Abstract
Serum neuropeptide levels may be linked to schizophrenia (SCZ) pathogenesis. This study aims to examine the relation between five serum neuropeptide levels and the cognition of patients with treatment-resistant schizophrenia (TRS), chronic stable schizophrenia (CSS), and in healthy controls (HC). Three groups were assessed: 29 TRS and 48 CSS patients who were hospitalized in regional psychiatric hospitals, and 53 HC. After the above participants were enrolled, we examined the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) and the blood serum levels of α-melanocyte stimulating hormone (α-MSH), β-endorphin (BE), neurotensin (NT), oxytocin (OT) and substance.P (S.P). Psychiatric symptoms in patients with SCZ were assessed with the Positive and Negative Syndrome Scale. SCZ patients performed worse than HC in total score and all subscales of the RBANS. The levels of the above five serum neuropeptides were significantly higher in SCZ than in HC. The levels of OT and S.P were significantly higher in CSS than in TRS patients. The α-MSH levels in TRS patients were significantly and negatively correlated with the language scores of RBANS. However, the BE and NT levels in CSS patients were significantly and positively correlated with the visuospatial/constructional scores of RBANS. Moreover, the interaction effect of NT and BE levels was positively associated with the visuospatial/constructional scores of RBANS. Therefore, abnormally increased serum neuropeptide levels may be associated with the physiology of SCZ, and may cause cognitive impairment and psychiatric symptoms, especially in patients with TRS.
Similar content being viewed by others
Introduction
According to the World Health Organization, schizophrenia (SCZ) is among the most impactful of major psychiatric diseases. Particularly, SCZ is estimated to affect about 1% of the worldwide population, with an age-standardized prevalence of 287.4 per 100,000 population, and is ranked as a major cause of disability due to mental disorders1,2. With population growth and aging, the burden of SCZ-induced illness continues to increase, especially in middle-income countries3. Patients with SCZ tend to have poor eating habits and experience weight gain, with male patients favoring smoking and co-morbid substance use, all of which contribute to a 13–15 year reduction in their life expectancy4. The main symptoms of the disease are considered both positive (e.g., delusions and hallucinations) and negative (e.g., blunted affect and social withdrawal) and include the loss of cognitive functions (e.g., memory and executive functions)5,6,7. A recent review of SCZ has concluded that so-called first-degree symptoms are not as important and that cognitive dysfunction is the key clinical symptom of the disorder8. Cognitive deficits not only occur during the acute onset of the disease but persist throughout the disease, especially in the chronic phase, leading to severe cognitive impairment9. Previous studies10,11 have found deficits observed in patients with SCZ in all domains of neuropsychological functioning, with deficits in executive function, memory and sustained attention being particularly prominent.
Despite the severity of cognitive impairment in patients with SCZ, current clinical treatment of SCZ remains primarily focused on the immediate reduction or suppression of psychotic deterioration, with the goal of improving general symptoms as well as improving basic life skills12. Antipsychotic drugs are primarily used in the pharmacological treatment of SCZ. A substantial amount of trial data supports the conclusion that these medications can reduce symptoms, particularly positive symptoms, and also to a limited extent negative symptoms, and thus enhance cognitive functioning13. However, these drugs also place a significant somatic burden on the patient, including sedation, weight gain, and especially extrapyramidal symptoms14. Despite the fact that antipsychotic drugs have been routinely used to treat SCZ for decades, over one-third of SCZ patients do not respond to therapy15,16. There is considerable evidence to support the hypothesis that these schizophrenic patients who do not respond well to medications appear to differ from others in terms of pathological processes and outcomes, particularly in terms of cognitive functioning17,18. The American Psychiatric Association defines treatment-resistant SCZ (TRS) as the presence of little or no symptom decrease after a 6-week trial of at least two antipsychotics at a therapeutic dose for a sufficient period of time19. In other words, the treatment of TRS is difficult. Even with the use of clozapine, which has a multi-receptor mechanism of action, little improvement is seen20. Therefore, there is a need to identify new pathological mechanisms to facilitate treatment of TRS with more objective and effective treatment options.
The development of antipsychotic drugs originated from the dopamine (DA) hypothesis more than 50 years ago21,22,23. According to the DA hypothesis, SCZ symptoms are caused by an imbalance in DA24. However, as clinical research has progressed, no single dysfunction or impairment has been found in patients’ brains which can fully explain the etiology of SCZ25. In addition to monoamines such as DA, neuropeptides are one of the most active and representative classes of neurotransmitters, consisting of neuroactive peptides involved in brain processes such as membrane excitability, synaptogenesis, local blood flow, glial cell structure, and so on26,27. Neuropeptides are produced in the cytoplasm of neurons, are stored in vesicles, and are released from vesicles upon electrical activation of neurons. They are broadly dispersed throughout the brain and are frequently co-localized and co-released with monoamine neurotransmitters like DA, glutamate, or γ-aminobutyric acid28,29. Neuropeptides exhibit reciprocal neurotransmitter connections or independent activity in preclinical animal models by colocalizing with traditional monoaminergic transmission30,31. According to studies in humans and animal models32,33, the pathophysiology of psychiatric disorders like SCZ may involve deficits in neuropeptide signaling, resulting in disrupted dopaminergic neurotransmission in mesostriatal and mesocorticolimbic circuits that are highly relevant to SCZ. Accordingly, reversal of abnormalities in neuropeptide signaling pathways may be a novel approach to treating SCZ34,35.
Current research on neuropeptides has focused on α-melanocyte stimulating hormone (α-MSH), β-endorphin (BE), neurotensin (NT), oxytocin (OT) and Substance.P (S.P), and their specific distribution and functions are presented in Table 1. α-MSH has been linked to anorexic function as well as systemic (i.e., peripheral and central) anti-inflammatory and cytoprotective mechanisms36,37, and it has also been linked to the metabolic syndrome in patients with psychiatric disorders38. BE, which is derived from agranulocortinogen along with α-MSH, are endogenous reinforcing mediators that increase midbrain limbic dopaminergic neurotransmission, and these findings suggest a possible opioid-DA interaction39,40. Furthermore, given the role of BT in regulating the stress response, many psychiatric disorders can be attributed (at least in part) to some abnormality in BE levels41,42. Moreover, BE is a peptide that acts throughout the body, especially in the brain.
Also widely distributed in the human brain and in the periphery is NT, which regulates neural circuits involved in SCZ43. Activation of NT receptors (NTS1) in the ventral tegmental area, for example, increased activity of mesocortical DA neurons, the creation of which brain circuits is connected with cognitive deficiencies in SCZ44. Evidence suggests that the centrally acting, peripherally given NT analog PD149163 can cure psychosis and anxiety in animal models45. Another neuropeptide that is associated with cognitive impairment in psychiatric disorders is OT. Acute OT treatment has been demonstrated in certain studies to enhance cognitive performance in people with SCZ46,47. In addition, Fiefel et al. (2012)48 discovered substantial gains in OT connected with short-term and long-term verbal memory, but not with working memory. Finally, another neuropeptide which like NT acts in both the central and peripheral nervous system is S.P. On the one hand, S.P can influence various central nervous system processes, including emotional behavior, stress, anxiety and depression;49 on the other hand, S.P, like BE, is embedded in the integration of stressful emotional reactions50. However, the relationship between S.P and SCZ is currently unclear and needs to be further explored.
Contrary to conventional assumption, males are somewhat more likely than women to suffer from SCZ8. Similarly, males outnumber women in China as long-term inpatients with SCZ, and male patients have earlier onset and more severe clinical symptoms (particularly cognitive impairment)51,52. Furthermore, sex hormonal influences on cognitive performance could potentially confound our results53. However, there are no published studies on neuropeptides in Chinese chronic male schizophrenic patients (especially with TRS). We hypothesized, as previously stated, that neuropeptides (including α-MSH, BE, NT, OT and S.P) may be related to pathological mechanisms in chronic patients with SCZ (especially TRS). Thus, the present study aimed to investigate (1) whether the levels of the above five neuropeptides are variable in chronic patients with SCZ, especially in TRS; (2) whether cognitive functions are variable in chronic patients with SCZ, especially in TRS; (3) whether there is an association between the levels of the above five neuropeptides and cognitive functions in chronic patients with SCZ, especially in TRS; and (4) whether certain neuropeptides are associated through interactions with cognitive function in chronic SCZ patients, especially those with TRS.
Results
Sociodemographic features
In this study, the participants were divided into three groups: a “TRS Group”, comprising 29 treatment-resistant male SCZ patients, a “CSS Group”, comprising 48 chronic stable SCZ patients, and an “HC Group”, comprising 53 healthy male participants. There was no statistically significant difference among the three groups in age, years of education, BMI and smoking status (all p > 0.05). Additionally, there were no discernible differences between the TRS and CSS groups in terms of age of onset, duration of illness or dose of CPZ equivalent (all p > 0.05). However, there were notable discrepancies in the PANSS total and subscale scores between the TRS and CSS groups (all p < 0.05; Table 2).
Cognitive performance in TRS patients, CSS patients and HC
The means and standard deviations of the RBANS scores of TRS patients, CSS patients and HC are shown in Table 3. Both TRS and CSS patients performed worse than HC in total score and all subscales (all p < 0.01). After controlling for age, education, BMI and smoking status, the differences remained significant for the RBANS total score and subscale scores (all p < 0.01). Even after Bonferroni correction, the difference in cognitive function among the three groups remained significant (Table 3).
The levels of serum neuropeptides in TRS patients, CSS patients and HC
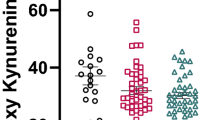
The levels of lg serum neuropeptides in TRS patients, CSS patients and HC are shown in Table 4. The lg serum neuropeptide levels, such as lg α-MSH levels and lg BE, lg NT, lg OT and lg S.P levels, were significantly higher in TRS and CSS patients than in HC (all p < 0.01). There were considerable differences in the lg serum neuropeptide levels in TRS patients, CSS patients and HC after adjusting for age, years of education, BMI and smoking status (all p < 0.01). Except for OT levels, significant differences in the remaining serum neuropeptides among the three groups persisted after application of the Bonferroni correction (Fig. 1).
Neuropeptides are synthesized within the cytoplasm of neurons, stored within vesicles, and subsequently discharged upon neuronal electrical stimulation. They exhibit extensive distribution throughout the brain and frequently co-localize and co-release alongside monoamine neurotransmitters, such as dopamine, glutamate, or γ-aminobutyric acid.
The activities of lg OT (F = 3.363, p = 0.003) showed significant differences, with diagnosis as a fixed variable and age, years of education, BMI, smoking status, age of onset, duration of illness and CPZ equivalency dosage as covariance factors between the TRS and CSS groups.
Associations between serum neuropeptide levels and cognitive impairment in TRS patients and CSS patients
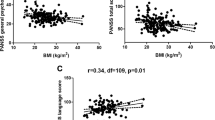
The lg α-MSH levels in TRS patients were significantly and negatively correlated with the language scores of RBANS (r = −0.398, p = 0.032, Fig. 2). In contrast, the lg BE levels in CSS patients were significantly and positively correlated with the visuospatial/constructional scores of RBANS (r = 0.294, p = 0.043, Fig. 3). Moreover, the lg NT levels in CSS patients were significantly and positively correlated with the visuospatial/constructional scores of RBANS (r = 0.288, p = 0.047, Fig. 4).
After controlling for possible influencing factors such as age, years of education, BMI, smoking status, age of onset, duration of illness and CPZ equivalence dose, language scores and lg α-MSH levels were found to be significantly correlated (R2 = 0.494, p = 0.019) in TRS patients by stepwise multiple regression analysis. In addition, after controlling for possible influencing factors such as age, years of education, BMI, smoking status, age of onset, duration of illness and CPZ equivalence dose, visuospatial/constructional scores and lg BE levels were found to be significantly correlated (R2 = 0.113, p = 0.030) in CSS patients by stepwise multiple regression analysis.
Interaction of lg NT and BE levels
Lg NT levels were significantly and positively correlated with lg BE in the CSS patients (r = 0.870, p < 0.001). After controlling for age, years of education, BMI, smoking, age of onset, duration of disease and CPZ equivalent dose, partial correlation analysis revealed that lg NT levels were significantly correlated with lg BE levels in the CSS group (r = 0.864, p < 0.001). Lg NT*BE interaction was found to have a positive correlation with the visuospatial/constructional scores of RBANS when the interaction term between lg NT and BE levels was developed (r = 0.299, p = 0.039).
Discussion
The following are the key findings of the current investigation on long-term medicated SCZ inpatients: (1) Serum neuropeptide levels, including α-MSH, BE, NT, OT and S.P, were significantly higher in patients with chronic SCZ on long-term medication than in HC, and patients in the CSS group had substantially higher levels of OT and S.P than the TRS group; (2) patients with chronic SCZ had considerably lower RBANS test scores than HC, and TRS had lower RBANS test scores than CSS, but the differences were not statistically significant. (3) α-MSH levels of TRS patients were significantly and inversely linked with their RBANS language scores; (4) NT and BE levels in CSS patients were significantly and positively correlated with their RBANS visuospatial/constructional scores; and (5) in the CSS group, there was a strong correlation between NT and BE levels. The RBANS visuospatial/constructional scores were found to positively correlate with the NT-BE interaction.
As predicted, levels of the five neuropeptides (α-MSH, BE, OT, NT and S.P) were significantly higher in chronic male SCZ patients than in healthy controls. However, these five neuropeptides have only recently been studied and previous results did not reveal significant differences between schizophrenic patients and HC54. Interestingly, there have also been studies showing significantly higher levels of BE in schizophrenic patients55,56, and others have concluded that low levels of OT are associated with SCZ57,58. Surprisingly, other studies have found that OT levels are high in patients with SCZ59,60,61. Moreover, low NT concentrations in the cerebrospinal fluid were linked to more severe psychopathology in SCZ, such as an increase in cognitive difficulties, delusions and hallucinations62,63, but changes in NT concentrations in the peripheral blood are likely to vary. Furthermore, a preclinical investigation utilizing a multiplex immunoassay found elevated NT and OT in hereditary and/or environmental SCZ-like rats64. In contrast, most of the previously published studies that contradict our findings had limitations such as small sample sizes, heterogeneity or short observation periods, which weakened their reliability. Taken together, findings regarding neuropeptide levels in patients with SCZ have been inconclusive and more research is needed to identify their role in SCZ.
In addition, our results showed that most of the neuropeptide levels in CSS were higher than in TRS, especially for OT and S.P. To the best of our knowledge, no studies have thus far explored the differences in neuropeptide levels between TRS and CSS. However, professional research on TRS has been undertaken65,66,67,68. Based on the results of previous studies with SCZ, schizophrenics can be grouped into a variety of patient segments69. Specifically, those with SCZ have diverse phenotypes, which are characterized by different indicators and symptoms of illness as well as life course, with various risk factors leading to disease, including a complex genetic load capacity, a broad spectrum of neurobiological characteristics implicating a pathophysiology of structure and function that is not shared by all patients, and a mixed range of expressed responses to treatment70. This demonstrates the heterogeneity which is characteristic of SCZ. It does, however, suggests new pathways involved in the pathogenesis of TRS. Compared with other patients with severe mental illness, TRS patients are difficult to treat, have poor social function recovery71, experience a heavy social and family economic burden72 and suffer from poor prognosis16. Furthermore, first-degree relatives of TRS have been shown to be at higher risk for SCZ compared with non-TRS patients and healthy controls73. In addition to hereditary variables and clinical symptoms, recent systematic studies have demonstrated that TRS patients have reduced gray matter, especially in the frontal lobe, increased white matter volume, and decreased striatal DA production compared to non-TRS patients74. Striatal synaptic DA has been implicated in the antipsychotic response, with 50% occupancy of D2 DA receptors required to alleviate clinical symptoms75. Similarly, we predicted that neuropeptides which co-localize and co-release with DA would endure similar modifications. Nevertheless, there is currently a lack of sufficient data to support this hypothesis. Further research may possibly establish that TRS has distinct neuropsychological traits.
In the present research, there is no doubt that cognitive function was significantly impaired in patients with SCZ who take antipsychotic drugs for a long period. This conclusion is consistent with a wealth of previous research evidence76,77,78,79. In terms of disease types, compared with other severe mental disorders, cognitive problems in patients with SCZ are most pronounced80. Virtually all people with SCZ experience cognitive impairment81. From the perspective of clinical symptoms, in comparison to positive symptoms such as hallucinations and delusions, cognitive function has been recognized as a therapy priority. While such recognition has been relatively late in coming, it is very crucial for SCZ patients’ long-term recovery81. For recovery from SCZ, cognitive deficiencies are apparent at the outset of disease82, must be endured into old age83, and are resistant to the effects of traditional medication for symptoms. Specifically, SCZ has been linked to substantial abnormalities in reasoning, planning, abstract thought and problem-solving, as well as impairments in working memory, attention, processing speed, and visual and linguistic acquisition84,85,86. However, studies in the field of cognitive function in TRS are scarce and findings suggest that cognitive deficits in TRS are more prevalent and severe87. Similarly, patients with TRS have been reported to have more prominent cognitive deficits in language domains, such as in speech, verbal intelligence, verbal fluency and verbal memory88. In contrast to the above findings, other authors have reported low scores on a nonverbal memory test of the Brief Cognitive Assessment of Schizophrenia in TRS patients in a cross-sectional study89. In a longitudinal study with at least 5 years of clinical follow-up, their findings suggested moderate improvement in cognitive function in TRS over time (Cognitive Index, mean effect size = 0.32)90. Nevertheless, overall cognitive deterioration (Cognitive Index, mean effect size = −0.64) was found to be more pronounced in TRS in a between-group comparison. Remarkably, our study was not statistically significant, although we found that the cognitive scores in TRS were lower than in CSS. Perhaps subsequent studies with larger samples will unveil the cognitive functioning domain of TRS.
Consistent with the latest emerging study54, we found that the level of cognitive function in schizophrenic patients was not implicated in neuropeptide levels such as S.P and OT. In contrast, our research found that the levels of NT, BE and α-MSH were significantly correlated with cognitive function. Evidence supporting the conclusions of our study has also been provided by Zak et al.91, who found that OT was not associated with executive function. Paradoxically, there is also a variety of clinical evidence pointing in the opposite direction, such as a randomized double-blind placebo-controlled trial47 in which OT recipients showed improvements in several cognitive indicators. Moreover, a recent study92 found a negative association (β = −0.46, p = 0.03) between plasma OT levels and cognitive function in patients with SCZ. Similarly, Urban-Kowalczyk et al.93 did not find any evidence linking S.P to cognitive function in patients with SCZ. Apparently, there has been little evidence reported regarding S.P and cognitive function in schizophrenic patients, and more evidence is required to confirm our conclusions.
Unlike S.P, which has received little attention, researchers have performed a great deal of work on BE and its relevance to the psychiatric field. The hypothesis of endogenous “hypermorphinergic pathology” has been proposed, suggesting that BE concentrations are significantly elevated in schizophrenic patients94. Increased BE levels are hypothesized to affect DA function, a precursor to endogenous opioids. Presynaptic opioid μ receptors, which also reduce DA pathway inhibition, enhance DA release95. Patricia Goldman-Rakic’s groundbreaking research96 revealed the neural circuitry required to generate mental representations in the dorsolateral prefrontal cortex for working memory, and she found that DA had a dramatic effect on this area of the brain, with DA depletion leading to severe impairment of working memory. Hence, enhancing beneficial behaviors with DA may be a reasonable way to improve cognitive deficits. Thus, elevated BE levels may improve cognitive deficits by affecting the DA pathway. Another neuropeptide that interacts closely with dopaminergic interactions is NT, and its mechanism of action has been extensively studied. In the nucleus ambiguus, NT receptors are co-localized with presynaptic and postsynaptic DA receptors. The binding effect of NT at these locations can be summarized as an antagonistic effect on D2 receptors. Notably, Fawaz et al.97 found that treatment with NT significantly enhanced DA release from the vomeronasal nucleus in rat brain slices during pulse string stimulation. Accordingly, we hypothesize that similar to BE, NT may have a similar effect on cognitive function. As expected, we discovered that the interaction of BE and NT was positively correlated with cognitive function. Consequently, we hypothesize that NT may affect cognitive function directly through the DA system or that NT may indirectly affect cognitive function through its interaction with BE.
In contrast to CSS, we found a significant correlation between α-MSH and cognitive function in TRS. However, similar studies are scarce, and a recent study54 has come to the opposite conclusion from our study. We currently have an understanding of the mechanisms underlying the role of α-MSH and the DA system in feeding behavior98, and other investigators have explored the role of α-MSH autoantibodies (autoAbs) in impaired cognitive function99. It has also been suggested that altered production of autoAbs which react with glycopeptides and α-MSH may be associated with Alzheimer’s disease-related peptidergic dysregulation. Increased levels of serum complex formation are associated with increased inhibition of α-MSH-mediated behavior compared to free α-MSH autoAbs100. As a result, elevated total and complexed galanin autoAbs may reflect enhanced inhibition of central galanin signaling in Alzheimer’s. Total and complexed IgG autoAb levels of galanin correlate positively with the Mini-mental State Examination score, showing that higher inhibition of galanin signaling may be associated with improved cognitive function101. Unfortunately, to date, the above hypothesis has not been confirmed in SCZ, and we could not verify that the correlation between α-MSH and cognitive function is yet another feature of TRS. However, it is likely that α-MSH will become a new approach to elucidate the pathological mechanism of schizophrenia.
There are several limitations that should be considered in the present research. First, our participants were on long-term antipsychotic medication, and we did not exclude the effect of long-term medication on neuropeptide levels. Second, our study was conducted with male subjects, and the findings need to be validated in the female population. Third, the sample sizes of our TRS and CSS patients were small, and the validity of our findings needs to be verified in a larger sample. Fourth, as a cross-sectional study, our findings have not been dynamically observed over time. Finally, although we observed some correlations between neuropeptide levels, cognitive function and treatment resistance, the underlying mechanisms remain to be investigated.
Conclusion
In conclusion, our research preliminarily demonstrates that chronic patients with SCZ have significantly higher serum neuropeptide levels compared to HC, including α-MSH, BE, NT, OT and S.P, while TRS patients have lower neuropeptide levels than CSS patients. Unexpectedly, cognitive function was significantly worse in chronic schizophrenic patients comparted to HC. In addition, the level of α-MSH in TRS was significantly and negatively correlated with cognitive function. BE and NT levels in CSS were significantly and positively correlated with cognitive function, and an interaction between BE and NT levels was observed. Considered together, we hypothesize that serum neuropeptides may have a specific modulatory action on particular aspects of cognitive function in SCZ. The mechanisms underlying the relationship between serum neuropeptides and SCZ deserve detailed investigation.
Material and methods
Participants
This study employed an observational, cross-sectional, retrospective design with a case-control approach, conducted from October 2018 to October 2020. A total of 29 patients diagnosed with TRS and 48 patients with chronic stable schizophrenia (CSS) were selected from of SCZ inpatients (all male) in local mental hospitals, specifically Lianyungang Fourth People’s Hospital. All patients met the following inclusion criteria: (1) aged 18–60 years old, Han Chinese; (2) diagnosed with schizophrenia based on the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV); (3) had a minimum illness duration of 2 years; and (4) received consistent administration of neuroleptic medications at stable doses for a minimum of 12 months, and (5) completed at least elementary school and demonstrated the ability to comprehend the questions posed by the investigators. Using accepted methods, antipsychotic drugs, including first- and second-generation antipsychotics, were translated into roughly similar daily milligram doses of chlorpromazine (CPZ) for each individual102. Exclusion criteria: first, patients who presented with somatic disorders or substance dependence were excluded. Second, patients who had experienced a major life event (e.g., divorce, widowhood, etc.) in the month prior to admission were excluded. In addition, patients who had not completed primary education were excluded from the study.
The local population of Lianyungang City was selected for the recruitment of 53 HC, all of whom were men and matched for age, education and BMI. To evaluate current mental state and any personal or family history of mental disorders, unstructured interviews were conducted. None of the healthy subjects presented a personal or family history of psychiatric disorders. Both the patients and control participants provided comprehensive medical histories (including use of sleep aids and antipsychotics), along with the results of physical examinations (e.g., vital signs, cardiopulmonary function, etc.) and laboratory tests (e.g., blood cell analysis, electrolytes, liver and kidney function, etc.). We thus ensured the inclusion of participants with normal results for these tests in our study. Subjects suffering from serious medical conditions were barred from participating. All healthy subjects were from the same ethnicity and geographic region as the patient group. A psychiatrist completely described the research protocol and procedures to each patient before they gave their written informed consent to participate in the study. The information regarding the study was conveyed in a manner that maximized comprehension, taking into account the subjects’ level of understanding and emotional readiness. In some cases, the researcher provided a detailed description to both the subjects and their parents or guardians. The Lianyungang Fourth People’s Hospital’s Institutional Review Board gave its approval to the research protocol. All methods were performed in accordance with the Declaration of Helsinki.
Defining TRS and CSS
The following criteria have been used in several earlier research studies to define TRS103,104,105,106: ineffectiveness of continuous treatment with two antipsychotic medications at an adequate dose for a duration exceeding 6 months; a minimum daily dose of chlorpromazine (CPZ) or its equivalent, equal to or greater than 600 mg; and a total score on the Positive and Negative Syndrome Scale (PANSS) of 70 or higher, with each of the eight individual PANSS items (P1, P2, P3, N1, N4, N6, G5, and G9) scoring at least 3. Likewise, the following criteria were used to define CSS based on the findings of numerous prior investigations103,104,105,106: continuous treatment with a single antipsychotic medication for a minimum of 6 months; CPZ or equivalent daily dose less than 600 mg; and a total PANSS score below 60, with each of the PANSS items described above scoring less than 3.
Application and scales
The diagnosis of those in the patient groups was first made by a DSM-IV-oriented clinical interview conducted by an experienced psychiatrist, and a sociodemographic data form was completed. Simultaneous assessment of disease symptoms and cognitive functions was performed on the first day of hospitalization (or within the first 3 days of hospitalization if the patient needed to adapt to the tests). In order to avoid the possible influence of medications (e.g., sleep aids) on the patients’ condition, we scheduled the assessment of psychiatric symptoms and cognition for the afternoon.
A sociodemographic and clinical data form was administered to collect sociodemographic data and disease history data from patients and control group participants. In the case of control group participants, the completion of the data form occurred subsequent to confirmation of the absence of any psychiatric diagnosis through a DSM-IV-oriented clinical interview. To evaluate cognitive functions, the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was utilized. The PANSS assesses positive and negative symptoms and general psychopathology in patients with psychiatric disorders and measures the severity of these symptoms. Two psychiatrists who had concurrently attended a training session in the use of the scale before the trial started independently assessed the psychopathology of the patients using the PANSS107 on the day of blood collection. Two separate PANSS scale interviews were conducted for each patient by the two psychiatrists after the training, guaranteeing the reliability and consistency of the obtained ratings throughout the study. In addition, replication of the PANSS total score assessment maintained an inter-rater reliability correlation coefficient >0.8. The two PANSS total scores of the same patient who met the requirements after enrollment were averaged as their final clinical symptom score.
Cognitive assessments
In this study, the Chinese version of RBANS108 was used to evaluate the cognitive abilities of SCZ patients and healthy controls. Given that RBANS takes about 30 min to complete and has demonstrated strong reliability and validity in individuals with psychosis, it was practical and realistic to employ it in a clinical setting. The cognitive domains under scrutiny encompassed five age-adjusted index scores, namely, the Immediate Memory Index, Visuospatial/Constructional Index, Language Index, Attention Index, and Delayed Memory Index, along with a composite total score. The initial neuropsychological variable scores were transformed to T-scores employing the established criteria provided in the relevant manuals. It is important to note that a higher RBANS score corresponds to an enhancement in cognitive performance.
Collection and analysis of biological samples
To guarantee a fasting state, all study participants fasted for 8 h prior to blood sampling, and between 7:00 and 9:30 a.m., 5 ml of elbow venous blood were drawn from each participant. The serum samples were kept frozen in a −80 °C refrigerator after the blood samples were centrifuged at 3000 r/min for 15 min to separate the upper serum layer.
Serum neuropeptides were measured using the Bio-Plex MAGPIX System (Human Neuropeptide Magnetic Bead Panel, item number HNPMAG-35K, Bio-Rad), a suspension microbead microarray platform based on the Luminex liquid phase suspension chip. Serum neuropeptides were expressed as one trillionth of a gram per milliliter plasma (pg/ml).
Statistical analysis
The study data were evaluated using the Statistical Product and Service Solutions 26.00 programme. After descriptive and frequency analysis, the groups were compared. Then, we examined the normality of the data using Q–Q plots and the Kolmogorov–Smirnov test. Given the non-normal distribution of serum neuropeptide levels among patients diagnosed with TRS, CSS, and HC (confirmed by the Kolmogorov–Smirnov test, all p < 0.05), we employed a logarithmic transformation (base 10) to convert the serum neuropeptide levels into normally distributed data. Categorical variables were evaluated by the chi-square test, while continuous variables were compared using Student’s t test or one-way analysis of variance. Data are presented as means ± standard deviation. All p values are two-tailed and the significance level was set at 0.05.
First, using diagnosis as a fixed factor and age, years of education, BMI and smoking status as covariates, multivariate analysis of covariance (MANCOVA) was carried out to compare differences in serum neuropeptide levels and RBANS scores (dependent variables) among TRS patients, CSS patients and HC. Second, using diagnosis set as a fixed factor and age, years of education, BMI, smoking status, age at onset, duration of illness and CPZ equivalence dose as covariates, analysis of covariance (ANCOVA) was used to compare differences in serum neuropeptides levels (dependent variables) between the TRS and CSS groups. In addition, multiple testing was adjusted with the Bonferroni correction (0.05/5). Third, Pearson’s correlation coefficient was used for correlation analysis. Finally, after adjusting for confounding variables such age, years of education, smoking status, BMI, age of onset, length of illness and CPZ equivalent dose, exploratory multiple regression analysis was used to determine the connection between serum neuropeptide levels and RBANS scores.
Data availability
The data supporting the results of this study are available upon request from the corresponding author.
References
Jongsma, H. E. et al. Treated incidence of psychotic disorders in the multinational EU-GEI study. JAMA Psychiatry 75, 36 (2018).
Rössler, W., Joachim Salize, H., van Os, J. & Riecher-Rössler, A. Size of burden of schizophrenia and psychotic disorders. Eur. Neuropsychopharmacol. 15, 399–409 (2005).
Charlson, F. J. et al. Global epidemiology and burden of schizophrenia: findings from the Global Burden of Disease Study 2016. Schizophr. Bull. 44, 1195–1203 (2018).
Hjorthøj, C., Stürup, A. E., McGrath, J. J. & Nordentoft, M. Years of potential life lost and life expectancy in schizophrenia: a systematic review and meta-analysis. Lancet Psychiatry 4, 295–301 (2017).
Dennerstein, L., Lehert, P., Bäckström, T. C. & Heinemann, K. Premenstrual symptoms—severity, duration and typology: an international cross-sectional study. Menopause Int. 15, 120–126 (2009).
Kalkstein, S., Hurford, I. & Gur, R. C. Neurocognition in schizophrenia. Curr. Top Behav. Neurosci. 4, 373–390 (2010).
Millan, M. J., Fone, K., Steckler, T. & Horan, W. P. Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur. Neuropsychopharmacol. 24, 645–692 (2014).
Jauhar, S., Johnstone, M. & McKenna, P. J. Schizophrenia. Lancet 399, 473–486 (2022).
McGregor, C., Riordan, A. & Thornton, J. Estrogens and the cognitive symptoms of schizophrenia: possible neuroprotective mechanisms. Front. Neuroendocrinol. 47, 19–33 (2017).
Reichenberg, A. The assessment of neuropsychological functioning in schizophrenia. Dialogues Clin. Neurosci. 12, 383–392 (2010).
Sheffield, J. M., Karcher, N. R. & Barch, D. M. Cognitive deficits in psychotic disorders: a lifespan perspective. Neuropsychol. Rev. 28, 509–533 (2018).
Rodríguez, B. et al. Neuropeptides and oligopeptidases in schizophrenia. Neurosci. Biobehav. Rev. 108, 679–693 (2020).
Haddad, P. M. & Correll, C. U. The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses. Ther. Adv. Psychopharmacol. 8, 303–318 (2018).
Leucht, S. et al. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet 373, 31–41 (2009).
Howes, O. D., McCutcheon, R., Owen, M. J. & Murray, R. M. The role of genes, stress, and dopamine in the development of schizophrenia. Biol. Psychiatry 81, 9–20 (2017).
Nucifora, F. C., Woznica, E., Lee, B. J., Cascella, N. & Sawa, A. Treatment resistant schizophrenia: clinical, biological, and therapeutic perspectives. Neurobiol. Dis. 131, 104257 (2019).
Frydecka, D., Beszłej, J. A., Gościmski, P., Kiejna, A. & Misiak, B. Profiling cognitive impairment in treatment-resistant schizophrenia patients. Psychiatry Res. 235, 133–138 (2016).
Pillinger, T. et al. Altered glutamatergic response and functional connectivity in treatment resistant schizophrenia: the effect of riluzole and therapeutic implications. Psychopharmacology 236, 1985–1997 (2019).
Lowe, P. et al. When the drugs don’t work: treatment-resistant schizophrenia, serotonin and serendipity. Ther. Adv. Psychopharmacol. 8, 63–70 (2018).
De Berardis, D. et al. Safety of antipsychotics for the treatment of schizophrenia: a focus on the adverse effects of clozapine. Ther. Adv. Drug Saf. 9, 237–256 (2018).
Creese, I., Burt, D. R. & Snyder, S. H. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 192, 481–483 (1976).
Johnstone, E. V. E. C., Frith, C. D., Crow, T. J., Carney, M. W. P. & Price, J. S. Mechanism of the antipsychotic effect in the treatment of acute schizophrenia. Lancet 311, 848–851 (1978).
Seeman, P., Lee, T., Chau-Wong, M. & Wong, K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature 261, 717–719 (1976).
Pogarell, O. et al. Dopaminergic neurotransmission in patients with schizophrenia in relation to positive and negative symptoms. Pharmacopsychiatry 45, S36–S41 (2012).
Pearlson, G. D. Neurobiology of schizophrenia. Ann. Neurol. 48, 556–566 (2000).
Cauli, B. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J. Neurosci. 24, 8940–8949 (2004).
Salio, C., Lossi, L., Ferrini, F. & Merighi, A. Neuropeptides as synaptic transmitters. Cell Tissue Res. 326, 583–598 (2006).
Hökfelt, T., Everitt, B. J., Theodorsson-Norheim, E. & Goldstein, M. Occurrence of neurotensinlike immunoreactivity in subpopulations of hypothalamic, mesencephalic, and medullary catecholamine neurons. J. Comp. Neurol. 222, 543–559 (1984).
LaCrosse, A. & Olive, M. Neuropeptide systems and schizophrenia. CNSNDDT 12, 619–632 (2013).
Binder, E. B., Kinkead, B., Owens, M. J. & Nemeroff, C. B. Neurotensin and dopamine interactions. Pharmacol. Rev. 53, 453–486 (2001).
Jomphe, C., Lemelin, P.-L., Okano, H., Kobayashi, K. & Trudeau, L.-E. Bidirectional regulation of dopamine D2 and neurotensin NTS1 receptors in dopamine neurons. Eur. J. Neurosci. 24, 2789–2800 (2006).
Cáceda, R., Kinkead, B. & Nemeroff, C. B. Involvement of neuropeptide systems in schizophrenia: human studies. Int. Rev. Neurobiol. 78, 327–376 (2007).
Lefevre, A., Hurlemann, R. & Grinevich, V. Imaging neuropeptide effects on human brain function. Cell Tissue Res. 375, 279–286 (2019).
Griebel, G. & Holsboer, F. Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat. Rev. Drug Discov. 11, 462–478 (2012).
McGonigle, P. Peptide therapeutics for CNS indications. Biochem. Pharmacol. 83, 559–566 (2012).
Fan, W., Boston, B. A., Kesterson, R. A., Hruby, V. J. & Cone, R. D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385, 165–168 (1997).
Getting, S. J. Melanocortin peptides and their receptors: new targets for anti-inflammatory therapy. Trends Pharmacol. Sci. 23, 447–449 (2002).
Raue, S., Wedekind, D., Wiltfang, J. & Schmidt, U. The role of proopiomelanocortin and α-melanocyte-stimulating hormone in the metabolic syndrome in psychiatric disorders: a narrative mini-review. Front. Psychiatry 10, 834 (2019).
Doron, R., Fridman, L. & Yadid, G. Dopamine-2 receptors in the arcuate nucleus modulate cocaine-seeking behavior. NeuroReport 17, 1633–1636 (2006).
Simmons, D. & Self, D. W. Role of Mu- and Delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology 34, 1946–1957 (2009).
Alonso, J. et al. Severe role impairment associated with mental disorders: results of the WHO World Mental Health Surveys International College Student Project. Depress. Anxiety 35, 802–814 (2018).
Charmandari, E., Tsigos, C. & Chrousos, G. Endocrinology of the stress response. Annu. Rev. Physiol. 67, 259–284 (2005).
Torruella-Suárez, M. L. & McElligott, Z. A. Neurotensin in reward processes. Neuropharmacology 167, 108005 (2020).
Woodworth, H. L., Brown, J. A., Batchelor, H. M., Bugescu, R. & Leinninger, G. M. Determination of neurotensin projections to the ventral tegmental area in mice. Neuropeptides 68, 57–74 (2018).
Feifel, D. et al. The reversal of amphetamine-induced locomotor activation by a selective neurotensin-1 receptor agonist does not exhibit tolerance. Psychopharmacology 200, 197–203 (2008).
Davis, M. C. et al. Effects of single dose intranasal oxytocin on social cognition in schizophrenia. Schizophr. Res. 147, 393–397 (2013).
Pedersen, C. A. et al. Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr. Res. 132, 50–53 (2011).
Feifel, D., MacDonald, K., Cobb, P. & Minassian, A. Adjunctive intranasal oxytocin improves verbal memory inpeople with schizophrenia. Schizophr. Res. 139, 207–210 (2012).
Kormos, V. & Gaszner, B. Role of neuropeptides in anxiety, stress, and depression: from animals to humans. Neuropeptides 47, 401–419 (2013).
Ebner, K. & Singewald, N. The role of substance P in stress and anxiety responses. Amino Acids 31, 251–272 (2006).
Mu, L. et al. Sex differences in association between clinical correlates and cognitive impairment in patients with chronic schizophrenia. J. Psychiatr. Res. 131, 194–202 (2020).
Zhu, R. et al. Sex difference in association between insomnia and cognitive impairment in patients with chronic schizophrenia. Schizophr. Res. 240, 143–149 (2022).
Halari, R. et al. The relationship of sex hormones and cortisol with cognitive functioning in Schizophrenia. J. Psychopharmacol. 18, 366–374 (2004).
Hidese, S. et al. Plasma neuropeptide levels in patients with schizophrenia, bipolar disorder, or major depressive disorder and healthy controls: a multiplex immunoassay study. Neuropsychopharmacol. Rep. 43, 57–68 (2023).
Urban-Kowalczyk, M., Kotlicka-Antczak, M., Strzelecki, D., Rudecka, E., & Śmigielski, J. The relationship between course of illness and β-endorphin plasma levels in patients with schizophrenia. Neuropsychiatr. Dis. Treat. 15, 3609–3614 (2019).
Urban-Kowalczyk, M., Kotlicka-Antczak, M., Strzelecki, D., Rudecka, E. & Śmigielski, J. Plasma β-endorphin concentration and antipsychotic treatment outcome in schizophrenia: 1-year follow-up. Med. Sci. Monit. 26, e924307 (2020).
Goldman, M., Marlow-O’Connor, M., Torres, I. & Carter, C. S. Diminished plasma oxytocin in schizophrenic patients with neuroendocrine dysfunction and emotional deficits. Schizophr. Res. 98, 247–255 (2008).
Jobst, A. et al. Oxytocin and vasopressin levels are decreased in the plasma of male schizophrenia patients. Acta Neuropsychiatr. 26, 347–355 (2014).
Beckmann, H., Lang, R. E. & Gattaz, W. F. Vasopressin—oxytocin in cerebrospinal fluid of schizophrenic patients and normal controls. Psychoneuroendocrinology 10, 187–191 (1985).
Strauss, G. P. et al. Factor structure of the Brief Negative Symptom Scale. Schizophr. Res. 142, 96–98 (2012).
Strauss, G. P. et al. Plasma oxytocin levels predict social cue recognition in individuals with schizophrenia. Schizophr. Res. 162, 47–51 (2015).
Breslin, N. A. et al. CSF concentrations of neurotensin in schizophrenia: an investigation of clinical and biochemical correlates. Schizophr. Res. 12, 35–41 (1994).
Sharma, R. P., Janicak, P. G., Bissette, G. & Nemeroff, C. B. CSF neurotensin concentrations and antipsychotic treatment in schizophrenia and schizoaffective disorder. Am. J. Psychiatry 154, 1019–1021 (1997).
Ni, P. et al. Plasma neuropeptides as circulating biomarkers of multifactorial schizophrenia. Compr. Psychiatry 94, 152114 (2019).
Kane, J. M. et al. Clinical guidance on the identification and management of treatment-resistant schizophrenia. J. Clin. Psychiatry 80, 18com12123 (2019).
Meltzer, H. Y., Share, D. B., Jayathilake, K., Salomon, R. M. & Lee, M. A. Lurasidone improves psychopathology and cognition in treatment-resistant schizophrenia. J. Clin. Psychopharmacol. 40, 240–249 (2020).
Siskind, D. et al. Rates of treatment-resistant schizophrenia from first-episode cohorts: systematic review and meta-analysis. Br. J. Psychiatry 220, 115–120 (2022).
Vita, A. et al. Treatment-resistant schizophrenia: genetic and neuroimaging correlates. Front. Pharmacol. 10, 402 (2019).
Bleuler, E. [Dementia praecox or the group of schizophrenias]. Vertex 21, 394–400 (2010).
Kinon, B. J. The group of treatment resistant schizophrenias. Heterogeneity in treatment resistant schizophrenia (TRS). Front. Psychiatry 9, 757 (2018).
Burton, C. Z. et al. Factor structure of the MATRICS Consensus Cognitive Battery (MCCB) in schizophrenia. Schizophr. Res. 146, 244–248 (2013).
Kennedy, J. L., Altar, C. A., Taylor, D. L., Degtiar, I. & Hornberger, J. C. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int. Clin. Psychopharmacol. 29, 63–76 (2014).
Joober, R. et al. Increased prevalence of schizophrenia spectrum disorders in relatives of neuroleptic-nonresponsive schizophrenic patients. Schizophr. Res. 77, 35–41 (2005).
Nakajima, S. et al. Neuroimaging findings in treatment-resistant schizophrenia: a systematic review: lack of neuroimaging correlates of treatment-resistant schizophrenia. Schizophr. Res. 164, 164–175 (2015).
Demjaha, A., Murray, R. M., McGuire, P. K., Kapur, S. & Howes, O. D. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am. J. Psychiatry 169, 1203–1210 (2012).
Dickinson, D., Ramsey, M. E. & Gold, J. M. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch. Gen. Psychiatry 64, 532–542 (2007).
Gebreegziabhere, Y., Habatmu, K., Mihretu, A., Cella, M. & Alem, A. Cognitive impairment in people with schizophrenia: an umbrella review. Eur. Arch. Psychiatry Clin. Neurosci. 272, 1139–1155 (2022).
Heinrichs, R. W. & Zakzanis, K. K. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 12, 426–445 (1998).
Schaefer, J., Giangrande, E., Weinberger, D. R. & Dickinson, D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr. Res. 150, 42–50 (2013).
Buchanan, R. W. et al. A summary of the FDA-NIMH-MATRICS workshop on clinical trial design for neurocognitive drugs for schizophrenia. Schizophr. Bull. 31, 5–19 (2005).
Mihaljević-Peleš, A. et al. Cognitive deficit in schizophrenia: an overview. Psychiatr. Danub 31, 139–142 (2019).
Kremen, W. S. et al. Cognitive decline in schizophrenia from childhood to midlife: a 33-year longitudinal birth cohort study. Schizophr. Res. 118, 1–5 (2010).
Irani, F., Kalkstein, S., Moberg, E. A. & Moberg, P. J. Neuropsychological performance in older patients with schizophrenia: a meta-analysis of cross-sectional and longitudinal studies. Schizophr. Bull. 37, 1318–1326 (2011).
Bowie, C. R. & Harvey, P. D. Cognition in schizophrenia: impairments, determinants, and functional importance. Psychiatr. Clin. North Am. 28, 613–633, 626 (2005).
Kayman, D. & Goldstein, M. Cognitive deficits in schizophrenia. Curr. Transl. Geriatr. Gerontol. 1, 45–52 (2012).
Kurtz, M. M., Gopal, S., John, S. & Thara, R. Cognition, social cognition and functional disability in early-stage schizophrenia: a study from southern India. Psychiatry Res. 265, 231–237 (2018).
Joober, R. et al. Neuropsychological impairments in neuroleptic-responder vs. -nonresponder schizophrenic patients and healthy volunteers. Schizophr. Res. 53, 229–238 (2002).
Iasevoli, F. et al. Treatment resistant schizophrenia is associated with the worst community functioning among severely-ill highly-disabling psychiatric conditions and is the most relevant predictor of poorer achievements in functional milestones. Prog. Neuropsychopharmacol. Biol. Psychiatry 65, 34–48 (2016).
de Bartolomeis, A. et al. Differential cognitive performances between schizophrenic responders and non-responders to antipsychotics: correlation with course of the illness, psychopathology, attitude to the treatment and antipsychotics doses. Psychiatry Res. 210, 387–395 (2013).
Spangaro, M. et al. Longitudinal course of cognition in schizophrenia: does treatment resistance play a role? J. Psychiatr. Res. 141, 346–352 (2021).
Zak, P. J., Kurzban, R. & Matzner, W. T. Oxytocin is associated with human trustworthiness. Horm. Behav. 48, 522–527 (2005).
Rubin, L. H. et al. Peripheral oxytocin and vasopressin are associated with clinical symptom severity and cognitive functioning in midlife women with chronic schizophrenia. Schizophr. Res. 195, 409–411 (2018).
Urban-Kowalczyk, M., Pigońska, J., & Śmigielski, J. Pain perception in schizophrenia: influence of neuropeptides, cognitive disorders, and negative symptoms. Neuropsychiatr. Dis. Treat. 11, 2023–2031 (2015).
Volavka, J., Davis, L. G. & Ehrlich, Y. H. Endorphins, dopamine, and schizophrenia. Schizophr. Bull. 5, 227–239 (1979).
Koneru, A., Satyanarayana, S. & Rizwan, S. Endogenous opioids: they physiological role and receptors. Global J. Pharmacol. 3, 149–153 (2009).
Brozoski, T. J., Brown, R. M., Rosvold, H. E. & Goldman, P. S. Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey. Science 205, 929–932 (1979).
Fawaz, C. S., Martel, P., Leo, D. & Trudeau, L.-E. Presynaptic action of neurotensin on dopamine release through inhibition of D(2) receptor function. BMC Neurosci. 10, 96 (2009).
Roseberry, A. G., Stuhrman, K. & Dunigan, A. I. Regulation of the mesocorticolimbic and mesostriatal dopamine systems by α-melanocyte stimulating hormone and agouti-related protein. Neurosci. Biobehav. Rev. 56, 15–25 (2015).
Costa, A. et al. Galanin and α-MSH autoantibodies in cerebrospinal fluid of patients with Alzheimer’s disease. J. Neuroimmunol. 240–241, 114–120 (2011).
Sinno, M. H. et al. Regulation of feeding and anxiety by alpha-MSH reactive autoantibodies. Psychoneuroendocrinology 34, 140–149 (2009).
Ogren, S. O., Kuteeva, E., Elvander-Tottie, E. & Hökfelt, T. Neuropeptides in learning and memory processes with focus on galanin. Eur. J. Pharmacol. 626, 9–17 (2010).
Woods, S. W. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry 64, 663–667 (2003).
Andreasen, N. C. et al. Remission in schizophrenia: proposed criteria and rationale for consensus. AJP 162, 441–449 (2005).
Howes, O. D. et al. Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group consensus guidelines on diagnosis and terminology. AJP 174, 216–229 (2017).
Østergaard, S. D., Foldager, L., Mors, O., Bech, P. & Correll, C. U. The validity and sensitivity of PANSS-6 in treatment-resistant schizophrenia. Acta Psychiatr. Scand. 138, 420–431 (2018).
Suzuki, T. et al. Defining treatment-resistant schizophrenia and response to antipsychotics: a review and recommendation. Psychiatry Res. 197, 1–6 (2012).
Kay, S. R., Fiszbein, A. & Opler, L. A. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophr. Bull. 13, 261–276 (1987).
Randolph, C., Tierney, M. C., Mohr, E. & Chase, T. N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J. Clin. Exp. Neuropsychol. 20, 310–319 (1998).
Acknowledgements
We sincerely appreciate everyone who participated in the research, both the patients and healthy controls. This work was supported by Suzhou Minsheng Technology Project (SYSD2020161), Key Diagnosis and treatment Program of Suzhou (LCZX202118), the Suzhou Clinical Medical Center for Mood Disorders (Szlcyxzx202109), the Suzhou Clinical Key disciplines for Geriatric Psychiatry (SZXK202116), Suzhou Science and Technology Development Programme Youth Project (SKYD2023160) and National Mentorship Training Programme for Young Health Professionals (Qngg2022027). The study’s funding sources had no influence on the study’s planning, data gathering, analysis, publication choice, or paper preparation.
Author information
Authors and Affiliations
Contributions
X.Z., G.Z., W.S. and T.J. were responsible for study design, statistical analysis and writing of the manuscript. H.Y. and J.L. were responsible for laboratorial analysis. Q.T., J.G. and R.P. were responsible for recruiting the patients, performing the clinical rating and collecting the samples. All authors have contributed to and have approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, W., Jin, T., Yang, H. et al. Alterations of serum neuropeptide levels and their relationship to cognitive impairment and psychopathology in male patients with chronic schizophrenia. Schizophr 10, 3 (2024). https://doi.org/10.1038/s41537-023-00425-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41537-023-00425-1