Abstract
Black, compared to white, women with residual estrogen receptor-positive (ER+) breast cancer after neoadjuvant chemotherapy (NAC) have worse distant recurrence-free survival (DRFS). Such racial disparity may be due to difference in density of portals for systemic cancer cell dissemination, called TMEM doorways, and pro-metastatic tumor microenvironment (TME). Here, we evaluate residual cancer specimens after NAC from 96 Black and 87 white women. TMEM doorways are visualized by triple immunohistochemistry, and cancer stem cells by immunofluorescence for SOX9. The correlation between TMEM doorway score and pro-metastatic TME parameters with DRFS is examined using log-rank and multivariate Cox regression. Black, compared to white, patients are more likely to develop distant recurrence (49% vs 34.5%, p = 0.07), receive mastectomy (69.8% vs 54%, p = 0.04), and have higher grade tumors (p = 0.002). Tumors from Black patients have higher TMEM doorway and macrophages density overall (p = 0.002; p = 0.002, respectively) and in the ER+/HER2- (p = 0.02; p = 0.02, respectively), but not in the triple negative disease. Furthermore, high TMEM doorway score is associated with worse DRFS. TMEM doorway score is an independent prognostic factor in the entire study population (HR, 2.02; 95%CI, 1.18–3.46; p = 0.01), with a strong trend in ER+/HER2- disease (HR, 2.38; 95%CI, 0.96–5.95; p = 0.06). SOX9 expression is not associated with racial disparity in TME or outcome. In conclusion, higher TMEM doorway density in residual breast cancer after NAC is associated with higher distant recurrence risk, and Black patients are associated with higher TMEM doorway density, suggesting that TMEM doorway density may contribute to racial disparities in breast cancer.
Similar content being viewed by others
Introduction
Breast cancer is the most common cancer and the second leading cause of cancer-related deaths among women1. Although breast cancer related deaths declined in the last three decades by approximately 40%, death rates declined less in Black, compared to non-Black, patients, resulting in persistent racial disparities in survival after a breast cancer diagnosis. Factors contributing to the racial gap in breast cancer mortality include: (i) advanced stage at presentation, (ii) higher incidence of triple-negative breast cancer (TNBC), (iii) higher comorbidity rates, (iv) poorer adherence to chemotherapy and endocrine therapy, and (v) adverse social determinants of health contributing to limitations in access to care2,3,4,5,6. Racial disparities are also present in clinical trial populations that are healthier and have access to care, indicating that social determinants of health and presentation with more advanced stage disease are the contributing factors7,8,9,10, and suggesting that other unknown factors may be contributing.
Due to more aggressive and advanced disease at presentation, Black patients with breast cancer are frequently treated with neoadjuvant chemotherapy (NAC) to downsize tumors and obtain information regarding treatment response. Tumor response to NAC is used as a pharmacodynamic biomarker that provides prognostic information and may be used to guide the choice of subsequent systemic adjuvant therapy after surgery11,12.
After NAC, the tumor microenvironment (TME) enters a reparative stage that results in a “cytokine storm” causing recruitment of bone marrow derived progenitors including pro-angiogenic TIE2+ monocytes and endothelial progenitor cells into TME13,14. In murine mammary carcinoma models, NAC leads to an increased density of tumor microenvironment of metastasis (TMEM) doorways, TMEM doorway-mediated cancer cell intravasation, and, ultimately, metastatic burden15,16. TMEM doorways are three-cell structures (composed of an actin regulatory protein mammalian-enabled [Mena] expressing tumor cell, a perivascular macrophage [TIE2 high], and an endothelial cell) and function as portals that surrounding tumor cells can use to intravasate into the blood stream17,18,19,20,21,22. Several large studies showed that TMEM doorway density is an independent prognostic marker for distant recurrence in patients with ER+/HER2- breast cancer who were treated with adjuvant systemic therapies20,22,23. It has been recently shown that TMEM doorways are also microanatomical niches enriched for cancer stem cells (CSCs) expressing SOX924. This finding is important because CSCs have the ability to initiate primary tumor growth and metastatic foci at distant sites25. However, the importance of CSC density for disease outcome is inconclusive, as several studies have found an association between CSCs and inferior clinical outcome in breast cancer patients26,27, whereas others have not28. Moreover, in ER+/HER2- disease, TMEM doorway density increased in the residual disease after NAC when compared with TMEM doorway density in the primary tumor before NAC15. This effect is now recognized as a mechanism of resistance to cytotoxic therapy wherein tumor dissemination is promoted, despite concomitant cytoreduction29. In a pooled analysis of 9702 women from 8 National Surgical Adjuvant Breast and Bowel Project (NSABP) trials treated with NAC, Black patients with residual ER+ breast cancer after NAC had inferior DRFS compared to white patients30.
We hypothesize that racial differences in TMEM doorway density may contribute to a racial difference in clinical outcome. Here we test this hypothesis using a prospective-retrospective study of TMEM doorway density and TMEM doorway-associated TME after NAC.
Results
Patient characteristics
We analyzed formalin-fixed paraffin-embedded tissue samples from 183 patients with residual invasive ductal breast cancer after NAC (Fig. 1). Tissue and outcome data from 96 (52.5%) Black and 87 (47.5%) white patients were included in the analysis (Table 1). There was no difference in neoadjuvant chemotherapy regimens (taxane only, taxane-containing, no-taxane containing regimens) between Black and white patients. Additionally, NAC only and NAC in combination with endocrine, HER2 inhibition, or radiation therapies were all similar in Black compared to white patients (Supplementary Table 1). When the cohort was stratified by breast cancer subtype, treatment regimens in Black and white patients with ER+/HER2- and TN disease were similar (Supplementary Table 2).
Demographic and tumor characteristics of the entire cohort, and for each breast cancer subtype, are described in Table 1 and Supplementary Table 3 respectively. Black, compared to white, patients were more likely to have distant recurrence (49% vs 34.5%, p = 0.07), receive mastectomy over breast conserving therapy (69.8% vs 54%, p = 0.04), and have a higher tumor grade (p = 0.002) in the entire cohort (Table 1). Age was similar among Black and white patients (51.6 vs 52.3). There was no difference in time to distant recurrence, tumor stage, lymph node status, or subtype between Black and white patients (Table 1).
When the cohort was stratified by breast cancer subtype, a racial difference in distant recurrence remained in ER+/HER2- (46.3% vs 28%) but did not reach statistical significance. However, this difference was lost in the TNBC (54.1% vs 54.5%) subtype (Supplementary Table 3). Black, compared with white, patients were more likely to get mastectomy (82.9% vs 52%, p = 0.004), have higher grade (p = 0.01), and positive lymph nodes (90.2% vs 66%, p = 0.01) in the ER+/HER2- subtype, but not in the TNBC subtype, indicating a racial disparity in the biology of ER+/HER2- but not TNBC disease. Black, compared to white, patients with ER+/HER2- breast cancer were slightly younger (49.6 vs 54), and patients with TNBC disease were slightly older (53.4 vs 50.9). Furthermore, there was no racial difference observed in the two breast cancer subtypes in time to distant recurrence and tumor stage (Supplementary Table 3).
Approach to evaluating Pro-metastatic tumor microenvironment and distant recurrence
Since NAC may increase the density of TMEM doorways in certain patients, we wanted to determine if there is racial disparity in this recently reported side-effect of NAC15,16,29. In addition, we wanted to determine if TMEM doorway score could be used as prognostic indicator of metastasis in the residual disease post-NAC similarly to treatment-naïve breast cancers20,22,23. Given that macrophages and blood vessels are components of TMEM doorways we also investigated racial disparity in macrophage and microvascular density and potential correlation with DRFS of these 2 parameters of TME. Lastly, we wanted to determine racial disparity in CSC density because CSCs are crucial for initiation of growth of metastatic foci and it was demonstrated that TMEM doorways may act as niches where CSCs preferentially reside24.
In all analyses we first examined potential disparity in pro-metastatic parameters between the two most prevalent breast cancer subtypes, ER+/HER2- and TNBC, and then the racial disparity between Black and white patients.
Disparity in pro-metastatic TME parameters
We used high nuclear expression of SOX9 to identify cells that activated stem program as previously reported24. Representative images of cancer cells expressing high levels of nuclear SOX9 (SOX9high) and low levels of nuclear SOX9 (SOX9low) are showed in Fig. 2a and representative images for TMEM doorway high vs mid/low analysis are shown in Supplementary Fig. 1. Tumors from patients with TNBC had a higher density of TMEM doorways (p = 0.004) (Fig. 2b), macrophages (p = 0.0002) (Fig. 2c), and CSCs (nuclear SOX9high cancer cells, p = 0.0002) (Fig. 2d) than tumors from patients with ER+/HER2- breast cancer. There was no difference in microvascular density between these two subtypes of breast cancer (Fig. 2e). Of note, two cases were excluded from the analysis of microvascular density because of large amount of highly vascularized intertumoral stroma.
a Representative images for SOX9-high cells (yellow arrows) and -low cells (white arrowheads). Scale bar, 10 µm. b TMEM doorway score (p = 0.004). c Macrophage density (p = 0.0002). d Cancer stem cells (p = 0.0002). e Microvascular density (p = 0.44) levels in patients with ER+/HER2- breast cancer (n = 91) vs TNBC (n = 59). ER+ Estrogen receptor positive, TNBC Triple negative breast cancer, TME Tumor microenvironment. Mann-Whitney U-test is used for comparison between two-independent groups and box and whisker diagram is used for plotting the data (b–e). Box represents median value, lower and upper quartile values. Whiskers show minimum and maximum data values. Outliers are single data points that are more than 1.5 times of upper or lower quartiles. ns: not statistically significant, p > 0.05, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
Black, compared to white, patients had higher density of TMEM doorways and macrophages in their TME overall (p = 0.002 and p = 0.002, respectively) and in ER+/HER2- subtype (p = 0.02 and p = 0.02, respectively) but not in TNBC (p = 0.74 and p = 0.31, respectively) (Fig. 3a–f). There was no racial difference in microvascular density (Fig. 3g–i) and density of CSCs (Fig. 3j–l) in either of the subtypes.
a–c TMEM doorway score by race in all subtypes (a, p = 0.002), ER+/HER2- (b, p = 0.02), and TNBC (c, p = 0.74). d–f Macrophage density by race in all subtypes (d, p = 0.002), ER+/HER2- (e, p = 0.02), and TNBC (f, p = 0.31). g–i Microvascular density by race in all subtypes (g, p = 0.06), ER+/HER2- (h, p = 0.09), and TNBC (i, p = 0.97). j–l Cancer stem cell percentage in all subtypes (j, p = 0.09), ER+/HER2- (k, p = 0.09), and TNBC (l, p = 0.73). Patient numbers as follows: all subtypes BP (n = 96), WP (n = 87); ER+/HER2- BP (n = 41), WP (n = 50); TNBC BP (n = 37), WP (n = 22). BP Black patients, WP White patients, ER+ Estrogen receptor positive, TNBC Triple negative breast cancer. Mann-Whitney U-test is used for comparison between two-independent groups and box and whisker diagram is used for plotting the data (a–l). Box represents median value, lower and upper quartile values. Whiskers show minimum and maximum data values. Outliers are single data points that are more than 1.5 times of upper or lower quartiles. ns: not statistically significant, p > 0.05, *p ≤ 0.05, **p ≤ 0.01.
Taken together, these data show enrichment of the pro-metastatic TME parameters TMEM doorway and macrophage density in TNBC compared to ER+/HER2-. Additionally, when separated by race and subtype, Black, compared to white, patients had higher TMEM doorway and macrophage density only in ER+/HER2- but not in TN subtype. We next tested how high density of these pro-metastatic TME parameters correlate with DRFS.
Correlation between DRFS and pro-metastatic TME parameters
Given that high TMEM doorway density is prognostic for DRFS in breast cancer patients receiving AC, we hypothesized that TMEM doorway density may also be prognostic for DRFS in patients with residual disease after NAC. We also wanted to examine if other pro-metastatic parameters in the residual disease post-NAC are associated with DRFS. All comparisons were made in the overall cohort as well as within ER+/HER2- and TNBC subtypes.
Since TMEM doorways contain macrophages and blood vessels, and since the areas around TMEM doorways have been found to be enriched for SOX9 expressing CSCs24, we first performed a Spearman correlation analysis among these pro-metastatic parameters and found significant positive correlation only between TMEM doorway score and macrophage density (Spearman Correlation Coefficient: 0.67) (Supplementary Fig. 2).
Next, we examined the racial disparity in DRFS and observed a trend towards inferior DRFS for Black compared to white patients with ER+/HER2- disease, but not with TNBC (Fig. 4a–c). Even though we collected patient samples from multiple institutions, follow-up time was similar between Black and white patients (median follow-up time 68.95 and 71.53 months, respectively, p = 0.9). Then we studied the correlation of DRFS and pro-metastatic TME parameters. We found that patients with high TMEM doorway score, compared to patients with mid/low TMEM doorway score, in their residual disease had worse DRFS overall (p = 0.008) (Fig. 4d) and trended towards inferior DRFS in ER+/HER2- (p = 0.08) (Fig. 4e), but not in TNBC (Fig. 4f). To investigate if NAC regimen is one of the driving factors affecting DRFS in TMEM doorway-high vs mid/low patients, we compared NAC treatment regimen between these two groups. There was no statistical difference in NAC treatment regimens in TMEM doorway-high and -mid/low groups (Supplementary Table 4). Even though, we observed racial disparity in macrophage density and correlation between TMEM doorway and macrophage density, there was no association between macrophage density and DRFS (Supplementary Fig. 3a). Although there was no association between DRFS and other pro-metastatic tumor markers (microvascular and CSCs density) (Supplementary Fig. 3), there was a trend towards worse DRFS in high compared to low microvascular density in TNBC (p = 0.06) (Supplementary Fig. 3f).
a–c DRFS in BP vs WP in the entire cohort (a, p = 0.21), ER+/HER2- breast cancer (b, p = 0.15), and TNBC (c, p = 0.6). d–f DRFS in TMEM-high vs TMEM-mid/low in patients in the entire cohort (d, p = 0.008), ER+/HER2- breast cancer (e, p = 0.08), and TNBC (f, p = 0.77). Kaplan-Meier survival curves and log-rank tests are used for DRFS analysis (a–f). g Cox regression model for covariates in the entire cohort (n = 175, patients with unknown status, n = 8, are excluded). Error bars represent 95% confidence intervals. Two-sided p-values are reported. DRFS: distant recurrence free survival, ER+ Estrogen receptor positive, TNBC Triple negative breast cancer, nucSOX9 Nuclear SOX9, BCT Breast conserving therapy, BP Black patients, WP White patients, HR Hazard Ratio, CI Confidence Interval.
We next performed a multivariate Cox regression model and found that TMEM doorway score (high vs mid/low) is an independent prognostic indicator (HR 2.02 [95% CI 1.18–3.46], p = 0.01), along with stage (T3 vs T1) (HR 1.97 [95% CI 1.03–3.76], p = 0.04), lymph node status (positive vs negative) (HR 3.88 [95% CI 1.92–7.86], p = 0.0002), grade (3 vs 2) (HR 2.91 [95% CI 1.43–5.92], p = 0.003), and subtype (TNBC vs ER+/HER2-) (HR 1.99 [95% CI 1.1–3.58], p = 0.02) (Fig. 4g). Of note, we included in the multivariate analysis patients whose tumors did not fall into TN or ER+/HER2- category as “other” category because one of the goals of the study was to determine if TMEM doorway density is an independent prognostic indicator of distant recurrence regardless of cancer subtype. Multivariate Cox regression analysis among patients with ER+/HER2- breast cancer showed that there is a positive association between DRFS and TMEM doorway score (high vs mid/low) with a slightly stronger magnitude (HR 2.38 [95% CI 0.96–5.95]) but with borderline significance (p = 0.06) compared to entire cohort analysis. Grade (3 vs 2) remained associated with DRFS in ER+/HER2- disease (HR 3.66 [95% CI 1.45–9.27], p = 0.006) upon cancer subtype stratification. Although the type of surgery (mastectomy vs breast conserving therapy) was not significantly associated with DRFS in entire cohort, it was associated with DRFS in ER+/HER2- disease (HR 0.35 [95% CI 0.13–0.95], p = 0.04) (Supplementary Fig. 4).
These data demonstrate that out of the four pro-metastatic parameters evaluated here, only TMEM doorway score was prognostic for DRFS in patients with residual disease post-NAC.
Discussion
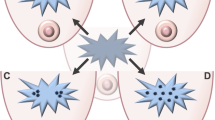
In this prospective-retrospective, multi-institutional study we investigated disparity in four pro-metastatic TME parameters in residual disease post-NAC according to breast cancer subtype (ER+/HER- vs TNBC) and race (Black vs white patients), as well as the association of high density of these parameters with DRFS in all patients. We found a higher density of TMEM doorways, macrophages, and CSCs in TNBC compared to ER+/HER2-. Furthermore, we found a higher density of TMEM doorways and macrophages in residual tumors from Black, compared to white, patients with an ER+/HER2- subtype, but not TNBC. We also showed that high TMEM doorway density in residual ER+/HER2-, but not TNBC, is associated with DRFS. Taken together, these findings show that a pro-metastatic response to chemotherapy depends on cancer subtype and race and is most unfavorable in Black patients with residual ER+/HER2- disease (Fig. 5).
Black patients with residual ER+/HER2- breast cancer after NAC have higher numbers of TMEM doorways and macrophages in their tumor microenvironment compared to white patients. TMEM doorways are localized, transient vascular openings formed by Mena expressing tumor cell, perivascular macrophage, and endothelial cell. Increased permeability around TMEM doorways helps cancer cells to intravasate and disseminate to other organs. Higher TMEM doorway score can be one of the mechanisms of inferior outcome and distant recurrence free survival in Black vs white patients with residual ER+/HER2- breast cancer. This original clipart was created with BioRender.com.
Over the last two decades many controlled clinical trials have demonstrated an association between Black patients and distant recurrence and several have indicated that racial disparity is more prominent in patients with ER+/HER2- disease. For example, in a randomized clinical trial including 4817 breast cancer patients, Black patients were strongly associated with inferior DRFS and overall survival in ER+/HER2- disease9. Likewise, in a single institution cohort of 3890 patients with invasive breast cancer, Black patients with ER+/HER2- disease had 2-fold increase in distant recurrence compared to white patients31. Moreover, the ECOG-ACRIN-5103 clinical trial of 4994 patients showed that Black patients with ER+/HER2- disease had worse disease free survival than white patients10. It needs to be emphasized that these three clinical trials have not found a racial disparity in distant recurrence in TNBC9,10,31. The recent NCI-sponsored trial, TAILORx, focused on ER+/HER2- disease alone, reported that Black patients were associated with a 1.60-fold increase in distant recurrence rate and an inferior overall survival8. Additionally, Albain et al. showed that Black patients with hormone-dependent cancers, such as pre- and post-menopausal ER+ disease, have worse outcome compared to white patients7. Furthermore, our group recently reported that racial disparities in DRFS for Black patients with residual ER+/HER2- but TNBC disease after NAC in a review of 8 NSABP trials30. However, in the present study, we only observed a trend towards worse DRFS in Black compared to white patients with residual ER+/HER2- disease. Most likely the difference in this current study did not attain statistical significance due to the tenfold smaller cohort size compared to the one by Kim et al.30. Consistent with the existing literature, we have not found in this current study a discrepancy in survival between Black and white patients with TNBC.
Among all breast cancer subtypes, TNBC has the poorest outcome due to a high risk of relapse, early recurrence with visceral organ metastases, and a lack of targeted therapies32,33,34. Furthermore, the aggressive nature of TNBC may mask other biological factors that would otherwise contribute to disparity in DRFS between Black and white patients. Indeed, in this current study we found that TMEM doorway score, as well as density of macrophages and CSCs, were higher in TNBC compared to ER+/HER2- disease, consistent with the higher metastatic potential associated with TNBC.
It has been shown that NAC induces repair signals in the tumor, leading to a recruitment of myeloid cells (particularly TIE2+ macrophages) into the TME13,14 and a subsequent increase in TMEM doorway score15,16. TIE2+ macrophages are crucial components of TMEM doorways, as they induce transient localized vascular openings via their secretion of vascular endothelial growth factor-A (VEGFA)17. Once TMEM doorways are open, MenaINV, an isoform of Mena (Mammalian Enabled, Actin regulatory protein) expressing tumor cells can intravasate19,35,36 and disseminate to other organs. Knowledge of this biology has important clinical implications since it has recently been demonstrated that TMEM doorway related vascular opening can be observed in patients in real time using a novel MRI approach37, and can be blocked by small molecule inhibitor of TIE238.
Several studies have reported a correlation between high macrophage density in the breast TME and worse prognosis39,40. Likewise, microvascular density has been shown to correlate with inferior survival and metastasis in patients with invasive breast carcinoma41. In the context of racial disparity, Martin et al. showed that Black patients with breast cancer have a higher macrophage and microvasculature density in the TME compared to white patients42. Although we also found a greater number of macrophages in Black, compared to white, patients, we did not observe racial disparity in microvascular density. Unlike in prior reports, neither macrophage nor microvascular density were associated with DRFS in our cohort.
It is currently unclear in which clinical scenarios CSC density correlates with outcome. Some reports clearly indicate an association with worse outcome, while others do not. For example, in 48 patients with stage IV breast cancer, a high percentage of circulating CSCs was associated with inferior treatment response, survival, and progression-free survival27. Similarly, a meta-analysis that included 12 studies showed an association between CSCs and inferior overall survival in breast cancer26. However, other studies have found that CSCs and clinical outcome do not correlate28, which accords with the results we report here. The lack of correlation we observed between TMEM doorway density and CSCs is an unexpected finding given the recently reported finding of a positive correlation between TMEM doorway score and a high percentage of CSCs24. The reasons for these discrepancies need to be further investigated.
The strengths of the current study are the inclusion of patients of different racial background (Black and white patients) from several large academic institutions with similar distribution of age, time to distant recurrence status, tumor stage, and lymph node status. Although the patients were treated at different institutions, their treatments were relatively uniform (Supplementary Tables 1, 2). Of note, the criteria for calling a case ER+ changed from 10% to 1% in 201043. However, about 97% of patients with ER+ disease have more than 10% of cancer cells positive for ER44. Therefore, subtyping cancer into ER+ category has not changed for most patients. Furthermore, the standard of care did not substantially change in the course of this study that included patients with HER2-negative disease. Anthracycline-taxane neoadjuvant chemotherapy was used in about 85% of patients, and those with ER-positive disease received adjuvant endocrine therapy for up to 10 years. An additional strength is the use of a multivariate Cox regression model that adjusts for potential confounders (Fig. 4 and Supplementary Fig. 4). Limitations are the relatively small cohort size, unknown treatment status in 10 Black and 4 white patients, and unclear effect of comorbidities (e.g., hypertension) on pro-metastatic parameters. As discussed in the results, relatively small cohort size might have underpowered our DRFS analysis and multivariate Cox regression model, especially considering nearly significant p values in ER+/HER2- group (Supplementary Fig 4). To overcome this limitation, future studies need to be designed with larger cohort size. Although the treatment status of 10 Black and 4 white patients was unknown, it is unlikely that this affected the results because for the vast majority of patients in the cohort (169) treatment regimen was known and no statistical difference in treatment status was found. Comorbidities could be one of the variates affecting survival of the patients in the study. We included potential cofounders in our multivariate Cox regression model from available patient data, however, comorbidities such as BMI or hypertension, when available, need to be taken into consideration in future studies. Additionally, knowing the adjuvant treatment such as additional endocrine therapy, radiation and chemotherapy after surgery could be affecting DRFS and would be help understand the data presented here. Unfortunately, the current sample is limited in size to control for too many potential confounders in the multivariate model. Further, one important limitation of this study is the absence of pre-chemotherapy tissue samples to observe the chemotherapy effect on TME for each patient individually. Now, we are focusing on new studies to validate our findings in larger cohorts and more detailed covariates (including comorbidities, adjuvant, endocrine, and radiation therapy regiments, etc.) with matched pre/post-chemotherapy tissue samples from patients with ER+/HER2- disease. This would guide us to investigate the impact of each limitation on our current findings and to understand the mechanism of the racial disparity in TME even further. Based on our previous work on inferior DRFS in Black patient with residual ER+/HER2- breast cancer, compared to white patients30, we only included Black and white patients in current study to investigate the biology of the racial disparity in these patient groups. Patients with other or unknown races were excluded in this study. To expand racial disparity, new studies could be designed including other races and/or ethnicities.
In conclusion, this study demonstrates the existence of a racial disparity in pro-metastatic TME in Black, compared to white, women with residual ER+/HER2- disease after NAC. This work provides a foundation for the development of companion diagnostics such as TMEM-MRI activity37 to facilitate the evaluation of systemic therapies on the metastatic dissemination, and therapies to block TMEM doorway activity38, which may ultimately contribute to reducing racial disparity in breast cancer.
Methods
Study design
This was a prospective-retrospective, multi-institutional case-control study performed in accordance with REMARK guidelines45,46. Patients were selected from four participating New York Pathology Oncology Group (NYPOG-https://einsteinmed.edu/research/groups/ny-pathology-oncology/) institutions (Montefiore Medical Center; New York-Presbyterian/Weill Cornell Medical Center; NYU Langone Health; Memorial Sloan Kettering Cancer Center). The study was approved by the Institutional Review Board of each institution. The written consent from patients was not obtained because we only used leftover archival tissue that was excised for standard clinical care for diagnostic or therapeutic purposes years before the initiation of this study. Due to the timing of the study many subjects are deceased. Female patients over age of 18, diagnosed with invasive ductal carcinoma (IDC) between 2004 and 2014, who received neoadjuvant chemotherapy and had sufficient residual cancer for staining (defined as >5 mm) were included. Patients with a prior personal history of breast or other cancers, bilateral cancers, and distant metastatic disease at the time of presentation were excluded from the study (Fig. 1). Cases were defined as patients with localized breast cancer treated with NAC who subsequently developed distant recurrence, whereas controls were defined as patients who did not develop distant recurrence. Detailed NAC regimens can be found in Supplementary Tables 1, 2.
Although it is now understood that race is a social construct, race was self-identified per the patient’s electronic medical records. Only patients of Black or white patients were included. Patients with ‘Other’ or unknown races were excluded from the study. Distant recurrence was defined as clinical or radiographic evidence of disease recurrence outside of the breast, chest wall, and axillary lymph nodes, with or without tissue confirmation. Primary endpoints were TMEM doorway score; percentage of tumor cells expressing high levels of SOX9, which indicates activation of stem program24; and time to distant recurrence, measured in months from date of diagnosis. Secondary endpoints were macrophage and microvascular density.
Cohort assembly and tissue collection
The tumor registry at each institution was queried and all cases were reviewed by a clinician to ensure that inclusion and exclusion criteria were met. A cohort of 216 patients who met the inclusion and exclusion criteria were assembled from the NYPOG institutions. The representative Formalin Fixed Paraffin Embedded (FFPE) blocks created at the time of surgical excision and stored in pathology archives at each institution were selected and five sequential sections were cut. Of the cohort 216 patients, 33 were excluded due to poor tissue quality, as determined by pathologists (MO, SA).
Slide staining & scanning
Of the five sequential sections, one was stained for TMEM doorways, and the other for the stem cell marker (SOX9) and DAPI (to mark nuclei).
TMEM doorways were visualized by triple immunostaining as per a previously validated protocol22,23 identifying Mena-overexpressing tumor cells, macrophages, and endothelial cells. Cancer stem cells were identified by immunofluorescent staining of SOX9 (anti-rabbit Millipore 3205915, 1:100 dilution) and DAPI (1:1000 dilution) on an adjacent sequential section. Alexa Fluor-546 goat anti-rabbit (H + L) (Thermofisher, Cat# A11035, 1:200 dilution) was used as a secondary antibody. The slides were digitally scanned at 20x on a 3D Histech P250 High-Capacity Slide Scanner.
TMEM doorway quantification
The digitally scanned slides were first imported into the Visiopharm image analysis software, Vis (Visiopharm, Hørsholm, Denmark). Next, in Vis, pathologists (MO, SA) identified approximately ten high power (20X) microscope fields best suitable for analysis based upon appropriate pathological criteria (e.g., lack of tumor necrosis, inflammation, tissue folds). Each 20X field covers a 660 × 880 µm2 area, which is 4 times the area of a 40X field previously used for TMEM doorway quantification23,47,48. Next, TMEM doorways were quantified using previously published algorithms47 in all the 20X microscope fields and the sum of TMEM doorways in these fields, divided by a conversion factor of 4, was used as the TMEM doorway score for the patient sample, which is consistent with previously published methods23,47,48. TMEM doorway-high patients were defined as those in the highest tertile of TMEM doorway score.
Quantification of macrophage and microvascular densities
In the original TMEM doorway quantification algorithm47, tumor, macrophage, and vessel areas were identified across entire region of interest (ROI). Macrophage area divided by the ROI area was used to determine the macrophage density. Likewise, vessel area divided by the ROI area determined the vascular density.
Nuclear SOX9 image analysis
We analyzed the percentage of nuclear SOX9 cells in Vis. We used the same ten, high-powered fields (20X) that were used for TMEM doorway quantification. The ROIs were captured using the screen capture tool and exported as bitmap (BMP) image files (lossless and uncompressed), allowing for accurate quantification of immunofluorescence intensities. Using the image analysis package, Fiji49, each image was split into separate color channels and the red (SOX9) and blue (nuclei) channels were used for further analysis. Again in Fiji, a machine learning algorithm, implemented via the Trainable Weka Segmentation plugin50 was used to segment the nuclei (blue) channel. The Trainable Weka Segmentation plugin was again run with separate training on the SOX9 (red) channel to segment the tumor regions. SOX9 mean fluorescence intensity measurements were made for nuclei in the tumor regions. Nuclear SOX9 intensity was quantified in each nucleus, and SOX9high cells above a specified intensity threshold were counted. It has been determined that CSCs constitute no more than 5% of cancer cells in untreated tumors24. Thus, the threshold for detecting CSCs was determined using cases expected to have the lowest CSC density - untreated ER+/HER2- breast cancers from white patients. We showed previously that CSC density correlates with TMEM doorway density24. In addition, we showed here that ER+/HER2- cancers have lower TMEM doorway density than TNBC and that breast cancers from white patients have lower TMEM doorway density than cancers from Black patients. Furthermore, TMEM doorway density may increase as a response to chemotherapy15. Hence the threshold was calculated using three different untreated ER+/HER2- cancers from white patients and the cutoffs averaged. This intensity threshold was then applied to all samples. SOX9high cell numbers were aggregated from all ten high-powered fields to get a single percentage of nuclear SOX9high cells number for each patient.
Sample size and power calculation
For each race group with at least N = 50 non-recurrences and N = 25 recurrences, with a two-sided type I error rate of not more than 5%, the study has 80% power to detect a difference of 0.61 SD in each marker between recurrences and non-recurrences. Thus, our study has adequate power to detect a moderate level of effect size.
Statistical analysis
Patient and tumor characteristics, including age (years); surgery type (breast conserving therapy vs mastectomy); post-NAC tumor size (> 5 cm, 2–5 cm, < 2 cm); post-NAC nodal status (positive vs. negative); Nottingham histologic grade (1, 2, 3); tumor subtype (TNBC vs ER+/HER2-); and tumor markers, including TMEM doorway score; density of SOX9High cancer cells; macrophage density; and microvascular density were compared between Black and white patients using Wilcoxon rank sum tests for continuous variables and chi-squared tests or Fisher’s exact tests for categorical variables. Spearman correlation was calculated between each pair of tumor markers.
The primary outcome variable was DRFS, defined as time from diagnosis to first distant relapse or a second primary cancer. Death prior to a distant recurrence or second primary cancer was censored. Kaplan-Meier survival curves and log-rank tests were used to compare DRFS between racial groups and between categorized pro-metastatic tumor characteristics (e.g., high vs low/mid tertile). A multivariate Cox proportional hazard model was used to examine the association between TMEM doorway score and DRFS, adjusting for race, age, surgery type, tumor size, nodal status, tumor grade, tumor subtype. The proportionality of the Cox model was examined using Schoenfeld residuals51,52.
Statistical significance was specified a priori as p < 0.05 and the two-side p-values were reported. All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC 2014) and GraphPad Prism version 9.1 (Dotmatics, Boston, MA).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data generated in this study are available upon request from the corresponding author.
References
Sung, H. et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin 71, 209–249 (2021).
Joslyn, S. A. & West, M. M. Racial differences in breast carcinoma survival. Cancer 88, 114–123 (2000).
Hershman, D. et al. Racial disparities in treatment and survival among women with early-stage breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 23, 6639–6646 (2005).
Tammemagi, C. M., Nerenz, D., Neslund-Dudas, C., Feldkamp, C. & Nathanson, D. Comorbidity and survival disparities among black and white patients with breast cancer. JAMA 294, 1765–1772 (2005).
Carey, L. A. et al. Race, breast cancer subtypes, and survival in the Carolina breast cancer study. JAMA 295, 2492–2502 (2006).
Partridge, A. H., Wang, P. S., Winer, E. P. & Avorn, J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 21, 602–606 (2003).
Albain, K. S., Unger, J. M., Crowley, J. J., Coltman, C. A. & Hershman, D. L. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J. Natl. Cancer Inst. 101, 984–992 (2009).
Albain, K. S. et al. Race, ethnicity, and clinical outcomes in hormone receptor-positive, HER2-negative, node-negative breast cancer in the randomized TAILORx trial. J. Natl. Cancer Inst 113, 390–399 (2021).
Sparano, J. A. et al. Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. J. Natl. Cancer Inst. 104, 406–414 (2012).
Schneider, B. P. et al. Impact of genetic ancestry on outcomes in ECOG-ACRIN-E5103. JCO Precis. Oncol. 2017, 1–9 (2017).
Killelea, B. K. et al. Racial differences in the use and outcome of neoadjuvant chemotherapy for breast cancer: Results from the National cancer data base. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 33, 4267–4276 (2015).
Mieog, J. S. D., van der Hage, J. A. & van de Velde, C. J. H. Neoadjuvant chemotherapy for operable breast cancer. Br. J. Surg. 94, 1189–1200 (2007).
Hughes, R. et al. Perivascular M2 macrophages stimulate tumor relapse after chemotherapy. Cancer Res 75, 3479–3491 (2015).
Chen, L. et al. Tie2 expression on macrophages is required for blood vessel reconstruction and tumor relapse after chemotherapy. Cancer Res. 76, 6828–6838 (2016).
Karagiannis, G. S. et al. Neoadjuvant chemotherapy induces breast cancer metastasis through a TMEM-mediated mechanism. Sci. Transl. Med. 9, eaan0026 (2017).
Chang, Y. S., Jalgaonkar, S. P., Middleton, J. D. & Hai, T. Stress-inducible gene Atf3 in the noncancer host cells contributes to chemotherapy-exacerbated breast cancer metastasis. Proc. Natl. Acad. Sci. USA. 114, E7159–E7168 (2017).
Harney, A. S. et al. Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discov 5, 932–943 (2015).
Arwert, E. N. et al. A unidirectional transition from migratory to perivascular macrophage is required for tumor cell intravasation. Cell Rep. 23, 1239–1248 (2018).
Pignatelli, J. et al. Macrophage-dependent tumor cell transendothelial migration is mediated by Notch1/MenaINV-initiated invadopodium formation. Sci. Rep. 6, 37874 (2016).
Robinson, B. D. et al. Tumor microenvironment of metastasis in human breast carcinoma: A potential prognostic marker linked to hematogenous dissemination. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 15, 2433–2441 (2009).
Karagiannis, G. S. et al. Assessing tumor microenvironment of metastasis doorway-mediated vascular permeability associated with cancer cell dissemination using intravital imaging and fixed tissue analysis. J. Vis. Exp. JoVE https://doi.org/10.3791/59633 (2019)
Rohan, T. E. et al. Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. JNCI J. Natl. Cancer Inst. 106, dju136 (2014).
Sparano, J. A. et al. A metastasis biomarker (MetaSite BreastTM Score) is associated with distant recurrence in hormone receptor-positive, HER2-negative early-stage breast cancer. NPJ Breast Cancer 3, 42 (2017).
Sharma, V. P. et al. Live tumor imaging shows macrophage induction and TMEM-mediated enrichment of cancer stem cells during metastatic dissemination. Nat. Commun. 12, 7300 (2021).
Nassar, D. & Blanpain, C. Cancer stem cells: Basic concepts and therapeutic implications. Annu. Rev. Pathol. 11, 47–76 (2016).
Zhou, L. et al. The prognostic role of cancer stem cells in breast cancer: a meta-analysis of published literatures. Breast Cancer Res. Treat. 122, 795–801 (2010).
Lee, C.-H. et al. Baseline circulating stem-like cells predict survival in patients with metastatic breast Cancer. BMC Cancer 19, 1167 (2019).
Ahmed, M. A. H. et al. A CD44−/CD24+ phenotype is a poor prognostic marker in early invasive breast cancer. Breast Cancer Res. Treat 133, 979–995 (2012).
DeMichele, A., Yee, D. & Esserman, L. Mechanisms of resistance to neoadjuvant chemotherapy in breast cancer. N. Engl. J. Med. 377, 2287–2289 (2017).
Kim, G. et al. Racial disparity in distant recurrence-free survival in patients with localized breast cancer: A pooled analysis of National surgical adjuvant breast and bowel project trials. Cancer 128, 2728–2735 (2022).
Kabat, G. C., Ginsberg, M., Sparano, J. A. & Rohan, T. E. Risk of recurrence and mortality in a multi-ethnic breast cancer population. J. Racial Ethn. Health Disparities 4, 1181–1188 (2017).
DeSantis, C. E. et al. Breast cancer statistics, 2019. CA. Cancer J. Clin. 69, 438–451 (2019).
Plevritis, S. K. et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US Women, 2000-2012. JAMA 319, 154–164 (2018).
Kennecke, H. et al. Metastatic behavior of breast cancer subtypes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 28, 3271–3277 (2010).
Pignatelli, J. et al. Invasive breast carcinoma cells from patients exhibit MenaINV- and macrophage-dependent transendothelial migration. Sci. Signal. 7, ra112 (2014).
Roussos, E. T. et al. Mena invasive (MenaINV) and Mena11a isoforms play distinct roles in breast cancer cell cohesion and association with TMEM. Clin. Exp. Metastasis 28, 515–527 (2011).
Karagiannis, G. S. et al. Assessment of MRI to estimate metastatic dissemination risk and prometastatic effects of chemotherapy. NPJ Breast Cancer 8, 101 (2022).
Harney, A. S. et al. The selective Tie2 inhibitor rebastinib blocks recruitment and function of Tie2Hi macrophages in breast cancer and pancreatic neuroendocrine tumors. Mol. Cancer Ther. 16, 2486–2501 (2017).
Bingle, L., Brown, N. J. & Lewis, C. E. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J. Pathol. 196, 254–265 (2002).
Wang, C. et al. The prognostic and clinical value of tumor-associated macrophages in patients with breast cancer: A systematic review and meta–analysis. Front. Oncol. 12, 905846 (2022).
Weidner, N., Semple, J. P., Welch, W. R. & Folkman, J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N. Engl. J. Med. 324, 1–8 (1991).
Martin, D. N. et al. Differences in the tumor microenvironment between African-American and European-American breast cancer patients. PloS One 4, e4531 (2009).
Hammond, M. E. H. et al. American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer (unabridged version). Arch. Pathol. Lab. Med. 134, e48–e72 (2010).
Yi, M. et al. Which threshold for ER positivity? A retrospective study based on 9639 patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 25, 1004–1011 (2014).
McShane, L. M. et al. Reporting recommendations for tumor marker prognostic studies (REMARK). J. Natl. Cancer Inst. 97, 1180–1184 (2005).
McShane, L. M. & Hayes, D. F. Publication of tumor marker research results: The necessity for complete and transparent reporting. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 30, 4223–4232 (2012).
Entenberg, D. et al. Validation of an automated quantitative digital pathology approach for scoring TMEM, a prognostic biomarker for metastasis. Cancers 12, E846 (2020).
Ye, X. et al. Combining TMEM doorway score and MenaCalc score improves the prediction of distant recurrence risk in HR+/HER2− breast cancer patients. Cancers 14, 2168 (2022).
Schindelin, J. et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Arganda-Carreras, I. et al. Trainable weka segmentation: A machine learning tool for microscopy pixel classification. Bioinforma. Oxf. Engl. 33, 2424–2426 (2017).
Schoenfeld, D. Partial residuals for the proportional hazards regression model. Biometrika 69, 239–241 (1982).
Grambsch, P. M. & Therneau, T. M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81, 515–526 (1994).
Acknowledgements
We would like to acknowledge New York Pathology Oncology Group (NYPOG) institutions (Montefiore, Cornell, NYU Langone, and MSKCC) for providing us the patient samples that was used for this study. We acknowledge the Analytical Imaging Facility at Albert Einstein College of Medicine for microscopy help. This work was supported by the following resources: DOH01-ROWLEY-2019-00037; NIH T32 CA200561; S10 OD026852, P30 CA013330; the Helen & Irving Spatz Family Foundation; the Evelyn Gruss Lipper Charitable Foundation, the Gruss-Lipper Biophotonics Center, and the Integrated Imaging Program for Cancer Research. This work was presented in part at the San Antonio Breast Cancer Symposium in 2021 and 2022.
Author information
Authors and Affiliations
Contributions
G.K.: Data curation, data organization, and writing – original draft. B.K.-F.: Data curation, data organization, and writing – original draft. J.Q.: Formal analysis and methodology. V.P.S.: Formal analysis and methodology. I.S.O.: Formal analysis and methodology. Y.L.: Data curation and methodology. X.Y.: Data curation, formal analysis, and methodology. S.A.: Data curation and methodology. J.M.P.: Clinical data curation. E.C.: Material collection and scientific proofing. N.L.: Scientific proofing. J.S.C.: Conceptualization, scientific proofing. E.A.: Material collection and scientific proofing. P.S.G.: Material collection and scientific proofing. T.D’A.: Material collection and scientific proofing. D.E.: Methodology discussion, study guidance. X.X.: Formal analysis and methodology. J.S.: Study guidance. M.H.O.: Conceptualization, study guidance, material collection and team guidance. G.K. and B.K.-F.: Co-first authors, contributed equally. All authors: Writing – review and editing.
Corresponding author
Ethics declarations
Competing interests
Joseph A. Sparano, is an associate editor of npjBreast Cancer. Other authors declare no potential conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, G., Karadal-Ferrena, B., Qin, J. et al. Racial disparity in tumor microenvironment and distant recurrence in residual breast cancer after neoadjuvant chemotherapy. npj Breast Cancer 9, 52 (2023). https://doi.org/10.1038/s41523-023-00547-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-023-00547-w
This article is cited by
-
Disparities in Hormone Receptor-Positive Breast Cancer
Current Breast Cancer Reports (2024)