dynamics of microorganisms. J. Environ. Sci. Health A 44, 1261–1273 (2009).
Häder, D. P. Photosynthesis in plants and algae. Anticancer Res. 42, 5035–5041 (2022).
Sims, D. A. & Gamon, J. A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 81, 337–354 (2002).
Biswall, B. Carotenoid catabolism during leaf senescence and its control by light. J. Photochem. Photobiol. B Biol. 30, 3–13 (1995).
Merzlyak, M. N. et al. Reflectance spectra of leaves and fruits during their development and senescence and under stress. Russ. J. Plant Physiol. 44, 614–622 (1997).
Ganugi, P., Martinelli, E. & Lucini, L. Microbial biostimulants as a sustainable approach to improve the functional quality in plant-based foods: a review. Curr. Opin. Food Sci. 41, 217–223 (2021).
Keshavarz Mirzamohammadi, H., Modarres-Sanavy, S. A. M., Sefidkon, F., Mokhtassi-Bidgoli, A. & Mirjalili, M. H. Irrigation and fertilizer treatments affecting rosmarinic acid accumulation, total phenolic content, antioxidant potential and correlation between them in peppermint (Mentha piperita L.). Irrig. Sci. 39, 671–683 (2021).
Baxter, C. J. et al. Comparison of changes in fruit gene expression in tomato introgression lines provides evidence of genome-wide transcriptional changes and reveals links to mapped QTLs and described traits. J. Exp. Bot. 56, 1591–1604 (2005).
Johnson, J. E. The role of carotenoids in human health. Nutr. Clin. Care. 5, 56–65 (2002).
Lin, D. et al. An overview of plant phenolic compounds and their importance in human nutrition and management of Type 2 Diabetes. Molecules 21, 1374 (2016).
Ghasemzadeh, A. & Ghasemzadeh, N. Flavonoids and phenolic acids: role and biochemical activity in plants and human. J. Med. Plants Res. 5, 6697–6703 (2011).
Directive 2011/65/EU of the European Parliament and of the Council, 08/06/2011 on the restriction of the use of certain hazardous substances in electrical and electronic equipment.
Costa. F. & Nebuloni, A. The Jetsons’ Kitchen. A Zero-Mile system for waste water recycling and cultivation (eds Costa, F. & Nebuloni, A.) 55–62 (Franco Angeli, 2021).
Brilli, F. et al. Plants for sustainable improvement of indoor air quality. Trends Plant Sci. 23, 507–512 (2018).
Rippka, R., Deruelles, J., Waterbury, J. B., Herdman, M. & Stanier, R. Y. Generic assignments, strain histories and properties of pure cultures of Cyanobacteria. J. Gen. Microbiol. 111, 1–61 (1979).
Chazotte, B. Labeling nuclear DNA using DAPI. Cold Spring Harb. Protoc. 2011, 80–82 (2011).
Di Pippo, F. & Congestri, R. Culturing toxic benthic blooms: the fate of natural biofilms in a microcosm system. Microorganisms 5, 46 (2017).
Di Gregorio, L., Rossetti, S., Tandoi, V., Congestri, R. & Di Pippo, F. Unravelling the core microbiome of biofilms in cooling tower systems. Biofouling 33, 793–806 (2017).
Di Pippo, F., Di Gregorio, L., Congestri, R., Tandoi, V. & Rossetti, S. Biofilm growth and control in cooling water industrial systems. FEMS Microbiol. Ecol. 94, fiy044 (2018).
APAT IRSA-CNR Azoto totale e fosforo totale in Metodi analitici per le acque (eds Belli, M. et al.) 29/2003, 537–540 (I.G.E.R. srl, 2004).
Takahashi, S., Tomita, J., Nishioka, K., Hisada, T. & Nishijima, M. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS One 9, e105592 (2014).
Bolyen, E. et al. QIIME 2: reproducible, interactive, scalable, and extensible microbiome data science. Nat. Biotechnol. 37, 852–857 (2019).
Callahan, B. J., McMurdie, P. J. & Holmes, S. P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 11, 2639–2643 (2017).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2012).
Wilson, D. Chemical sensors for farm-to-table monitoring of fruit quality. Sensors 21, 1634 (2021).
Magwaza, L. S. & Opara, U. L. Analytical methods for determination of sugars and sweetness of horticultural products—a review. Sci. Hortic. 184, 179–192 (2015).
Braglia, R. et al. Phytochemicals and quality level of food plants grown in an aquaponics system. J. Sci. Food Agric. 102, 844–850 (2022).
Jaywant, S. A., Singh, H. & Arif, K. M. Sensor and instruments for Brix measurement: a review. Sensors 22, 2290 (2022).
Lichtenthaler, H. K. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes in Methods in enzymology (eds Colowick, S. P. & Kaplan, N. O.) Vol 148, 350–382 (Academic Press, 1987).
Di Marco, G. et al. Tetracycline accumulates in Iberis sempervirens L. through apoplastic transport inducing oxidative stress and growth inhibition. Plant Biol. 16, 792–800 (2014).
Chang, C. C., Yang, M. H., Wen, H. M. & Chern, J. C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 10, 178–182 (2002).
Acknowledgements
The authors are grateful to Giorgio Buratti, Matteo Meraviglia, Attilio Nebuloni, Cristina Sambinelli and Giorgio Vignati (Polytechnic of Milan) for the development of the Zero Mile demonstrator, to Greta Ponsecchi (Tor Vergata—Ph.D. Programme in Evolutionary Biology and Ecology) for her advice in microbial test settings, and to Ilaria Spagnolo and Noluthando Tshuma (Tor Vergata Master of Science students) for the technical help in microbial and plant tests. A.A. and S.F. had a 3 years of Ph.D. scholarship from the University of Rome: A.A. had an industrial Doctorate in collaboration with the Algares s.r.l.; S.F. had a PON Doctorate in collaboration with the GT50 s.r.l. company. Data elaboration has been performed thanks to the ISCRA CINECA High Performance Computing Class C Project named ‘The Jetsons’ Kitchen project’ (HP10CF8YO) on Galileo100 server. Authors wish to thank the Centre of Advanced Microscopy ‘P. Albertano’ (Department of Biology, University of Rome ‘Tor Vergata’, Rome, Italy) for the analyses at the Confocal Laser Scanning Microscope. Last but not least, the authors are obliged to Prof. Gabriele Gentile for hosting the Zero Mile demonstrator in one of the laboratories of his research group, allowing this experimentation.
Author information
Authors and Affiliations
Contributions
A.A., R.C., F.C., M.M.D. and L.M. designed the study; A.A., S.F. and V.C. performed the microbial and plant tests; A.A., M.M.D. and S.F. performed data collection and data analysis; F.C. designed and produced the Zero Mile demonstrator; A.A. and R.C. performed the analysis by Confocal Laser Scanning Microscopy (CLSM); S.S. supervised the nutrient analyses; L.M. and M.M.D. supervised the statistical analyses; R.B. and F.S. analysed plant quality descriptors. A.A. and L.M. drafted the manuscript. All authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript, based on the accuracy/integrity with which the work has been performed in all its part.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alabiso, A., Frasca, S., Cantelmo, V. et al. A dirty job: dishwasher wastewater reuse and upcycle through an ad hoc engineered microbial consortium. npj Clean Water 6, 66 (2023). https://doi.org/10.1038/s41545-023-00280-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41545-023-00280-8