Abstract
Spaceflight associated neuro-ocular syndrome (SANS) alters the vision of astronauts during long-duration spaceflights. There is controversy regarding SANS being similar to patients with idiopathic intracranial hypertension (IIH). IIH has been shown to be due to an elevation in venous sinus pressure. The literature suggests an increase in jugular vein pressure secondary to a headward shift of fluid occurs in SANS but this may not be enough to significantly alter the intracranial pressure (ICP). The literature regarding cardiac output and cerebral blood flow (CBF) in long-duration spaceflight is contradictory, however, more recent data suggests increased flow. Recent modelling has shown that an increase in CBF can significantly increase sinus pressure. The purpose of the present paper is to review the SANS vascular dynamics literature and through mathematical modelling suggest the possible underlying cause of SANS as an elevation in venous sinus pressure, secondary to the redistribution of fluids towards the head, together with a significant increase in pressure drop across the venous system related to the CBF.
Similar content being viewed by others
Introduction
Spaceflight associated neuro-ocular syndrome (SANS) refers to the pathological effects of long-term microgravity on the eyes and orbital physiology of astronauts. The clinical manifestations of SANS include unilateral and bilateral optic disc oedema, globe flattening, choroidal and retinal folds, hyperopic refractive error shifts, and focal areas of ischaemic retina1. Although 15% of astronauts have clinically significant optic disc oedema, subclinical oedema has been detected in many astronauts returning from long-duration flights1.
It has been suggested the findings in SANS may be similar to terrestrial idiopathic intracranial hypertension (IIH)1, however, others disagree2. IIH is characterised by an increased intracranial pressure (ICP) in the absence of parenchymal brain lesions, vascular malformations, hydrocephalus or central nervous system infection3. To diagnose IIH, a cerebrospinal fluid (CSF) pressure above 25 cm H2O (18.4 mmHg) is required according to the published guidelines4. A patient is suggested of having IIH if they have 3 out of 4 additional imaging criteria i.e. compression of the pituitary, flattening of the posterior globe, distention of the optic nerve sheath diameter (ONSD) or transverse venous sinus stenosis4. In long flight astronauts, globe flattening was seen in 26%, optic nerve protrusion (optic disc oedema) in 15% and pituitary flattening in 11%5. Seven astronauts with globe flattening had a mean ONSD of 7.2 mm compared to 20 without globe flattening who had a mean ONSD of 5.8 mm (p = 0.01)5. Although it should be acknowledged there was no pre-flight control data, the ONSD findings in the seven with orbital flattening are above the level associated with IIH5. Thus, three of the imaging criteria for IIH are found in long flight veterans. Three of the astronauts underwent post-flight lumbar puncture showing pressures of 23, 28 and 29 cm H2O5 (inflight data is not available), with 2 of these pressures being in the diagnostic range for IIH4. A counterpoint paper suggests there is no evidence of ONSD enlargement secondary to spaceflight6 but the study was underpowered and had only one individual who developed SANS. This individual had an increase in ONSD in the range suggestive of IIH.
It can be seen that by utilising the revised IIH criteria, there are some astronauts who would fulfil the criteria for IIH. The purpose of this perspective is to review the literature in SANS with respect to the blood and CSF dynamics. Following this, computational fluid dynamics modelling will be utilised to suggest the possible causes of SANS in astronauts undergoing long-duration space flight and to suggest a vascular aetiology for SANS which is similar to IIH.
Discussion
There are similarities between SANS and IIH, however, there are also differences. Terrestrial symptoms of IIH include headache and pulsatile tinnitus, but astronauts with SANS do not complain of these symptoms7. IIH presents more often as bilateral eye changes, predominately in women, but SANS has a higher asymmetric or unilateral presentation and is more common in men1. Transient visual obscurations or diplopia secondary to a non-localising sixth nerve palsy have never been reported in astronauts with SANS, unlike IIH1. Despite these differences, there remain enough similarities to suggest some commonality may exist in the pathophysiology of both.
The main criteria for IIH is an elevation in the ICP which is modelled using Davson’s equation
where FRCSF is the CSF formation rate, Rout is the CSF outflow resistance and SSSp is the superior sagittal sinus pressure. The sagittal sinus pressure is analogous to Ohm’s law, i.e. pressure is the product of the outflow resistance and blood flow through the outflow plus the jugular bulb pressure8. So Eq. (1) can be expanded to
where TCBF is the total blood flow leaving the capillaries to enter the venous system, Rven is the venous outflow resistance from the sagittal sinus to the jugular bulbs and JBP is the jugular bulb pressure. The CSF formation rate is normal in IIH and decreases as the ICP increases9. The pressure gradient between the CSF and sagittal sinus was found to low-normal at 2.34 mmHg in one paper in IIH10 and 2.7 mmHg in another11. Rearranging Eq. (1) by subtraction, the ICP to sagittal sinus pressure gradient is equal to the FRcsf x Rout, and if this term is normal, then the elevation in ICP in IIH can only be due to elevated venous pressure. This discussion tends to validate the original assertion made by Karahalios et al. i.e. “an elevated venous pressure is the universal mechanism underlying IIH”12. Is a venous pressure elevation also the universal cause of SANS?
The normal ICP is 15.6 cm H2O or 11.5 mmHg in the lateral decubitus position in adults between 20 and 49 years13. The criteria for IIH is an ICP of 18.4 mmHg (or 25 cm H2O)4. Thus a prolonged increase in venous sinus pressure of approximately 7 mmHg would be required if SANS were analogous to IIH. As previously described, the venous sinus pressure depends on the combination of the jugular bulb pressure, venous outflow resistance and the total blood flow passing through the venous system. In adults with IIH, 71% are obese14. Obesity raises the jugular bulb pressure by up to 20 mmHg15. Up to 90% of adults with IIH have been found to have outflow stenosis mostly sited at the middle of the transverse sinus, raising the venous resistance and therefore the venous pressure16. Sixty-six per cent of the increase in ICP in IIH is due to venous stenosis and 34% is due to obesity17. Astronauts are not obese1. A recent paper comparing the venous sinus volumes, both before and after spaceflight, showed a significant increase in the size of the superior sagittal, transverse and sigmoid sinuses (not stenosis) in those who developed SANS, compared to those who did not18. In another paper, in three long-duration astronauts with borderline or elevated CSF opening pressures, the MRV images were reported as normal with no evidence of stenosis or thrombosis19.
Therefore, if the physiology of SANS is similar to IIH, there must be other factors elevating the venous sinus pressure.
Current hypotheses regarding SANS aetiology
The main hypothesis regarding the cause of SANS centres on a rise in ICP due to the cephalad fluid shifts occurring during long-duration spaceflight1. Similar to obesity, this would have the effect of directly raising the venous sinus pressure. A second hypothesis suggests that CSF accumulates in the optic nerve sheath due to a one-way valve mechanism20. This hypothesis would explain the eye findings but not the elevated ICP or pituitary compression found in some astronauts. A third hypothesis suggests that SANS is due to upward shift of the brain putting tension on the optic nerve sheath21. This hypothesis is not necessarily mutually exclusive of hypothesis 1 but of itself does not explain the elevation in ICP.
Cephalad fluid shift hypothesis
Ideally, all hypotheses would be tested on astronauts whilst in space, during a long-duration flight. However, the numbers of subjects available are limited, an astronaut’s time in space is precious and the technique used must be achievable in space and must match the expertise of the subjects. For example, performing magnetic resonance imaging (MRI) or lumbar punctures on board the International Space Station is not feasible. Thus, ground-based space analogues are utilised. Analogues such as supine bed rest, head-down tilt bed rest (HDTBR), wet and dry immersion, lower-extremity limb suspension and parabolic flight have been tried22.
One space-based study used ultrasound-guided jugular vein compression. The terrestrial jugular venous pressure varies between the two-thirds of the day one spends upright and the one-third supine. If one weighted the pressures found (66% of the upright figure plus 33% of the supine) then the 24-h weighted average jugular venous pressure was found to be 9.2 mmHg when on the ground and increased by 6.6 mmHg at day 150 during long term weightlessness23. Note that this is very close to the 7 mmHg increase in pressure required to trigger terrestrial IIH. Head-down tilt experiments, similar to the jugular vein compression study, indicate that a headward redistribution of fluid brings about a dilatation of the jugular veins with some evidence of increased ICP24. Strict head-down tilt experiments can cause retinal thickening and chorioretinal folds but no changes in refractive error or perimetry developed25. This suggests the headward shift is able to produce a syndrome similar to mild SANS.
Cardiac output during long-term spaceflight and HDTBR
During spaceflight, the cardiac output (CO) appears to depend on which technique is used. Two studies using ultrasound echocardiography observed a decrease in stroke volume and CO during flight26,27. Hughson et al. used the continuous finger blood pressure contour technique and found no changes in stroke volume28. However, Norsk et al. found significant increases in stroke volume and CO of as much as 35 and 41%, respectively, between 3 and 6 months of flight on the ISS29. A foreign gas rebreathing technique was used29. Hughson et al. later measured CO and stroke volume by the same method as Norsk et al. and found similar increases30. They concluded that their previously unchanged stroke volume and CO estimations had been incorrect30. The increase in CO of 41–56% after months29,30 is higher than the increase measured by the same technique i.e.18–26% reported from shorter week long-Space Shuttle missions31,32,33. Thus, long durations in space produce higher stroke volumes and COs than a short duration. Norsk comments, both foreign gas rebreathing and ultrasound techniques have been validated thoroughly and multiple times against invasive more direct, gold standard techniques. The foreign gas rebreathing method is observer-independent and obtains data over a longer time period (20 s) than the ultrasound Doppler (three cardiac cycles) and that the breathing frequency and depth are controlled and kept constant during the measurements. Thus, he suggests the gas technique is probably correct34. The cerebral blood flow (CBF) at rest is 15–20% of the total CO35 and the renal blood flow is 20% of CO36, In a low powered study of only 5 individuals, the renal plasma flows increased by 17% at 1 week in orbit but this was not significantly different to preflight values37. If a larger cohort had found this difference to be significant then this would be similar to the 18–26% increase in CO found by Norsk31,32,33. The CO must all pass through a capillary bed to return to the heart. If the CO is increased by 41% then the renal and CBFs cannot be normal.
CBF in space flight and HDTBR
Similar to the findings in CO, the CBF findings seem to depend on the technique being used. A 5-day flight of a Rhesus monkey on the Cosmos 1514 biosatellite fitted with a carotid flow sensor showed a sustained 8 cm/s increase in blood velocity38. Impedance rheoencephalography has shown increased CBF both during and immediately after flight39,40,41,42,43. Transcranial Doppler (TCD) showed an increase in straight sinus velocity in 9/13 astronauts, with flow velocities ranging from 30–47 cm/sec and the normal range being 14–28 cm/sec44.
The transcranial Doppler of the middle cerebral arterial literature in spaceflight is contradictory. Some studies suggest an increase in cerebral blood velocity45,46,47,48, some unchanged49,50 and some decreased flow51,52. The divergence between the previously described measures of CBF and the transcranial Doppler velocity is striking. The original validation studies for TCD showed the correlation between the arterial velocity and the gold standard for CBF (xenon 133 CT) was weak at r = 0.4253. This is because the CBF depends on both the velocity of the blood and the cross-sectional area of the vessel. If the middle cerebral artery is constricted, the CBF will fall but the velocity will increase and in reverse, the velocity will drop but the flow increases when the diameter increases54. The middle cerebral artery lumen can increase in size by 45% by altering carbon dioxide (CO2) levels from low-normal to high-normal55. Theoretically, if the CBF increased purely due to an increase in diameter, then the TCD could underestimate the effect by 45%. Indeed, Coverdale et al. found a change in middle cerebral artery cross-sectional area during acute hypercapnia resulted in a CBF underestimation by TCD of 18%56.
A recent study has shown a significant increase in the middle cerebral vein velocity on day 150 of spaceflight and ascribed this to a reduction in the vein diameter with a resulting normal venous flow volume57. However, the 84% increase in velocity found would require a 46% reduction in vein diameter to give a normal blood flow volume57. This would require the ICP to approach cerebral perfusion pressure58. This is because as the ICP increases the collapse of veins begins close to their outflow with the more proximal sections (where TCD measures velocity) being increased in size59. Only once the pressure reaches perfusion pressure will the whole vein collapse. No one suggests ICP approaches perfusion pressure in space as this would be lethal. Roberts et al. found some elevation of the brain and crowding of the sulci over the vertex and suggested this could lead to venous narrowing or arachnoid granulation obstruction. However, static pressures are equally distributed throughout a water bath and the reduction in the sulci was mild and unlikely to be water tight58. If the TCD vein diameter is normal then an increase in velocity must indicate an increase in blood flow volume.
Similar to the TCD findings, HDTBR studies tend to show a reduced CBF. This is perhaps not surprising as HDTBR also produces a reduction in cardiac stroke volume and CO60. There appears to be a distinct physiological difference between spaceflight and HDTBR. Thirteen days of space flight in a mouse model led to both a decrease in myogenic vasoconstrictor tone and an increase in arterial distention, leading to a 20% increase in resting cross-sectional area61. This would be consistent with lower vascular resistance and higher CBF during space flight61. This compared to control head-down tilt mice where there was no effect on vasoconstriction, passive pressure-diameter response or maximal diameter61. Using an MRI arterial spin labelling (ASL) technique, 30 days of head-down tilt plus increased CO2, demonstrated the expected decrease in CBF62. However, the five participants who developed optic disc oedema had higher CBF (approaching the baseline levels) compared with the non-oedema group62. This suggests that variations in vasoreactivity and autoregulatory responses to CO2 may play a role in SANS development62. Acute hypercapnia increases the CBF but the effect is short-lived being corrected within a few hours63. However, there is a delayed, more long-lasting vasodilatation occurring with chronic CO2 elevation which appears to be genetically mediated63. In chronic obstructive pulmonary disease, patients retain CO2. A reduction in the CO2 by non-invasive positive pressure ventilation reduced the middle cerebral artery TCD velocity by 23% without affecting the oxygen levels64. It should be noted TCD may have underestimated this effect. In a series of patients undergoing ASL MRI, those found to have an elevated CO2 had a grey matter CBF double the patients with CO2 in the normal range65. The effect was not statistically different between acute and chronic hypercapnia65. Overall, there was a 6.7% increase in CBF per 1-mmHg rise in partial pressure of CO265. Interestingly, women tended to be more resistant to the effect of CO2 than men65 which may point to a reason why men are more prone to develop SANS than women.
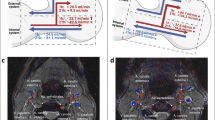
Thus, an increase in CO and CBF in long-term space flight may be related to CO2 in long-duration spaceflight. Technical constraints maintain carbon dioxide levels at nearly 10 times earth normal levels1. Air convection is also significantly reduced in microgravity and pockets of CO2 may develop around the nose and mouth of astronauts. At an ambient partial pressure of CO2 of 3.8 mmHg during flight, the expired CO2 of a cohort of astronauts was 6.1 mmHg higher than pre-flight levels66. There is a 6–6.7% increase in CBF for each 1-mmHg rise in partial pressure of CO265,67. This indicates the CBF could be increased by the 41% increase in CO noted by Norsk et al.29. Forty-five per cent of children with IIH have cerebral hyperaemia17. A study in patients with IIH showed a 56% increase in CO2 led to a 166% increase in superior sagittal sinus pressure68 indicating the effect of CO2 on sinus pressure is non-linear. In a modelling study, the increase in blood returning through the venous system significantly increased the pressure drop from the sagittal sinus to the jugular bulbs because the pressure response to CBF of the venous system was quadratic in nature69. The cause of the greater than the linear response of the venous system to blood flow is due to the development of rotational or vortex flow induced by the unusual geometry. Could an increased blood flow in long-term space flight increase the venous pressure is SANS? The normal arterial inflow for a group of average age 43 years is 792 mls/min70. A 40% increase in arterial inflow secondary to the elevated CO2 would lead to a mean arterial inflow of 1110 mls/min. If we were to recruit the 5 normal individuals who had their normal venous outflow modelled with computational fluid dynamics (CFD) in our original paper69, and place them in orbit for 6 months, then we can see from Fig. 1 that there would be a variable increase in the venous pressure. The normal pressure drop across the cerebral venous system is 2.5 mmHg in middle age17. IIH needs an increase in venous pressure of 7 mmHg to develop. We can see in Fig. 1 that under hyperaemic conditions patient 4 would have no significant increase in pressure drop across the venous system compared to normal and presumably not be at high risk for IIH. Patients 5 and 1 would have pressure drops of 1.7 and 1.8 mmHg, respectively, above the normal figure, giving an increase in sinus pressure overall (once the 6.6 mmHg increase in jugular vein pressure from the headward fluid shift is added) of 8.3 and 8.4 mmHg and be at moderate risk of IIH and perhaps SANS. Patients 3 and 2 would have sinus pressure drops of 4.5 and 7.3 mmHg above normal, giving total sinus pressure increases of 11.1 and 13.9 mmHg and presumably be at a high risk of developing IIH and possibly SANS. Fifteen per cent of long-duration space flight astronauts have SANS and 20% have choroidal folds1, perhaps correlating with the 20% of our venous blood flow models which have suggested a large increase in pressure with increased CBF. The fivefold variation in the pressure response to the same increase in blood flow noted suggests that there is a wide variation in response to cerebral hyperaemia in normal individuals. Therefore, there may be some utility in performing CFD modelling studies on the venous outflow of prospective long-duration astronauts to try and stratify the risk of developing SANS.
A graph of the venous pressure response vs. blood flow for the five patients modelled in reference69. The two vertical lines represent the normal flow rate at middle age on the left (792 mls/min) and a 40% increase in flow on the right (1110 mls/min). Note the pressure drop across the venous system is close to the normal figure of 2.5 mmHg at a normal flow rate for 3 of the 5 patients but elevated in 2. At a flow rate of 1110 mls/min there is a wide variation in response meaning there is a variable risk for the development of SANS if this condition is analogous to IIH. IVJ internal jugular vein, SSS superior sagittal sinus.
Methods
Initially, a literature search was undertaken in PUBMED looking for all research papers written in the English language with the search criteria “spaceflight associated neuro-ocular syndrome” or “vision impairment intracranial pressure”. Ninety-eight responses were obtained. Papers were chosen for review on the basis of discussing the CSF or blood flow dynamics. After the review, mathematical estimation of venous pressure in long term space flight was undertaken to utilise the computational fluid dynamic modelling undertaken by one of the authors (A.B.). The modelling data consists of 5 computational fluid dynamic models of the cerebral venous outflow from the sagittal sinus to the jugular veins obtained from the CT venogram images of patients who did not have evidence of cerebral disease, please see the original modelling study for further details69. Some of this data has been previously published69. Informed consent was obtained from all five patients who had their CT venogram data modelled to provide Fig. 1. The study original modelling study was approved by the Hunter New England Area Health Ethics Committee, therefore, the original study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. The authorisation number HREG/10/NHE/132 was issued.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Lee, A. G. et al. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: a review and an update. NPJ Microgravity 6, 7 (2020).
Kesserwani, H. Space flight-associated neuroocular syndrome, idiopathic intracranial hypertension, and pseudotumor cerebri: phenotypic descriptions, pathogenesis, and hydrodynamics. Cureus 13, e14103 (2021).
Cleves-Bayon, C. Idiopathic intracranial hypertension in children and adolescents: an update. Headache 58, 485–493 (2018).
Friedman, D. I., Liu, G. T. & Digre, K. B. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology 81, 1159–1165 (2013).
Kramer, L. A., Sargsyan, A. E., Hasan, K. M., Polk, J. D. & Hamilton, D. R. Orbital and intracranial effects of microgravity: findings at 3-T MR imaging. Radiology 263, 819–827 (2012).
Rohr, J. J. et al. Quantitative magnetic resonance image assessment of the optic nerve and surrounding sheath after spaceflight. NPJ Microgravity 6, 30 (2020).
Ong, J., Lee, A. G. & Moss, H. E. Head-down tilt bed rest studies as a terrestrial analog for spaceflight associated neuro-ocular syndrome. Front. Neurol. 12, 648958 (2021).
Bateman, G. Hyperemic hydrocephalus: a new form of childhood hydrocephalus analogous to hyperemic intracranial hypertension in adults. J. Neurosurg. Pediatr. 5, 20–26 (2010).
Guess, H. A., Charlton, J. D., Johnson, R. N. & Mann, J. D. A nonlinear least-squares method for determining cerebrospinal fluid formation and absorption kinetics in pseudotumor cerebri. Comput. Biomed. Res. 18, 184–192 (1985).
Lalou, A. D. et al. Coupling of CSF and sagittal sinus pressure in adult patients with pseudotumour cerebri. Acta Neurochir. 162, 1001–1009 (2020).
Liu, K. C. et al. Venous sinus stenting for reduction of intracranial pressure in IIH: a prospective pilot study. J. Neurosurg. 127, 1126–1133 (2017).
Karahalios, D. G., Rekate, H. L., Khayata, M. H. & Apostolides, P. J. Elevated intracranial venous pressure as a universal mechanism in pseudotumor cerebri of varying etiologies. Neurology 46, 198–202 (1996).
Fleischman, D. et al. Cerebrospinal fluid pressure decreases with older age. PLoS ONE 7, e52664 (2012).
Rowe, F. J. & Sarkies, N. J. The relationship between obesity and idiopathic intracranial hypertension. Int. J. Obes. Relat. Metab. Disord. 23, 54–59 (1999).
Sugerman, H. J., DeMaria, E. J., Felton, W. L. 3rd, Nakatsuka, M. & Sismanis, A. Increased intra-abdominal pressure and cardiac filling pressures in obesity-associated pseudotumor cerebri. Neurology 49, 507–511 (1997).
De Simone, R. et al. Dural sinus collapsibility, idiopathic intracranial hypertension, and the pathogenesis of chronic migraine. Neurol. Sci. 40, 59–70 (2019).
Bateman, G. A., Subramanian, G. M., Yap, S. L. & Bateman, A. R. The incidence of obesity, venous sinus stenosis and cerebral hyperaemia in children referred for MRI to rule out idiopathic intracranial hypertension at a tertiary referral hospital: a 10 year review. Fluids Barriers CNS 17, 59 (2020).
Rosenberg, M. J. et al. Comparison of dural venous sinus volumes before and after flight in astronauts with and without spaceflight-associated neuro-ocular syndrome. JAMA Netw. Open 4, e2131465 (2021).
Stenger, M. B. et al. Evidence report: risk of spaceflight associated neuro-ocular syndrome (SANS). https://humanresearchroadmap.nasa.gov.au/evidence/reports/SANS.pdf.2017 (2017).
Killer, H. E. et al. Cerebrospinal fluid dynamics between the intracranial and the subarachnoid space of the optic nerve. Is it always bidirectional? Brain 130, 514–520 (2007).
Shinojima, A., Kakeya, I. & Tada, S. Association of space flight with problems of the brain and eyes. JAMA Ophthalmol. 136, 1075–1076 (2018).
Pandiarajan, M. & Hargens, A. R. Ground-based analogs for human spaceflight. Front. Physiol. 11, 716 (2020).
Marshall-Goebel, K. et al. Assessment of jugular venous blood flow stasis and thrombosis during spaceflight. JAMA Netw. Open 2, e1915011 (2019).
Watkins, W., Hargens, A. R., Seidl, S., Clary, E. M. & Macias, B. R. Lower-body negative pressure decreases noninvasively measured intracranial pressure and internal jugular vein cross-sectional area during head-down tilt. J. Appl Physiol. 123, 260–266 (2017).
Laurie, S. S. et al. Optic disc edema and chorioretinal folds develop during strict 6 degrees head-down tilt bed rest with or without artificial gravity. Physiol. Rep. 9, e14977 (2021).
Herault, S. et al. Cardiac, arterial and venous adaptation to weightlessness during 6-month MIR spaceflights with and without thigh cuffs (bracelets). Eur. J. Appl. Physiol. 81, 384–390 (2000).
Hamilton, D. R. et al. On-orbit prospective echocardiography on International Space Station crew. Echocardiography 28, 491–501 (2011).
Hughson, R. L. et al. Cardiovascular regulation during long-duration spaceflights to the International Space Station. J. Appl Physiol. 112, 719–727 (2012).
Norsk, P., Asmar, A., Damgaard, M. & Christensen, N. J. Fluid shifts, vasodilatation and ambulatory blood pressure reduction during long duration spaceflight. J. Physiol. 593, 573–584 (2015).
Hughson, R. L., Peterson, S. D., Yee, N. J. & Greaves, D. K. Cardiac output by pulse contour analysis does not match the increase measured by rebreathing during human spaceflight. J. Appl Physiol. 123, 1145–1149 (2017).
Prisk, G. K., Guy, H. J., Elliott, A. R., Deutschman, R. A. 3rd & West, J. B. Pulmonary diffusing capacity, capillary blood volume, and cardiac output during sustained microgravity. J. Appl Physiol. 75, 15–26 (1993).
Shykoff, B. E. et al. Cardiovascular response to submaximal exercise in sustained microgravity. J. Appl Physiol. 81, 26–32 (1996).
Norsk, P. et al. Vasorelaxation in space. Hypertension 47, 69–73 (2006).
Norsk, P. Adaptation of the cardiovascular system to weightlessness: Surprises, paradoxes and implications for deep space missions. Acta Physiol. 228, e13434 (2020).
Kety, S. S. Circulation and metabolism of the human brain in health and disease. Am. J. Med. 8, 205–217 (1950).
Botti, R. E., Razzak, M. A., MacIntyre, W. J. & Pritchard, W. H. The relationship of renal blood flow to cardiac output in normal individuals as determined by concomitant radioisotopic measurements. Cardiovasc. Res. 2, 243–246 (1968).
Leach, C. S. et al. Regulation of body fluid compartments during short-term spaceflight. J. Appl. Physiol. 81, 105–116 (1996).
Sandler, H. et al. Cardiovascular results from a rhesus monkey flown aboard the Cosmos 1514 spaceflight. Aviat. Space Environ. Med. 58, 529–536 (1987).
Moskalenko, Y. E., Weinstein, G. B. & Semernja, V. N. Investigation of human cerebral circulation in spaceflight conditions. Aviat. Space Environ. Med. 46, 1023–1026 (1975).
Turchaninova, V. F., Egorov, A. D. & Domracheva, M. V. Central and regional hemodynamics in long space flights. Kosm. Biol. Aviakosm Med. 23, 19–26 (1989).
Vasil’eva, T. D., Iarullin, K. & Zhuĭko, V. I. Regional hemodynamic changes following space flights lasting up to 8 days. Kosm. Biol. Aviakosm Med. 16, 12–17 (1982).
Iarullin, K., Vasil’eva, T. D., Turchaninova, V. F., Sokolova, I. V. & Vikharev, N. D. Compensatory-adaptive reactions of regional hemodynamics to weightlessness during a long space flight. Kosm Biol. Aviakosm Med. 18, 22–28 (1984).
Yegorov, A. D. et al. Studies of cardiovascular system in prolonged spaceflights aboard Salyut orbital stations. Izvestiya. Akad. Nauk. SSSR Seriya. Biol. 4, 485–497 (1982).
Mayasnikov, V. & Stepanova, S. in Orbital Station MIR 300–305 (Institute of Biomedical Problems: State Scientific Center of Russian Federation, 2008).
Iwasaki, K. I. et al. Long-duration spaceflight alters estimated intracranial pressure and cerebral blood velocity. J. Physiol. 599, 1067–1081 (2021).
Arbeille, P., Achaibou, F., Fomina, G., Pottier, J. M. & Porcher, M. Regional blood flow in microgravity: adaptation and deconditioning. Med. Sci. Sports Exerc. 28, S70–S79 (1996).
Arbeille, P. et al. Adaptation of the left heart, cerebral and femoral arteries, and jugular and femoral veins during short- and long-term head-down tilt and spaceflights. Eur. J. Appl. Physiol. 86, 157–168 (2001).
Pourcelot, L. et al. in Life Sciences Research in Space: Proceedings of the Second European Symposium (ESA SP-212) 119–123 (ESA Scientific and Technical Publications, 1984).
Arbeille, P., Fomina, G., Achaibou, F., Pottier, J. & Kotovskaya, A. Cardiac and vascular adaptation to 0g with and without thigh cuffs (Antares 14 and Altair 21 day Mir spaceflights). Acta Astronaut 36, 753–762 (1995).
Bagian, J. P. & Hackett, P. Cerebral blood flow: comparison of ground-based and spaceflight data and correlation with space adaptation syndrome. J. Clin. Pharm. 31, 1036–1040 (1991).
Zuj, K. A. et al. Impaired cerebrovascular autoregulation and reduced CO(2) reactivity after long duration spaceflight. Am. J. Physiol. Heart Circ. Physiol. 302, H2592–H2598 (2012).
Blaber, A. P., Goswami, N., Bondar, R. L. & Kassam, M. S. Impairment of cerebral blood flow regulation in astronauts with orthostatic intolerance after flight. Stroke 42, 1844–1850 (2011).
Bishop, C. C., Powell, S., Rutt, D. & Browse, N. L. Transcranial Doppler measurement of middle cerebral artery blood flow velocity: a validation study. Stroke 17, 913–915 (1986).
Brauer, P. et al. Correlation of transcranial Doppler sonography mean flow velocity with cerebral blood flow in patients with intracranial pathology. J. Neurosurg. Anesthesiol. 10, 80–85 (1998).
Du Boulay, G. H. & Symon, L. The anaesthetist’s effect upon the cerebral arteries. Proc. R. Soc. Med. 64, 77–80 (1971).
Coverdale, N. S., Gati, J. S., Opalevych, O., Perrotta, A. & Shoemaker, J. K. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J. Appl Physiol. 117, 1090–1096 (2014).
Arbeille, P. et al. Lower body negative pressure reduces jugular and portal vein volumes and counteracts the elevation of middle cerebral vein velocity during long-duration spaceflight. J. Appl Physiol. 131, 1080–1087 (2021).
Bateman, G. A. & Bateman, A. R. Spaceflight-associated increase in middle cerebral vein velocity: collapse, collateral flow, or hyperemia? J. Appl Physiol. 131, 1392–1393 (2021).
Si, Z. et al. MRI-based investigation on outflow segment of cerebral venous system under increased ICP condition. Eur. J. Med. Res. 13, 121–126 (2008).
Nicogossian, A. E., Huntoon, E. L. & Pool, S. L. (Lea and Febiger, USA, 1989).
Taylor, C. R. et al. Spaceflight-induced alterations in cerebral artery vasoconstrictor, mechanical, and structural properties: implications for elevated cerebral perfusion and intracranial pressure. FASEB J. 27, 2282–2292 (2013).
Roberts, D. R. et al. Altered cerebral perfusion in response to chronic mild hypercapnia and head-down tilt Bed rest as an analog for Spaceflight. Neuroradiology https://doi.org/10.1007/s00234-021-02660-8 (2021).
Najarian, T. et al. Prolonged hypercapnia-evoked cerebral hyperemia via K(+) channel- and prostaglandin E(2)-dependent endothelial nitric oxide synthase induction. Circ. Res. 87, 1149–1156 (2000).
Cannizzaro, G., Garbin, L., Clivati, A. & Pesce, L. I. Correction of hypoxia and hypercapnia in COPD patients: effects on cerebrovascular flow. Monaldi Arch. Chest Dis. 52, 9–12 (1997).
Pollock, J. M. et al. Hypercapnia-induced cerebral hyperperfusion: an underrecognized clinical entity. AJNR Am. J. Neuroradiol. 30, 378–385 (2009).
Prisk, G. K., Fine, J. M., Cooper, T. K. & West, J. B. Vital capacity, respiratory muscle strength, and pulmonary gas exchange during long-duration exposure to microgravity. J. Appl Physiol. 101, 439–447 (2006).
Vavilala, M. S., Lee, L. A. & Lam, A. M. Cerebral blood flow and vascular physiology. Anesthesiol. Clin. North Am. 20, 247–264 (2002).
Tschoe, C. et al. Changes in mean arterial pressure and end-tidal carbon dioxide content affect venous sinus pressures in patients with idiopathic intracranial hypertension: a randomized study. J. Neurointerv. Surg. 12, 906–910 (2020).
Bateman, A. R., Bateman, G. A. & Barber, T. The relationship between cerebral blood flow and venous sinus pressure: can hyperemia induce idiopathic intracranial hypertension? Fluids Barriers CNS 18, 5 (2021).
Bateman, G. A., Lechner-Scott, J. & Lea, R. A. A comparison between the pathophysiology of multiple sclerosis and normal pressure hydrocephalus: is pulse wave encephalopathy a component of MS? Fluids Barriers CNS 13, 18 (2016).
Author information
Authors and Affiliations
Contributions
Conceptualisation and design: G.A.B. and A.R.B. Mathematical analysis: A.R.B. Writing original draft: G.A.B. Review and editing: all authors. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bateman, G.A., Bateman, A.R. A perspective on spaceflight associated neuro-ocular syndrome causation secondary to elevated venous sinus pressure. npj Microgravity 8, 3 (2022). https://doi.org/10.1038/s41526-022-00188-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41526-022-00188-6