Abstract
Accumulating evidence suggests that imbalanced oxidative stress (OS) may contribute to the mechanism of schizophrenia. The aim of the present study was to evaluate the associations of OS parameters with psychopathological symptoms in male chronically medicated schizophrenia (CMS) and treatment-resistant schizophrenia (TRS) patients. Levels of hydrogen peroxide (H2O2), hydroxyl radical (·OH), peroxidase (POD), α-tocopherol (α-toc), total antioxidant capacity (TAC), matrix metalloproteinase-9 (MMP-9), and tissue inhibitor of metalloproteinases-1 (TIMP-1) were assayed in males with CMS and TRS, and matched healthy controls. Schizophrenia symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS). The results demonstrated significant differences in the variables H2O2 (F = 5.068, p = 0.008), ·OH (F = 31.856, p < 0.001), POD (F = 14.043, p < 0.001), α-toc (F = 3.711, p = 0.027), TAC (F = 24.098, p < 0.001), and MMP-9 (F = 3.219, p = 0.043) between TRS and CMS patients and healthy controls. For TRS patients, H2O2 levels were correlated to the PANSS positive subscale (r = 0.386, p = 0.032) and smoking (r = −0,412, p = 0.021), while TAC was significantly negatively correlated to the PANSS total score (r = −0.578, p = 0.001) and POD and TAC levels were positively correlated to body mass index (r = 0.412 and 0.357, p = 0.021 and 0.049, respectively). For patients with CMS, ·OH levels and TAC were positively correlated to the PANSS general subscale (r = 0.308, p = 0.031) and negatively correlated to the PANSS total score (r = −0.543, p < 0.001). Furthermore, H2O2, α-toc, and ·OH may be protective factors against TRS, and POD was a risk factor. Patients with CMS and TRS exhibit an imbalance in OS, thus warranting future investigations.
Similar content being viewed by others
Introduction
Schizophrenia is a chronic, severe, and often life-long mental disorder in which approximately half of patients do not respond well to medication, resulting in significant functional deficits1,2. Although atypical antipsychotics can be used to treat schizophrenia, 20–50% of patients are defined as having treatment-resistant schizophrenia (TRS), resulting in increased costs by 3−11 fold as compared to patients in remission3,4. Furthermore, clozapine is prescribed 8% more often for men with TRS than women, indicating the prevalence of TRS among males5,6. However, the underlying etiopathology of TRS remains relatively unknown, although interactions among multiple etiologies are suspected.
Neurobiological mechanisms potentially associated with TRS include dopamine super-sensitivity, hyperdopaminergic subtypes, normal dopaminergic subtypes, dysregulation of glutamate and serotonin, oxidative stress (OS), and inflammation7. A previous study suggested that combined deletion polymorphisms of glutathione S-transferase theta 1 and glutathione S-transferase mu 1 were associated with a 4.6-fold greater risk of TRS8. OS is a normal and complex physiological process in healthy individuals involving the production and neutralization of reactive oxygen species (ROS) and reactive nitrogen species, of which hydrogen peroxide (H2O2), catalase (CAT), superoxide dismutase (SOD), malondialdehyde (MDA), glutathione peroxidase (GSH-Px), nitric oxide synthase, and total antioxidant capacity (TAC) are important parameters of physiological functions9. As shown in Fig. 1. Many previous studies have reported that an imbalance of OS plays a vital role in the pathophysiological mechanism of schizophrenia. In these studies, abnormal levels of OS parameters, such as SOD, MDA and total oxidant status, were associated with the severity of clinical symptoms of schizophrenia10,11,12. Regarding the non-enzymatic antioxidant system, α-tocopherol (α-toc, vitamin E) levels are reportedly elevated during the acute phase of schizophrenia, while decreased in patients with chronic schizophrenia13. OS is thought to be associated with imbalances in specific neurotransmitter systems, which may exacerbate psychotic symptoms and reduce treatment responsiveness in patients with TRS7,14. TRS patients might have compromised antioxidative defenses, rendering them more susceptible to free radical damage that can aggravate their condition15,16. Long-term antipsychotic treatment may increase the production of free radicals or decrease the levels of antioxidants, disrupting the balance between oxidation and antioxidation17. Therefore, therapeutic strategies targeting OS, such as supplementing with antioxidants like vitamin E and N-acetylcysteine18,19,20, may benefit patients with schizophrenia and those with chronic-medicated schizophrenia (CMS).
In some conditions, under the action of Nitric Oxide Synthase (NOS) and Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidases, superoxide anions (O2−) are formed within the body. These O2− are rapidly converted into H2O2 by the enzyme superoxide dismutase (SOD). H2O2 is then decomposed into H2O, oxygen (O2), or hydroxyl radicals (·OH) through the action of enzymes like catalase (CAT), glutathione peroxidase (GPx), and peroxidase (POD). H2O2 participates in various signaling pathways of the oxidation and antioxidant systems. These mechanisms collectively maintain redox balance, protecting the organism from oxidative stress (OS) damage.
Furthermore, accumulating evidence suggests that serum levels of redox biomarkers, such as CAT, GSH-Px, reduced glutathione (GSH), activator protein 1, TAC, kynurenine, and total oxidant status, could be useful as additional parameters to differentiate schizophrenia patients from healthy individuals21. Xie et al. found that plasma TAC levels were significantly lower in first-episode drug-naïve schizophrenic patients and associated with cognitive deficits in some domains22. TAC was reportedly elevated at both 8 and 26 weeks in schizophrenia patients who received n-3 polyunsaturated fatty acids as an add-on therapy23. Decreased TAC indicates impaired antioxidant defenses, providing support for the involvement of OS in the altered pathophysiology of schizophrenia.
H2O2 and hydroxyl radicals (·OH) are crucial ROS, with H2O2 playing a particularly significant role in reversible oxidation of key redox-sensitive cysteine residues of target proteins24. H2O2 is converted to ·OH by a variety of enzymes, such as CAT and peroxidase (POD), but can cause damage to lipids, proteins, and DNA, resulting in cellular dysfunction and even death25. Non-classical mechanisms mediated by H2O2 inhibit the release of dopamine that is otherwise modulated by neurotransmitters such as glutamate and γ-aminobutyric acid (GABA), and this modulation of DA release by glutamate and GABA depends on H2O2 generated downstream from AMPA receptors26, suggesting the potential involvement of H2O2 in the pathology of schizophrenia.
An imbalance in OS and even interactions with other mechanisms, such as neuroinflammation, in the pathophysiology of schizophrenia have, therefore, been the focal points of intense investigations. A systematic and comprehensive review showed that increased production of ROS stimulated by abnormal signals of the central nervous system, imbalance of redox potential, altered gene expression, neurotransmitter abnormalities, and immune dysfunction during OS were related to schizophrenia27. In glutamate-cysteine ligase modifier knockout mice, a vicious cycle between OS and neuroinflammation was found to have long-term effects on parvalbumin fast-spiking interneurons and integrity of perineuronal nets, which have been implicated in neural synchronization, in addition to cognitive, emotional, social, and sensory deficits28. Disrupted redox responses lead to the activation of matrix metalloproteinase-9 (MMP-9), triggering a complex interplay among MMP-9, receptor for advanced glycation end-products shedding, proinflammatory cytokine activation, and microglial cell activation29. This intricate network forms the link between OS and neuroinflammation.
MMP-9 is a member of the matrix metalloproteinase family, which comprises a group of zinc-dependent proteases that degrade various components of the extracellular matrix, leading to tissue remodeling and repair30. Tissue inhibitor of metalloproteinases-1 (TIMP-1) acts as an endogenous inhibitor that tightly controls the activity of MMP-9 and prevents excessive degradation of the extracellular matrix31,32. Furthermore, MMP-9 is regarded as a significant neuroinflammatory factor that plays a pivotal role in learning, memory, and cortical plasticity. Meanwhile, dysregulation of MMP-9 has been linked to multiple disorders, including schizophrenia, autism spectrum disorders, and epilepsy33,34,35. Several studies have reported that MMP-9, either alone or in conjunction with TIMP-1 or brain-derived neurotrophic factor, holds promise as a potential biomarker for specific conditions36.
Although multiple clinical and preclinical studies have demonstrated an imbalance in OS, dysfunction in oxidative defense, and abnormal neuroinflammation in patients with schizophrenia, the results are inconsistent. These discrepancies may be related to multiple factors, such as sex, age, cohort size, and the type of prescribed antipsychotic medication. In addition, the findings vary across different stages of the disorder, including patients with acute-phase schizophrenia, first-episode drug-naïve schizophrenia, and long-term medicated schizophrenia, as relatively few studies have specifically focused on TRS37.
As previously mentioned, OS parameters (i.e., H2O2, ·OH, POD, TAC, α-toc), MMP-9, and TIMP-1 may be involved in the underlying pathophysiology of schizophrenia. Therefore, male patients with TRS and CMS were recruited to determine (1) whether there are differences in OS parameters, MMP-9, and TIMP-1 between schizophrenia patients and healthy controls; (2) whether alterations to OS parameters, MMP-9, and TIMP-1 are related to the severity of clinical symptoms; (3) whether serum MMP-9 or TIMP-1 and plasma OS parameters levels are independently or interactively correlated with the clinical features of schizophrenia patients; and (4) whether OS parameters and MMP-9 and TIMP-1 levels are predictive of the prognosis of TRS. To the best of our knowledge, this is the first study to investigate the relationships among H2O2, ·OH, POD, TAC, α-toc, MMP-9, and TIMP-1, and the clinical symptoms of male patients with TRS.
Results
Comparison of demographic and general clinical data
The demographic information and clinical data of TRS and CMS patients and the healthy controls are presented in Table 1. There were no significant differences of age, education, BMI, and smoking status among the TRS, CMS and healthy control groups (all p > 0.05). Also, there was no significant differences in age of onset between the TRS and CMS groups (F = 1.428, p = 0.236). However, there were significant differences in the duration of illness, PANSS total score, PANSS subscale scores, and equivalent dose of chlorpromazine (all, p < 0.05).
Plasma levels of OS parameters, MMP-9, and TIMP-1
The results of multivariate analysis of covariance showed that the effect of diagnosis was significant (Wilks’s lambda, F = 6.719, p < 0.001). ANOVA revealed significant differences in the variables H2O2 (F = 5.068, p = 0.008), ·OH (F = 31.856, p < 0.001), POD (F = 14.043, p < 0.001), α-toc (F = 3.711, p = 0.027), TAC (F = 24.098, p < 0.001), log MMP-9 (F = 3.219, p = 0.043), and log TIMP-1 (F = 0.604, p = 0.548) among the TRS, CMS, and healthy control groups (Table 2).
Post hoc comparisons with the Bonferroni correction method indicated that H2O2 levels were significantly lower in CMS patients than the healthy controls (p = 0.007), ·OH and TAC were significantly lower in TRS and CMS patients as compared to the healthy controls (p < 0.001), and α-toc levels were lower in TRS patients than the healthy controls (p = 0.028), while there were no significant differences in H2O2, ·OH, α-toc, and TAC levels between patients with TRS and CMS (all, p > 0.05). Additionally, POD activities were significantly decreased in CMS patients as compared to TRS patients (p = 0.004) and healthy controls (p < 0.001), while there was no significant difference between the TRS patients and healthy controls (p = 0.442). After Bonferroni correction, there were no significant differences in log MMP-9 and log TIMP values (all, p > 0.05) (Fig. 2).
Furthermore, the results of analysis of covariance revealed significant differences in plasma levels of ·OH (F = 27.852, p < 0.001), POD (F = 12.742, p < 0.001), α-toc (F = 4.141, p = 0.018), and TAC (F = 20.895, p < 0.001), and log MMP-9 values (F = 3.123, p = 0.047) among the TRS, CMS, and healthy control groups after correction for age, education, BMI, and smoking status. However, there were no significant differences in levels of H2O2 and log TIMP among the TRS, CMS, and healthy control groups after multiple corrections (F = 2.962 and 0.754, p = 0.055 and 0.472, respectively).
Correlations among OS parameters, MMP-9, and TIMP-1 and clinical symptoms in TRS and CMS patients
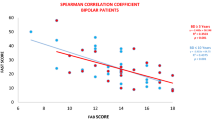
For TRS patients, correlation analyses showed that the H2O2 concentration was associated with the PANSS positive symptoms score (r = 0.386, p = 0.032) (Fig. 3A) and smoking status (r = −0,412, p = 0.021), while POD activities were positively correlated with BMI (r = 0.412, p = 0.021) and TAC was significantly negatively associated with the PANSS total score (r = −0.578, p = 0.001) (Fig. 3B) and weakly positively correlated with BMI (r = 0.357, p = 0.049). Levels of α-toc were significantly correlated with age of onset (r = 0.525, p = 0.002). After controlling for confounders, multiple regression analysis revealed that the H2O2 concentration was positively correlated to the PANSS positive subscale (B = 0.380, t = 2.171, p = 0.039), while TAC levels were negatively significantly correlated to the PANSS total score (B = −0.010, t = −4.132, p < 0.001) and α-toc levels were associated with age of onset (B = 0.183, t = 2.505, p = 0.018). However, there were no significant associations among ·OH, POD, log MMP-9, and log TIMP-1 and PANSS total score and the PANSS subscales (all, p > 0.05).
For CMS patients, ·OH levels were positively associated with the PANSS subscale scores (r = 0.308, p = 0.031) (Fig. 4A), while TAC was negatively correlated to the PANSS total score (r = −0.543, p < 0.001) (Fig. 4B). The log MMP-9 value was negatively associated with age of onset (r = −0.301, p = 0.036). There were no correlations among the H2O2, POD, and α-toc levels, log TIMP-1 values, PANSS total score, and PANSS subscale scores (all, p > 0.05). After controlling for confounding factors (i.e., age, years of education, BMI, smoking status, age of onset, duration of illness, and chlorpromazine equivalent dose), multiple regression analysis revealed that ·OH was associated with the PANSS subscale scores (B = 0.033, t = 2.233, p = 0.031), TAC, and PANSS total score (B = −0.008, t = −4.438, p < 0.001). The log MMP-9 value was correlated with age of onset (B = −0.011, t = −2.161, p = 0.036).
Independent variables predictive of TRS
TRS and healthy controls were defined as dichotomous variables, modified Poisson regression analysis demonstrated that H2O2 (B = −0.008, RR = 0.992, 95%CI:0.986–0.999, p = 0.021), α-toc (B = −0.113, RR = 0.893, 95%CI: 0.834−0.956, p = 0.001), and ·OH (B = −0.328, RR = 0.265, 95%CI: 0.143−0.493, p < 0.001) were independent variables protective of TRS, while TAC (B = −2.084, RR = 0.124, 95%CI: 0.013−1.230, p = 0.075) and POD (B = 0.037, RR = 1.037, 95%CI: 0.964−1.116, p = 0.326) were not observed. With TRS and CMS being considered as dichotomous variables, modified Poisson regression analysis revealed that POD (B = 0.113, RR = 1.120, 95% CI: 1.031−1.217, p = 0.007) was a risk factor for TRS.
Discussion
The present study showed that (1) levels of H2O2, ·OH, POD, α-toc, and TAC were decreased, and MMP-9 levels were increased in male patients with TRS and CMS as compared to healthy controls; (2) H2O2 levels in patients with TRS were positively associated with the PANSS positive psychopathology subscore, TAC was negatively correlated with the PANSS total score, ·OH activities were positively association with the PANSS general psychopathology subscore, and TAC was negatively correlated to the PANSS total score in patients with CMS; and (3) H2O2, α-toc, and ·OH may be protective factors against TRS, and POD was a risk factor. To date, this is the first study to report the relationships among H2O2 and ·OH levels, TAC, and clinical psychopathology in male patients with TRS.
In contrast to previous studies that measured antioxidant enzyme activities12,38,39, the current study directly assessed levels of peroxidation products, which could serve as more direct indicators of damage to the antioxidant system. In vivo, two primary sources of H2O2 are mitochondrial production and superoxides generated during oxidation of nicotinamide adenine dinucleotide phosphate (NADPH) by NADPH oxidase in the presence of SOD40. H2O2 can follow two pathways: (1) conversion to non-toxic substances through the actions of CAT and GSH, and (2) conversion to ·OH via POD25,41. When either pathway is disrupted, OS occurs, leading to various psychiatric disorders42,43. Mitochondrial dysfunction plays a pivotal role in the OS observed in schizophrenia, potentially serving as both a precursor and a consequence of the disorder14. Genetic and environmental factors induce OS within neurons, leading to mitochondrial anomalies and a compromised inflammatory response, which in turn generate further OS and neuronal damage14,28,29. A pathological “vicious cycle” among OS, neuroinflammation, and mitochondrial dysfunction may underlie the development of schizophrenia. Our previous studies indicated that inflammatory cytokines and OS may be involved in the pathophysiology of TRS and chronic stable schizophrenia44, and future studies will focus on the interplay between these factors and mitochondrial dysfunction.
Additionally, POD plays a significant role in innate immunity, apoptosis, and cell signaling, while abnormal peroxidase activities contribute to oxidative damage to cells and tissues, leading to the development of various diseases45. A previous study by our group found that plasma CAT activities and decreased levels of GSH-Px, SOD, and MDA were elevated in TRS and chronic patients as compared to healthy controls44. In the current study, H2O2 and ·OH levels were significantly reduced in TRS and CMS patients as compared to healthy controls, while POD activity was significantly decreased in CMS patients as compared to healthy controls. Moreover, levels of α-toc, a non-enzymatic antioxidant, were decreased in patients with TRS. It is noteworthy that previous study has found that the serum OS markers, H2O2 and MDA, are twice as high in obese women compared to those of normal weight and that they increase as the BMI increases46. Although the analyses included correction for BMI, we postulated that a higher BMI could be a contributing factor to the elevated H2O2 and ·OH levels observed in the healthy control group as well as TRS and CMS patients. Our study provides a perspective that abnormalities in oxidative and antioxidant responses may have some connection with the pathophysiology of TRS and CMS. However, as this study is cross-sectional, such associations cannot be directly interpreted as causal relationships, and these links could potentially be subject to bidirectional interactions or confounding factors. Future research should seek to verify these findings through more rigorous control of confounding factors or longitudinal studies.
Moreover, plasma levels of TAC were decreased in TRS and CMS patients, although these findings were not consistent across different types of schizophrenia. A previous study reported no significant difference in TAC levels among drug-free, medicated, and short-term-treated schizophrenia patients versus controls12. In contrast, serum TAC levels during admission and discharge were lower in patients with acute paranoid schizophrenia and chronic disease than those experiencing a first episode of schizophrenia21,47. Plasma Trolox-equivalent antioxidant capacity was comparable between treatment-responsive patients and the control group48. Prior research also noted decreased total antioxidant status in both drug-naïve first-episode and chronic schizophrenia patients22,49. Various factors can contribute to variations in OS parameters, including age, diet, smoking, sample size, assay sampling method (e.g., serum or plasma), disease stage, antipsychotic medication, illness duration, and sex50,51. Variations in TAC may potentially indicate compensatory effects or past OS damage within cells and may be influenced not only by various confounding factors, but also the dynamic states of antioxidant enzymes39. Therefore, these studies from different perspectives provide further evidence of the relationships between dysregulation of oxidative defense system and schizophrenia. In the present study, TRS was associated with changes to TAC, although the exact pathophysiological process remains unclear, thus warranting further investigations.
Furthermore, in this study, plasma MMP-9 levels were elevated in TRS patients, thereby providing an important supplementary finding to previous research52. The close relationship between MMP-9 and schizophrenia has been validated in various studies, including human and animal investigations of the expression and activity of MMP-9 in the central nervous system, synaptic plasticity, and gene polymorphisms53. In line with the findings of Yamamori et al.54, the results of the present study found that MMP-9 was not significantly correlated to the PANSS total score or PANSS subscale scores. As a possible explanation for these findings, MMP-9 activity may be regulated by TIMP-1, as suggested by previous research36. Additionally, Niitsu et al. proposed that peripheral blood levels of MMP-9 in male schizophrenia patients are associated with older age and smoking55. These findings indicate the need for further studies.
OS is reported to significantly impact the clinical characteristics of schizophrenia56. In this study, decreased H2O2 levels were positively correlated to symptoms of TRS, while decreased TAC levels were negatively associated with the total PANSS score of patients with TRS and CMS. Meanwhile, ·OH, a metabolite of H2O2, was positively associated with general symptoms of CMS. H2O2 is widely recognized as a direct indicator of OS and TAC is considered an indirect marker of OS. Imbalances between antioxidant enzymes, such as CAT, SOD, and GSH-Px, and levels of H2O2 and MDA contribute to OS, while antioxidant treatment has been shown to ameliorate the symptoms of schizophrenia57,58. In a previous study, doxycycline administered with antioxidant and anti-inflammatory agents reduced ketamine-induced schizophrenia-like behavior and synergistically improved the therapeutic efficacy of the antipsychotic risperidone in a murine model of schizophrenia59. Li et al. reported that decreased plasma TAC levels in schizophrenia patients were associated with deficits in cognitive function60. These results suggest that H2O2, and TAC may play important roles in the severity of clinical symptoms, indicating that changes to H2O2, and TAC levels are correlated with the pathophysiology of TRS. However, a previous study reviewed the range of chemical constituents, both direct and indirect, in cigarette smoke that may have pro-inflammatory effects and disrupt the equilibrium between antioxidants and oxidants, thereby precipitating OS61. The study conducted by Xiu et al. showed that smoking affects the activities of SOD, GSH-Px, and CAT, thereby influencing their predictive association with the improvement of clinical symptoms in patients with schizophrenia before and after treatment62. We still should take into account that smoking status may confound the relationship between H2O2 concentration and PANSS positive symptoms.
Further analysis revealed that decreased TAC levels were associated with the psychopathology of TRS, suggesting that disruption of antioxidant capacity may be associated with the occurrence of TRS. However, numerous factors that have been linked to TRS contribute to the difficulty of treatment, such as social isolation, pre- and postnatal inflammation, younger age of onset, family history, smoking, sex, types of prescribed antipsychotic medications, disease classification, clinical profiles, neuroimaging, and neurobiological factors3,6,63. Therefore, these findings may provide new perspectives for alternative treatment strategies for TRS.
Previous studies have indicated that α-toc supplementation can alleviate motor retardation in schizophrenia patients treated with haloperidol23,64, while higher or lower TAC levels reflect the degree of functioning of the oxidative defense system65,66. The present study provides evidence of the protective effects of TAC and α-toc against TRS, suggesting that reduced TAC and α-toc levels indicate compromised functioning of the oxidative defense system in TRS. On the other hand, POD and ·OH are influenced by H2O2 and various enzymes, although further studies are needed for confirmation. Nonetheless, these findings support the involvement of OS in the pathophysiology of TRS.
Interestingly, TAC levels were positively correlated to BMI in TRS patients. Previous studies have suggested associations between TAC and BMI in women, indicating a potential link between endogenous oxidative and antioxidant enzyme activities and lifestyle or metabolic factors67,68,69. A systematic review and meta-analysis reported a significant impact of BMI on the detection of 8-hydroxy-2-deoxyguanosine, a biomarker of oxidative DNA damage70. Brain imaging studies of schizophrenia and BMI have suggested that OS may contribute to decreased prefrontal myelin formation, while obesity may be associated with alterations to the frontal and temporal regions, as well as ventricular structures71,72. These overlapping findings provide valuable insights into the potential relationships among OS, metabolic factors, and brain structures in the pathophysiology of schizophrenia. On the other hand, existing research indicates that obesity may disrupt antioxidant enzymes73, with schizophrenia patients showing altered SOD and MDA levels related to weight changes post-antipsychotic treatment74. and a BMI-clinical symptom correlation in males, as identified by Wei et al.75. Although our study statistically established a relationship between TAC and BMI, and found no direct relationship between BMI and PANSS scores, it is important to consider that BMI may act as a confounding factor in the association between TAC and clinical symptoms.
There were several limitations to this study that should be addressed. First, male patients with TRS and CMS exhibited longer durations of clinical symptoms and had more compounding factors as compared to first-episode and drug-naïve patients with schizophrenia. Second, the sample size in this study was small and numerous parameters were tested. Although the Bonferroni correction method was employed to correct for multiple comparisons between groups, and several findings showed highly significant differences, the results reported for this manuscript warrant further validation with large samples to confirm these findings and mitigate the risk of potential Type II errors. Third, the causal relationships between OS parameters and PANSS scores could not be determined through cross-sectional studies, thus longitudinal studies are required. Additionally, analysis of the demographic data of only males potentially limits the generalizability of the results.
Conclusion
In conclusion, these preliminary findings provide evidence that the levels of OS parameters (i.e., H2O2, ·OH, POD, α-toc, and TAC) and MMP-9 significantly differed among male patients with TRS and CMS. Variations in H2O2, ·OH, and TAC levels were associated with the severity of clinical symptoms. H2O2, α-toc, and ·OH were identified as protective factors against TRS. These results provide additional evidence that OS and MMP-9 may be involved in the pathophysiology of TRS and CMS.
Subjects and methods
Subjects and assessments
Participants were recruited from the Mental Disorder Department of the Fourth People’s Hospital of Lianyungang (Lianyungang, China). Diagnoses of schizophrenia were confirmed using the Structured Clinical Interview of the Diagnostic and Statistical Manual-IV. Patient data, including age, education, smoking status, body mass index (BMI), age of onset, and illness duration, were collected through a questionnaire. Dosages of antipsychotic medications were standardized to chlorpromazine equivalents. The severity of psychotic symptoms was assessed by two experienced psychiatrists utilizing the positive and negative syndrome scale (PANSS), with an inter-rater correlation coefficient exceeding 0.8. The study protocol was approved by the Ethics Committee of the Fourth People’s Hospital of Lianyungang and written informed consent was obtained from all participants or their guardians.
The TRS group was defined as conforming to the following criteria: (1) poor effects of two different antipsychotics medications for at least 6 months with a minimum dose of chlorpromazine at ≥600 mg/day or equivalent and (2) scores of each of the six items of the PANSS (P1, P2, P3, N1, N4, and N6) ≥ 3 and PANSS total score ≥703,76,77,78. The CMS group was defined as having received (1) treatment with one antipsychotic medication with stable disease symptoms for >6 consecutive months with chlorpromazine at <600 mg/day or equivalent and (2) scores of each of the six items of PANSS (P1, P2, P3, N1, N4, and N6) < 3 with a PANSS total score <6076,77,78,79.
Patients with comorbid neurological disorders (e.g., mental retardation, dementia, epilepsy, degenerative disease, and/or traumatic brain injury) and endocrine disorders (e.g., thyroid dysfunction, diabetes mellitus, and/or alcoholic or substance dependence/abuse) were excluded from the study.
Fifty-three healthy controls, matched for age, sex, education, BMI, and smoking status, were recruited from the Lianyungang community through advertisements. Healthy controls who met the Diagnostic and Statistical Manual-IV Axis I criteria for a major disease or had a family history of mental disorder or alcohol abuse/dependence were excluded from the study. Physical examinations and laboratory tests were conducted to assess the health status of all participants.
Blood sampling and biochemical assays
Blood samples were obtained from healthy controls and patients in the morning between 07:00 and 09:00 following overnight fasting. Blood samples were collected in anticoagulant-coated tubes and centrifuged at 3000 rpm for 15 min. The plasma was extracted from the anticoagulant tubes and stored at −80 °C until analysis. A technician blinded to the sample identity and clinical status performed duplicate assays for all blood samples. The coefficients of variation for OS parameters ranged from 3.4% to 7.2% for both intra- and inter-assay measurements.
Plasma levels of H2O2, ·OH, POD, α-toc, and TAC were measured using commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the manufacturer’s instructions. Plasma levels of H2O2 (mmol/L), ·OH (U/mL), POD (U/mL), and α-toc (μg/mL) were measured using a colorimetric method, and TAC (mmol/L) was determined using an antioxidant capacity assay to assess ferric reducing antioxidant potential22. To obtain more explicit results for ·OH, the unit was switched from U/mL to U/μL. Serum levels of MMP-9 (ng/mL) and TIMP-1 (ng/mL) were measured with a Luminex liquid suspension chip detection assay (R&D Systems, Minneapolis, MN, USA) in accordance with the instructions provided by the manufacturer.
Statistical analysis
Statistical analyses were conducted using IBM SPSS Statistics for Windows, version 19.0. (IBM Corporation, Armonk, NY, USA). The Kolmogorov–Smirnov test was used to assess the normal distribution of variables. Continuous variables with a normal distribution were analyzed using one-way analysis of variance and are presented as the mean ± standard deviation (SD). Non-normally distributed data were assessed with the Mann–Whitney U-test and are summarized as the median with 25th and 75th quartiles. Categorical variables were analyzed using the chi-square test. A probability (p) value < 0.05 was considered statistically significant. To address the non-normal distribution, logarithmic transformation was applied to serum MMP-9 and TIMP-1 levels, resulting in normally distributed data. Cohen’s d values were used to report the effect size, where 0.2 was considered a small effect size, 0.5 was a medium effect size, and 0.8 was a large effect size80.
To mitigate the risk of type I errors arising from potential interactions among multiple continuous dependent variables, multivariate analysis of covariance was conducted as the initial step. In this model, H2O2, ·OH, POD, α-toc, TAC, MMP-9, and TIMP-1 were dependent variables, with diagnoses (TRS, CMS, and healthy controls) as fixed factors, and age, years of education, BMI, and smoking status as covariates. Analysis of covariance was used to determine the significance of differences of each parameter among the TRS, CMS and healthy controls group. Multiple comparisons were adjusted using the Bonferroni correction method. Potential correlations of the normally and non-normally distributed data were identified using the Pearson’s correlation coefficient and Spearman’s correlation coefficient, respectively. Subsequently, stepwise multivariate regression analyses were conducted to evaluate the correlation between the OS parameters, serum levels of MMP-9 and TIMP-1, and PANSS subscale scores, while accounting for potential confounding variables. Relative risk factors for TRS were predicted using modified Poisson regression.
Data availability
The data supporting the results of this study are available upon request from the corresponding author.
References
Ayano, G., Tesfaw, G. & Shumet, S. The prevalence of schizophrenia and other psychotic disorders among homeless people: a systematic review and meta-analysis. BMC Psychiatry 19, 370 (2019).
Stepnicki, P., Kondej, M. & Kaczor, A. A. Current concepts and treatments of schizophrenia. Molecules 23, 2087 (2018).
Nucifora, F. C. Jr., Woznica, E., Lee, B. J., Cascella, N. & Sawa, A. Treatment resistant schizophrenia: Clinical, biological, and therapeutic perspectives. Neurobiol. Dis. 131, 104257 (2019).
Zhang, T. et al. Further evidence that antipsychotic medication does not prevent long-term psychosis in higher-risk individuals. Eur. Arch. Psychiatry Clin. Neurosci. 272, 591–602 (2022).
Wellesley Wesley, E. et al. Gender disparities in clozapine prescription in a cohort of treatment-resistant schizophrenia in the South London and Maudsley case register. Schizophr. Res. 232, 68–76 (2021).
Smart, S. E., Kepinska, A. P., Murray, R. M. & MacCabe, J. H. Predictors of treatment resistant schizophrenia: a systematic review of prospective observational studies. Psychol. Med. 51, 44–53 (2021).
Potkin, S. G. et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. npj Schizophr. 6, 1 (2020).
Pinheiro, D. S. et al. GSTM1/GSTT1 double-null genotype increases risk of treatment-resistant schizophrenia: A genetic association study in Brazilian patients. PLoS One 12, e0183812 (2017).
Wadhwa, R., Gupta, R. & Maurya, P. K. Oxidative stress and accelerated aging in neurodegenerative and neuropsychiatric disorder. Curr. Pharm. Des. 24, 4711–4725 (2018).
An, H. et al. Obesity, altered oxidative stress, and clinical correlates in chronic schizophrenia patients. Transl. Psychiatry 8, 258 (2018).
Bahceci, B. et al. Elevated nucleosome level and oxidative stress in schizophrenia patients. Bratisl Lek Listy 116, 587–590 (2015).
Bai, Z. L. et al. Serum oxidative stress marker levels in unmedicated and medicated patients with schizophrenia. J. Mol. Neurosci. 66, 428–436 (2018).
Solberg, D. K., Refsum, H., Andreassen, O. A. & Bentsen, H. A five-year follow-up study of antioxidants, oxidative stress and polyunsaturated fatty acids in schizophrenia. Acta Neuropsychiatr. 31, 202–212 (2019).
Beeraka, N. M., Avila-Rodriguez, M. F. & Aliev, G. Recent reports on redox stress-induced mitochondrial DNA variations, neuroglial interactions, and NMDA receptor system in pathophysiology of schizophrenia. Mol. Neurobiol. 59, 2472–2496 (2022).
Mishra, A., Reeta, K. H., Sarangi, S. C., Maiti, R. & Sood, M. Effect of add-on alpha lipoic acid on psychopathology in patients with treatment-resistant schizophrenia: a pilot randomized double-blind placebo-controlled trial. Psychopharmacology 239, 3525–3535 (2022).
Lin, C. H., Chen, Y. M. & Lane, H. Y. Novel treatment for the most resistant schizophrenia: Dual activation of NMDA receptor and antioxidant. Curr. Drug Targets 21, 610–615 (2020).
De Simone, G. et al. Schizophrenia synaptic pathology and antipsychotic treatment in the framework of oxidative and mitochondrial dysfunction: translational highlights for the clinics and treatment. Antioxidants 12, 975 (2023).
Arvindakshan, M., Ghate, M., Ranjekar, P. K., Evans, D. R. & Mahadik, S. P. Supplementation with a combination of omega-3 fatty acids and antioxidants (vitamins E and C) improves the outcome of schizophrenia. Schizophr. Res. 62, 195–204 (2003).
Bentsen, H., Osnes, K., Refsum, H., Solberg, D. K. & Bohmer, T. A randomized placebo-controlled trial of an omega-3 fatty acid and vitamins E+C in schizophrenia. Transl. Psychiatry 3, e335 (2013).
Neill, E. et al. N-Acetylcysteine (NAC) in schizophrenia resistant to clozapine: a double-blind, randomized, placebo-controlled trial targeting negative symptoms. Schizophr. Bull. 48, 1263–1272 (2022).
Juchnowicz, D. et al. Pro/Antioxidant state as a potential biomarker of schizophrenia. J. Clin. Med. 10, 4156 (2021).
Xie, T. et al. Plasma total antioxidant status and cognitive impairments in first-episode drug-naïve patients with schizophrenia. Cogn. Neurodyn. 13, 357–365 (2019).
Pawełczyk, T., Grancow-Grabka, M., Trafalska, E., Szemraj, J. & Pawełczyk, A. Oxidative stress reduction related to the efficacy of n-3 polyunsaturated fatty acids in first episode schizophrenia: Secondary outcome analysis of the OFFER randomized trial. Prostaglandins Leukot. Essent. Fatty Acids 121, 7–13 (2017).
Lennicke, C. & Cochemé, H. M. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol. Cell 81, 3691–3707 (2021).
Chainy, G. B. N. & Sahoo, D. K. Hormones and oxidative stress: an overview. Free Rad. Res. 54, 1–26 (2019).
Avshalumov, M. V., Chen, B. T., Marshall, S. P., Peña, D. M. & Rice, M. E. Glutamate-dependent inhibition of dopamine release in striatum is mediated by a new diffusible messenger, H2O2. J. Neurosci. 23, 2744–2750 (2003).
Ermakov, E. A. et al. Oxidative stress-related mechanisms in schizophrenia pathogenesis and new treatment perspectives. Oxid. Med. Cell Longev. 2021, 8881770 (2021).
Cuenod, M. et al. Caught in vicious circles: a perspective on dynamic feed-forward loops driving oxidative stress in schizophrenia. Mol. Psychiatry 27, 1886–1897 (2021).
Bitanihirwe, B. K. Y. & Woo, T.-U. W. A conceptualized model linking matrix metalloproteinase-9 to schizophrenia pathogenesis. Schizophr. Res. 218, 28–35 (2020).
Shahzad, A., Rink, L. & Wessels, I. Regulation of matrix metalloproteinase-9 during monopoiesis and zinc deficiency by chromatin remodeling. J. Trace Elem. Med. Biol. 78, 127162 (2023).
Umbricht, D. Matrix metalloproteinase 9 levels and parvalbumin positive interneuron dysfunction. Neuropsychopharmacology 47, 429–429 (2021).
Jäsberg, H., Tervahartiala, T., Sorsa, T., Söderling, E. & Haukioja, A. Probiotic intervention influences the salivary levels of Matrix Metalloproteinase (MMP)-9 and Tissue Inhibitor of metalloproteinases (TIMP)-1 in healthy adults. Arch. Oral Biol. 85, 58–63 (2018).
Vafadari, B., Salamian, A. & Kaczmarek, L. MMP-9 in translation: from molecule to brain physiology, pathology, and therapy. J. Neurochem. 139, 91–114 (2016).
Dwir, D. et al. MMP9/RAGE pathway overactivation mediates redox dysregulation and neuroinflammation, leading to inhibitory/excitatory imbalance: a reverse translation study in schizophrenia patients. Mol. Psychiatry 25, 2889–2904 (2019).
Cui, N., Hu, M. & Khalil, R. A. Biochemical and biological attributes of matrix metalloproteinases. in Matrix Metalloproteinases and Tissue Remodeling in Health and Disease: Cardiovascular Remodeling 1-73 (2017).
Cabral-Pacheco, G. A. et al. The roles of matrix metalloproteinases and their inhibitors in human diseases. Int. J. Mol. Sci. 21, 9739 (2020).
Chien, Y.-L. et al. Clinical implications of oxidative stress in schizophrenia: Acute relapse and chronic stable phase. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 99, 109868 (2020).
Zhang, X. Y. et al. Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophr. Res. 81, 291–300 (2006).
Wu, J. Q., Kosten, T. R. & Zhang, X. Y. Free radicals, antioxidant defense systems, and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 46, 200–206 (2013).
Emiliani, F. E., Sedlak, T. W. & Sawa, A. Oxidative stress and schizophrenia. Curr. Opin. Psychiatry 27, 185–190 (2014).
Smaga, I., Frankowska, M. & Filip, M. N-acetylcysteine as a new prominent approach for treating psychiatric disorders. Br. J. Pharmacol. 178, 2569–2594 (2021).
Kim, Y. et al. Mitochondria, metabolism, and redox mechanisms in psychiatric disorders. Antioxid. Redox Signal 31, 275–317 (2019).
Nandi, A., Yan, L. J., Jana, C. K. & Das, N. Role of catalase in oxidative stress- and age-associated degenerative diseases. Oxid. Med. Cell. Longev. 2019, 9613090 (2019).
Yang, H. et al. Catalase and interleukin-6 serum elevation in a prediction of treatment-resistance in male schizophrenia patients. Asian J. Psychiatr. 79, 103400 (2023).
Vlasova, I. Peroxidase activity of human hemoproteins: keeping the fire under control. Molecules 23, 2561 (2018).
Leanza, G. et al. Oxidative stress in postmenopausal women with or without obesity. Cells 12, 1137 (2023).
Morera-Fumero, A. L., Díaz-Mesa, E., Abreu-Gonzalez, P., Fernandez-Lopez, L. & Cejas-Mendez, M. D. R. Low levels of serum total antioxidant capacity and presence at admission and absence at discharge of a day/night change as a marker of acute paranoid schizophrenia relapse. Psychiatry Res. 249, 200–205 (2017).
Buosi, P. et al. Oxidative stress biomarkers in treatment-responsive and treatment-resistant schizophrenia patients. Trends Psychiatry Psychother. 43, 278–285 (2021).
Zhang, X. Y. et al. Plasma total antioxidant status and cognitive impairments in schizophrenia. Schizophr. Res. 139, 66–72 (2012).
Zhu, M. et al. Sex difference in the interrelationship between TNF-alpha and oxidative stress status in first-episode drug-naive schizophrenia. J. Neuroinflamm. 18, 202 (2021).
Mahadik, S. P., Evans, D. & Lal, H. Oxidative stress and role of antioxidant and omega-3 essential fatty acid supplementation in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 25, 463–493 (2001).
Domschke, K. et al. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS ONE 5, e9166 (2010).
Lepeta, K. & Kaczmarek, L. Matrix Metalloproteinase-9 as a novel player in synaptic plasticity and schizophrenia: Table 1. Schizophr. Bull. 41, 1003–1009 (2015).
Yamamori, H. et al. Plasma levels of mature brain-derived neurotrophic factor (BDNF) and matrix metalloproteinase-9 (MMP-9) in treatment-resistant schizophrenia treated with clozapine. Neurosci. Lett. 556, 37–41 (2013).
Niitsu, T. et al. A positive correlation between serum levels of mature brain-derived neurotrophic factor and negative symptoms in schizophrenia. Psychiatry Res. 215, 268–273 (2014).
Hendouei, N. et al. Alterations in oxidative stress markers and its correlation with clinical findings in schizophrenic patients consuming perphenazine, clozapine and risperidone. Biomed. Pharmacother. 103, 965–972 (2018).
Collin, F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 20, 2407 (2019).
Bitanihirwe, B. K. & Woo, T. U. Oxidative stress in schizophrenia: an integrated approach. Neurosci. Biobehav.Rev. 35, 878–893 (2011).
Ben-Azu, B. et al. Doxycycline prevents and reverses schizophrenic-like behaviors induced by ketamine in mice via modulation of oxidative, nitrergic and cholinergic pathways. Brain Res. Bull. 139, 114–124 (2018).
Li, J., Li, D., Guo, J., Wang, D. & Zhang, X. Age of onset moderates the association between total antioxidant capacity and cognitive deficits in patients with drug-naïve schizophrenia. Antioxidants 12, 1259 (2023).
Astori, E. et al. Antioxidants in smokers. Nutr. Res. Rev. 35, 70–97 (2021).
Xiu, M. et al. Smoking affects the predictive roles of antioxidant enzymes in the clinical response to risperidone in schizophrenia: a large-scale cohort study. Curr. Neuropharmacol. 21, 2151–2158 (2023).
Moller, M., Swanepoel, T. & Harvey, B. H. Neurodevelopmental animal models reveal the convergent role of neurotransmitter systems, inflammation, and oxidative stress as biomarkers of schizophrenia: implications for novel drug development. ACS Chem. Neurosci. 6, 987–1016 (2015).
Bošković, M., Vovk, T., Koprivšek, J., Plesničar, B. K. & Grabnar, I. Vitamin E and essential polyunsaturated fatty acids supplementation in schizophrenia patients treated with haloperidol. Nutr. Neurosci. 19, 156–161 (2016).
Silvestrini, A., Meucci, E., Ricerca, B. M. & Mancini, A. Total antioxidant capacity: biochemical aspects and clinical significance. Int. J. Mol. Sci. 24, 10978 (2023).
Zahedi Avval, F. et al. Determining pro-oxidant Antioxidant Balance (PAB) and Total Antioxidant Capacity (TAC) in patients with schizophrenia. Iran. J. Psychiatry 13, 222–226 (2018).
Mahasneh, A. A., Zhang, Y., Zhao, H., Ambrosone, C. B. & Hong, C.-C. Lifestyle predictors of oxidant and antioxidant enzyme activities and total antioxidant capacity in healthy women: a cross-sectional study. J. Physiol. Biochem. 72, 745–762 (2016).
Fakhari, M., Fakhari, M. & BamBaeichi, E. The effects of pilates and flavanol-rich dark chocolate consumption on the total antioxidant capacity, glycemic control and BMI in diabetic females with neuropathy complications. J. Bodyw. Mov. Ther. 26, 294–299 (2021).
CÖMert, T. K., Akpinar, F., Erkaya, S., Durmaz, B. & Durmaz, R. The effect of gestational weight gain on serum total oxidative stress, total antioxidant capacity and gut microbiota. Biosci. Microbiota Food Health 41, 160–167 (2022).
Graille, M. et al. Urinary 8-OHdG as a biomarker for oxidative stress: a systematic literature review and meta-analysis. Int. J. Mol. Sci. 21, 3743 (2020).
Maas, D. A., Vallès, A. & Martens, G. J. M. Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia. Transl. Psychiatry 7, e1171–e1171 (2017).
McWhinney, S. R. et al. Obesity and brain structure in schizophrenia – ENIGMA study in 3021 individuals. Mol. Psychiatry 27, 3731–3737 (2022).
Ozata, M. et al. Increased oxidative stress and hypozincemia in male obesity. Clin. Biochem. 35, 627–631 (2002).
Liu, H. et al. Antioxidant enzymes and weight gain in drug-naive first-episode schizophrenia patients treated with risperidone for 12 weeks: a prospective longitudinal study. Curr. Neuropharmacol. 20, 1774–1782 (2022).
Wei, C. W., Chen, Y. Q., Ma, M., Xiu, M. H. & Zhang, X. Y. Sex differences in the association of body mass index with symptoms and cognitive deficits in Chinese patients with chronic schizophrenia. Transl. Psychiatry 10, 18 (2020).
Suzuki, T. et al. Defining treatment-resistant schizophrenia and response to antipsychotics: a review and recommendation. Psychiatry Res. 197, 1–6 (2012).
Howes, O. D. et al. Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am. J. Psychiatry 174, 216–229 (2017).
Ostergaard, S. D., Foldager, L., Mors, O., Bech, P. & Correll, C. U. The validity and sensitivity of PANSS-6 in treatment-resistant schizophrenia. Acta Psychiatr. Scand. 138, 420–431 (2018).
Andreasen, N. C. et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am. J. Psychiatry 162, 441–449 (2005).
Faul, F., Erdfelder, E., Lang, A. G. & Buchner, A. G. Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. methods 39, 175–191 (2007).
Acknowledgements
We would like to thank the participants in the study. The study was supported by the Suzhou Key Technologies Program (SKY2021063), Suzhou clinical Medical Center for mood disorders (No. Szlcyxzx202109), Suzhou Clinical Key disciplines for Geriatric Psychiatry (SZXK202116), Lianyungang Science and Technology Bureau of Social Development Key R&D Projects (SF2208) and General Program of Lianyungang Health Committee (NO.202130). The funding sources of this study had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Author information
Authors and Affiliations
Contributions
H.Y. and X.Z. wrote the manuscript; X.Z. was responsible for study design; H.Y., W.S., and J.L. performed the statistical analysis; H.Y., M.Y., and J.Z. were responsible for performing the clinical rating, recruiting the patients, and collecting the samples. All authors have contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
We declare that all experiments on human subjects were conducted in accordance with the Declaration of Helsinki and that all procedures were carried out with the adequate understanding and written consent of the subjects. All experimental protocols were approved by the Ethics Committee of Lian Yun Gang Fourth People’s Hospital. Informed consent was obtained from all the participants and/or their legal guardians. All methods were carried out in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, H., Sun, W., Yang, M. et al. Variations to plasma H2O2 levels and TAC in chronical medicated and treatment-resistant male schizophrenia patients: Correlations with psychopathology. Schizophr 10, 45 (2024). https://doi.org/10.1038/s41537-024-00468-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-024-00468-y