Abstract
Planktonic cultures, of a rationally designed consortium, demonstrated emergent properties that exceeded the sums of monoculture properties, including a >200% increase in cellobiose catabolism, a >100% increase in glycerol catabolism, a >800% increase in ethanol production, and a >120% increase in biomass productivity. The consortium was designed to have a primary and secondary-resource specialist that used crossfeeding with a positive feedback mechanism, division of labor, and nutrient and energy transfer via necromass catabolism. The primary resource specialist was Clostridium phytofermentans (a.k.a. Lachnoclostridium phytofermentans), a cellulolytic, obligate anaerobe. The secondary-resource specialist was Escherichia coli, a versatile, facultative anaerobe, which can ferment glycerol and byproducts of cellobiose catabolism. The consortium also demonstrated emergent properties of enhanced biomass accumulation when grown as biofilms, which created high cell density communities with gradients of species along the vertical axis. Consortium biofilms were robust to oxic perturbations with E. coli consuming O2, creating an anoxic environment for C. phytofermentans. Anoxic/oxic cycling further enhanced biomass productivity of the biofilm consortium, increasing biomass accumulation ~250% over the sum of the monoculture biofilms. Consortium emergent properties were credited to several synergistic mechanisms. E. coli consumed inhibitory byproducts from cellobiose catabolism, driving higher C. phytofermentans growth and higher cellulolytic enzyme production, which in turn provided more substrate for E. coli. E. coli necromass enhanced C. phytofermentans growth while C. phytofermentans necromass aided E. coli growth via the release of peptides and amino acids, respectively. In aggregate, temporal cycling of necromass constituents increased flux of cellulose-derived resources through the consortium. The study establishes a consortia-based, bioprocessing strategy built on naturally occurring interactions for improved conversion of cellulose-derived sugars into bioproducts.
Similar content being viewed by others
Introduction
Sustainable, cost-effective production of fuels and chemicals is a major societal challenge. Lignocellulosic biomass is a promising feedstock for bioprocesses because of the large global supply, low cost, and the flexibility of the monomers to be converted into value-added products, including fuels, chemicals, and materials1,2. Consolidated, one pot, bioprocessing where lignocellulose depolymerization and product formation occur in a single vessel, is proposed to be a cost-effective strategy for producing fuels and chemicals due to process simplicity3,4. Biological routes for lignocellulose depolymerization are environmentally and economically attractive due to high substrate conversion and mild operating conditions as compared to the high energy and harsh chemical requirements of thermochemical processes5.
Traditional bioprocessing efforts have focused primarily on using a single “superbug” to achieve all desired chemistries. However, using single organisms for consolidated bioprocess often leads to low product titers, yields, and productivities6,7,8,9. It is difficult to optimize all necessary traits simultaneously due to tradeoffs in resource allocation10. Resources allocated to one function are not available to optimize additional functions; this concept forms the basis of the “Darwinian Demon” ecological thought experiment11,12. Evolution and natural selection have addressed the challenge of complex, multistep processes, like lignocellulose deconstruction via consortia using division of labor8,13,14,15,16,17,18. Natural and assembled consortia have been used for degrading lignocellulosic substrates8,19,20,21,22,23,24. The assembled consortia have used combinations of fungi or fungi and bacteria. For example, Minty et al.25 have used Escherichia coli and Trichoderma reesei to produce isobutanol from cellulose while Jin et al.23 and Zuroff et al.21 have assembled consortia comprised of Clostridium phytofermentans (a.k.a. Lachnoclostridium phytofermentans) and Saccharomyces cerevisiae to produce ethanol from cellulosic feedstocks.
Biofilms are microbial aggregates encapusulated in self-produced polymers and are typically associated with an interface like a solid surface; in nature, most microorganisms reside in biofilms26. The biofilm phenotype is distinct from the planktonic phenotype. Rate imbalances between biotic reactions and abiotic diffusion create gradients in chemicals and metabolic activity. These gradients are largely responsible for the structure and physiology of biofilms and can be viewed as control parameters for bioprocess applications27,28,29. Biofilms have competitive properties for bioprocessing including high cell densities (200–300 g cell dry weight L−1), high volumetric productivities, reduced requirements for water, no need for energy intensive agitation, facilitated separation of biomass from supernatant, and high tolerance to stresses like pH or inhibitors10,30,31.
There is considerable scientific interest in improving the catalytic efficiency of natural processes like nutrient cycling and applied processes like biofuel synthesis. Harnessing the emergent properties of microbial interactions has the potential to achieve this catalytic goal9. However, the biological compentents and interactions necessary to achieve emergent properties are not well understood. Natural systems are often extremely complex in terms of the number of species and the number of interactions, confounding the basis of emergent properties. Synthetic and artifical ecology have ability to decode the requirements of nonlinear, emergent properties15. In this work, an artificial consortium comprised of C. phytofermentans and E. coli was constructed. Here, the term artifical consortium is used to describe a consortium comprised of wild-type organisms that are not thought to cooccur in nature; alternatively, a synthetic consortium is defined as a consortium with at least one genetically modified population32. C. phytofermentans is a mesophilic, obligate anaerobe that grows on both soluble and insoluble components of lignocellulosic feedstocks33. C. phytofermentans is remarkable among the Clostridium genus due to its ability to catabolize a broad range of substrates. Its genome encodes over 169 carbohydrate-active enzymes, the largest number among sequenced clostridia, and its efficient ethanol production makes it a model system for cellulosic biofuel production21,23,34,35,36,37. E. coli is a well studied, facultative anaerobe capable of fermenting a broad range of substrates including glucose and glycerol which is a widely available waste product from biodiesel production21,23,38. E. coli is also a convenient host for metabolic engineering and can be modified to produce a wide range of biochemical products39,40. The C. phytofermentans and E. coli consortium was assembled to leverage common ecological motifs including cooccurrence of primary and secondary-resource specialists, metabolite exchange with positive feedback, and the flux of nutrients and energy between trophic levels through the catabolism of lysed biomass known as necromass27,41,42,43,44,45,46. Additionally, when grown as a biofilm, E. coli consumes O2 creating an anoxic environment for C. phytofermentans. The role of each consortium member, the mechanisms of interaction, and the spatial and temporal analysis of system function were considered in this study quantifying the enhanced consortium productivity. The metrics used to quantify the emergent properties of the consortium were (1) enhanced depletion of cellulosic sugar, (2) enhanced production of ethanol as a proxy biofuel and bioproduct molecule, and (3) enhanced production of microbial biomass.
Results
Planktonic monocultures and consortium properties
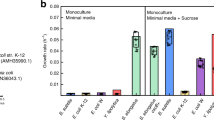
A consortium of C. phytofermentans and E. coli was assembled based on compatible physiologies, culturing conditions, and the possibility of synergistic interactions. The consortium was characterized as an anoxic planktonic culture to facilitate analysis of phenotypes, consortium member roles, and intercellular metabolite exchanges. The consortium demonstrated the emergent properties of enhanced substrate depletion, enhanced ethanol secretion, and enhanced biomass production as compared to monocultures (Fig. 1a, c, e and Table 1). The consortium consumed 8.84 ± 0.06 mM of cellobiose over 72 h of cultivation which was a 240% increase over C. phytofermentans monocultures (2.60 ± 0.11 mM). E. coli monocultures did not catabolize cellobiose, as expected; E. coli does not possess a functional cellobiase47. C. phytofermentans monocultures accumulated glucose during batch growth while free glucose was not measured during consortium cultivation, presumably due to rapid catabolism of the monosaccharide by E. coli. E. coli monocultures fermented 9.85 ± 0.89 mM of glycerol over 72 h while C. phytofermentans monocultures did not catabolize glycerol (Fig. 1d). E. coli catabolism of glycerol doubled to 19.89 ± 1.33 mM under consortium cultivation. mGS-2 medium contained citrate as an ion chelator; E. coli readily fermented citrate in the presence of glycerol while C. phytofermentans did not oxidize citrate (Supplementary Table 1 and Supplementary Fig. 1A). The observed, emergent properties were robust to changes in culture medium. Four different formulations of mGS-2 medium were analyzed; all resulted in similar, enhanced cellobiose (Fig. 1c), ethanol (Fig. 1e), and biomass properties (Fig. 1a and Supplementary Figs. 2–4).
a Optical density (OD600), b pH, c cellobiose concentration, d glycerol concentration, e ethanol concentration, f acetate concentration, g glucose concentration, and h formate concentration. Ec + Cp indicates the sum of E. coli and C. phytofermentans monoculture properties. Trends are only shown where relevant. All experiments were performed using mGS-2 medium. Error bars represent the standard deviation from three biological replicates.
Increased catabolism of cellobiose and glycerol resulted in higher titers of byproducts. The consortium produced 26.71 ± 0.61 mM ethanol, 25.40 ± 0.02 mM acetate, and 7.49 ± 0.54 mM formate (Table 1). Cultures produced small (<1.2 mM), but measurable, amounts of lactate (Supplementary Fig. 1B); no succinate was observed. Consortium pH values where lower than the monocultures reflecting the increased catabolism of cellobiose and glycerol and increased secretion of acidic byproducts. Consortium pH dropped to 5.8 over the course of 72 h while the C. phytofermentans and E. coli monoculture pH dropped to 6.7 and 6.6, respectively (Fig. 1b).
E. coli cultures accumulated biomass for approximately 12 h while C. phytofermentans cultures also accumulated biomass for 12 h but continued to catabolize substrate and secrete byproducts for 72 h (Fig. 1a, c, e). The consortium had a 41% increase in optical density (OD600) relative to the sum of the monocultures. Biomass productivity of the consortium was substantially larger than the sum of monoculture cell dry weights, increasing 121% (Table 1). Quantitative relationships between OD600, colony-forming units (CFU) per liter and gram cell dry weight per liter can be found in the materials and methods.
Biofilm phenotypes of consortium, with culturing perturbations
C. phytofermentans and E. coli monocultures and the consortium were grown as biofilms, a common, naturally occurring, growth state and potentially useful phenotype for bioprocesses31,48. Biofilm cultures were grown for 10 days using one of three cultivation strategies: completely anoxic, completely oxic, or an anoxic to oxic switch (AOS) after 6 days of cultivation (Fig. 2a–d). The AOS strategy was designed to quantify the robustness of the consortium to perturbations and to induce O2-based, lysis of C. phytofermentans cells to produce necromass.
OX: 10 days of oxic only conditions, AN: 10 days of anoxic only conditions, and AOS: 6 days anoxic and 4 days oxic growth (denoted with gray shading). a C. phytofermentans cell number per monoculture biofilm, b E. coli cell number per monoculture biofilm, c C. phytofermentans cell number per consortium biofilm, d E. coli cell number per consortium biofilm, e Total biofilm mass (biomass + extracellular material) for monoculture and consortium biofilm cultures. Black hashed area represents sum of monoculture data for AOS condition. Data in e collected after 10 days of cultivation. Error bars represent the standard deviation from three biological replicates. Statistical significance at **p < 0.01, T-test.
Monoculture biofilms of C. phytofermentans grew under anoxic conditions and during the anoxic phase of the AOS cultivation (Fig. 2a). There was no biomass accumulation when the monoculture biofilms were incubated for 10 days in the presence of O2 (Fig. 2a). The AOS cultures had a decrease in cell number, based on qPCR, after 2 days of oxic cultivation. The initial increase in cell number during oxic culturing likely reflected growth prior to O2-based lysis. The cell number data were based on the presence of chromosomal DNA and not necessarily viable cells.
E. coli monoculture biofilms produced more biomass under oxic conditions relative to anoxic, as anticipated. The presence of O2 made more substrate energy bioavailable (Fig. 2b). The effect of O2 was especially apparent during AOS cultivation; the introduction of O2 after 6 days resulted in rapid, biomass accumulation as the fermentation byproducts like acetate and nonfermentable amino acids found in the medium were likely oxidized aerobically (Fig. 2b).
Anoxic and AOS consortium growth increased the biomass productivity of both C. phytofermentans and E. coli relative to monoculture biofilms (Fig. 2c, d). The enhanced growth also implied an enhanced use of available substrates. C. phytofermentans grown as a consortium biofilm more than doubled biomass productivity as compared to the monoculture biofilm (Fig. 2a, c). During AOS cultivation, C. phytofermentans cell number, as detected by qPCR, increased initially following transition to oxic conditions before decreasing. The time delay, before the cell number decreased, could have been a result of O2 diffusion, cell lysis, and DNA degradation kinetics. The largest increase in E. coli biomass accumulation occurred during cultivation of the consortium under AOS conditions (Fig. 2b, d). There was a rapid increase in E. coli biomass upon switching to an oxic environment where accumulated fermentation byproducts could be catabolized and additionally, the O2-lysed C. phytofermentans biomass was available for E. coli catabolism (Fig. 2d). E. coli growth under AOS conditions exceeded E. coli growth under continuous O2 exposure, quantifying how the temporal partitioning of metabolism can enhance culture performance.
The consortium, grown under completely oxic conditions, did not show enhanced biomass productivity. C. phytofermentans was inhibited by O2 at inoculation, leading to a biofilm that was functionally, an E. coli monoculture (Fig. 2b–d).
Biofilm productivity was also analyzed using direct, gravimetric analysis on day 10 (Fig. 2e). C. phytofermentans and E. coli have different cellular geometries and, therefore, a comparison of cell number does not reflect total cell mass. Additionally, qPCR quantifies copy number of DNA sequences and would not quantify the production of other biofilm components like extracellular polymeric substance (EPS). C. phytofermentans monoculture biofilms produced over 2 mg cellular material (biomass and EPS) per biofilm during anoxic cultivation; no biomass accumulation was observed under oxic conditions and the AOS condition had an intermediate mass of cellular material. E. coli monoculture biofilms had a mass of 0.7–1.2 mg cellular material per biofilm depending on cultivation strategy; AOS and oxic cultivation produced the larger masses. AOS cultivation resulted in a large increase in consortium biomass. AOS consortium accumulated 6.25 mg cellular material per biofilm which was 153% more material than the sum of the E. coli and C. phytofermentans monocultures grown under AOS conditions (Table 2).
Spatially resolved analysis of biofilm cultures
Spatially resolved in situ O2 concentrations were measured within the biofilms on day 10 (Fig. 3). The AOS cultivation strategy produced the thickest biofilm (275–475 µm) (Figs. 3 and 4d and Supplementary Fig. 5); the in situ O2 concentration was below detection 50 µm from the oxic interface, creating a large anoxic zone for C. phytofermentans. The oxic conditions produced the thinnest biofilms (17–34 µm) which were oxic from top to bottom (≥75% of saturation) (Fig. 3 and Supplementary Fig. 5). The consortium biofilms, cultivated for 10 days anoxically, consumed O2 as soon as they were removed from the anoxic incubator and reduced O2 concentrations below detection within 100 µm from the oxic surface (Fig. 3) (it took ~15 min to remove the biofilms from the incubator, to transport the biofilms to the microelectrode equipment, and to make the O2 measurements). This rapid response indicated the E. coli had the enzymatic machinery to respire O2 expressed, even though the cultures were not exposed to the electron acceptor for 10 days. As a reference calculation, the abiotic diffusion of O2 through a 150–200 µm biofilm (Fig. 4d) would be predicted to take approximately 14–25 s, assuming the effective diffusion coefficient of O2 within a biofilm was 8 × 10−6 cm2 s−1 49 and assuming there were no O2 consuming reactions. Therefore, the observed O2 profiles reflected biological consumption and not solely a diffusion process.
OX: grown for 10 days oxically, AN: grown for 10 days anoxically, AOS: grown for 6 days anoxically followed by 4 days of oxic growth. The O2 concentrations within biofilm were measured using 25 µm diameter microelectrode O2 probes. A depth of 0 μm is the top surface of the biofilm. Error bars represent the standard deviation from three biological replicates.
a–c Consortium biofilms grown anoxically (AN) for 10 days and d–f consortium biofilms grown anoxically for 6 days followed by 4 days of oxic growth (AOX). Species distributions were measured using laser microdissection and qPCR analysis of 16S gene copy number. Biomass percentage was calculated from cell number data converted to mass using conversion factors listed in the “Materials and methods”. Cell data based on biofilm samples taken from three vertical positions (top, middle, bottom) and four to six radial positions from a single biofilm. Error bars represent the standard deviation of samples. Micrograph scale bars = 100 μm.
The spatial distributions of species and cell concentrations were measured using a combination of biofilm cryosectioning, laser microdissection, and qPCR. Samples were collected from three vertical locations: top, middle, and bottom of the biofilms at four to six radial positions (Fig. 4a, d). During anoxic cultivation, C. phytofermentans accounted for ~30% of the total cell number, based on qPCR, and ~70% of the total cell mass at the top and bottom of the biofilm. E. coli accounted for >70% of the total cell number and cell mass in the middle section of the biofilm, suggesting an optimal, spatial environment where glucose and C. phytofermentans necromass were available (Fig. 4b, c).
AOS cultivation showed different results. First, the biofilms were more than twofold thicker than the anoxic biofilm based on the cryosectioned samples (Fig. 4a, d) and optical coherence tomography analysis of hydrated biofilms (Supplementary Fig. 5). Second, C. phytofermentans resided primarily in the anoxic bottom of the biofilm, where it represented ~55% of the total cell number and ~84% of the total cell mass (Fig. 4e, f). E. coli comprised more than 99% of the total cell number and total cell mass at the top, oxic layer of the biofilm, and >75 % of the total cell number and cell mass in the middle of the biofilm.
The cellular distributions provided data for calculating the total cell number and total cellular mass as a function of spatial position in the biofilm (Fig. 5a–e). The total cell number peaked in the middle of the biofilm for both the AN and AOS biofilms, reaching approximately 2–2.5 × 1011 cells per mL of biofilm. The cellular mass concentration was highest at the bottom of the biofilm with densities of 0.25–0.30 g biomass per mL.
a, c Consortium biofilms grown for 10 days anoxically (AN) and b, d consortium biofilms grown anoxically for 6 days followed by 4 days of oxic growth (AOS). Data are from day 10. Cryosectioned biofilms had cells samples excised using laser microdissection from three vertical positions (top, middle, bottom) from four to six radial positions. Cell number was calculated using qPCR. Biomass concentrations were calculated using conversion factors listed in “Materials and methods”. Error bars represent the standard deviation of samples.
Mechanisms of enhanced consortium performance: role of cellobiase and glucose inhibition
Possible mechanisms responsible for enhanced consortium performance were tested including the role of product inhibition on cellobiose degradation. Many cellulose degradation processes are inhibited, at either an enzyme activity- or regulation level, by the accumulation of degradation products such as glucose50,51. Planktonic, C. phytofermentans monocultures accumulated glucose during growth on cellobiose suggesting the release of cellobiase into the medium (Fig. 1g). Culture supernatants were collected during the stationary phase from C. phytofermentans monocultures grown on mGS-2 medium supplemented with either glucose (5 g L−1), cellobiose (5 g L−1), or carboxymethyl cellulose (CMC) (5 g L−1). Samples were filtered through 0.2 µm pore membranes to remove cells. Fresh cellobiose (5 g L−1) was added to the filtered supernatants and glucose production was monitored to measure cellobiase activity (Fig. 6a).
a Volumetric cellobiase activity represented as liberated glucose concentration plotted as a function of time, b specific cellobiase activity represented as liberated glucose concentration normalized to culture OD600 plotted as a function of time, c C. phytofermentans growth (OD600) on cellobiose (5 g L−1), glucose (5 g L−1), and a mixture of sugars (5 g L−1 each), d Cellobiose consumption in C. phytofermentans monocultures with and without the presence of glucose (5 g L−1). Error bars represent the standard deviation from three biological replicates.
C. phytofermentans monocultures grown on CMC had the highest volumetric, cellobiase activity followed by the cellobiose- and the glucose-grown monocultures. This trend was further emphasized when the cellobiase activity was analyzed on a specific basis (volumetric activity normalized to culture OD600). CMC-grown monocultures had ~eight-fold higher specific cellobiase activity than the cellobiose-grown cultures (Fig. 6b). Monocultures grown on glucose containing medium did not produce statistically significant cellobiase activity. Enzyme activity was stable in the presence of O2; the cellobiase assays were performed under oxic conditions for 72 h.
The copresence of glucose and cellobiose negatively affected C. phytofermentans biomass accumulation and the degradation of cellobiose (Fig. 6c, d). This property was based on reduced production of cellulolytic enzymes (Fig. 6b) and likely due to some uncharacterized, catabolite repression mechanism. The C. phytofermentans genome contains three, annotated cellobiase/β-glucosidase genes. A candidate gene (ABX42305) for the C. phytofermentans cellobiase activity was identified based on similar extracellular activity, similar substrate repression, and the protein sequence alignment with enzyme BglA (AAQ00997) from Clostridium cellulovorans52. An alignment of the C. phytofermentans enzyme with the C. cellulovorans enzyme had 96% protein coverage, 31.4% protein identity, and an E-value of 5e−56.
Mechanism of enhanced consortium performance: catabolism of C. phytofermentans necromass
The catabolism of C. phytofermentans necromass by E. coli was evaluated as another potential mechanism driving enhanced consortium performance. Necromass refers to released biomass components including macromolecules and free metabolites from lysed cells21,53. This cellular material could have served as a substrate for E. coli.
C. phytofermentans necromass was produced from monocultures, grown anoxically to mid-exponential phase. The cultures were harvested by centrifugation, washed in M9 medium54 with no carbon source, and then exposed to ambient air for 24 h to induce cell lysis. C. phytofermentans readily lysed in the presence of O2, as documented with microscopy (Fig. 7a, b). E. coli growth on C. phytofermentans necromass was tested under oxic conditions. Different concentrations of C. phytofermentans necromass were added to M9 minimal medium as the sole carbon source (Fig. 7c). E. coli produced more biomass, as quantified using qPCR, with increasing concentrations of necromass. The control E. coli culture, with no added C. phytofermentans necromass, showed an increase in DNA, likely due to cellular division based on storage compounds like polyglucose. The abundance of C. phytofermentans DNA, as quantified by qPCR, decreased with time potentially due to abiotic DNA degradation similar to environmental DNA degradation or due to released DNase enzymes (Fig. 7d)55,56,57,58.
a Epifluorescence micrograph of C. phytofermentans cultured anoxically. b Epifluorescence image of lysed C. phytofermentans after 24 h of ambient air exposure. c Aerobic E. coli growth on different amounts of C. phytofermentans necromass, see main text for details. d C. phytofermentans necromass abundance, expressed as qPCR-based cell number, during aerobic E. coli growth on lysed C. phytofermentans biomass. Cp100, Cp50, Cp10, and Cp0 refer the percentage of medium comprised of C. phytofermentans necromass solution, see text for more details. Error bars represent the standard deviation from three biological replicates. Micrograph scale bars = 10 μm.
E. coli biomass yield on C. phytofermentans necromass was estimated with respect to two normalizations, cell number and cell mass. On a cell number basis, producing one E. coli cell required 2.0–2.2 cells of C. phytofermentans and on a mass basis, 1 g of E. coli biomass required 8.5–9.1 g of C. phytofermentans biomass. The differing values reflect the difference in E. coli and C. phytofermentans cell geometry and volume: E. coli cells are approximately 2 μm long while C. phytofermentans cells are approximately 10 μm long. The presented biomass yields and published biomass yields on necromass components suggest ~17–23% of the C. phytofermentans necromass was bioavailable for E. coli41. Free metabolite pools account for ~5% of cellular mass so some macromolecule degradation likely occurred59,60. This mechanism is believed to have played a large role in the enhanced productivity of the AOS grown biofilm cultures (Fig. 2e). Although, it is also proposed to play a role under anoxic conditions. CFU analyses suggested a large fraction of the C. phytofermentans culture formed spores during late exponential growth phase, lysing the vegetative cells, and releasing biomass components which would have been available for E. coli catabolism (Fig. 8b)21,53.
a C. phytofermentans cell concentration during monoculture and binary consortium growth, based on qPCR analysis. b C. phytofermentans growth as a monoculture or binary consortia. Consortia growth tested both with and without E. coli necromass. c E. coli growth as an anoxic, monoculture and binary consortium based on qPCR analysis. Cell death occurred after 10–12 h of growth likely releasing necromass. d C. phytofermentans monoculture growth on different amino acid sources. No growth was observed from casamino acid-based medium nor on a nutritionally complete, chemically defined medium CSP which contained only free amino acids. Yeast extract (YE) contained peptides in addition to free amino acids. e C. phytofermentans monoculture growth on different concentrations of peptide-containing YE. f C. phytofermentans growth on different concentrations (High and Low) of E. coli necromass produced via sonication-induced lysis. Error bars represent the standard deviation from three biological replicates. See text for more details.
Mechanism of enhanced consortium performance: catabolism of E. coli necromass
Catabolism of E. coli necromass by C. phytofermentans was explored; this was an additional mechanism for enhancing consortium productivity under anoxic conditions. C. phytofermentans cultures had a large increase in cell number when grown in a consortium, as compared to monoculture growth (Fig. 8a); the binary consortium had >10-fold more C. phytofermentans cells than the monoculture, based on qPCR. When the C. phytofermentans monocultures were analyzed using CFU analysis, the monocultures lost cell viability after 12 h of incubation with CFUs falling approximately 90% by 48 h of cultivation (Fig. 8b). However, the C. phytofermentans grown in consortia increased in CFUs until 24 h of incubation and retained >2.0 × 108 CFU per biofilm at 48 h of cultivation (Fig. 8b). E. coli CFU counts decreased after exponential phase in both the monoculture and consortia experiments (Fig. 8c). Collectively, the results suggested resources from E. coli, potentially necromass, were promoting growth and sustaining viability of C. phytofermentans.
E. coli necromass was tested directly as a potential growth enhancer. C. phytofermentans did not grow on CSP chemically defined medium containing individual amino acids (Supplementary Table 2) nor did it grow on casamino acids, presumably requiring peptides supplied in the mGS-2 medium or from lysed cells (Fig. 8d). C. phytofermentans biomass accumulation increased with the addition of yeast extract which contained peptides along with other potential growth factors including trace metals and vitamins (Fig. 8e). E. coli necromass was generated by collecting biomass via centrifugation from mid-exponential phase, oxic monocultures. The biomass was washed twice with fresh mGS-2 medium and sonicated (Microson XL 2000) in an ice bath for 15 min at the maximum power setting to lyse the E. coli cells. The lysis solution was filtered using a 0.2 µm membrane to remove intact E. coli cells and the filtrate was used as a necromass source.
C. phytofermentans growth on E. coli necromass was evaluated under anoxic conditions as either a monoculture or binary consortium (Fig. 8b, f). C. phytofermentans monocultures had increased biomass accumulation which scaled with the addition of E. coli necromass (Fig. 8f). C. phytofermentans growth also increased when E. coli necromass was added to the binary consortium containing viable E. coli (Fig. 8b). The enhanced C. phytofermentans growth provides a basis for estimating the biomass yield of C. phytofermentans on E. coli necromass. On a cell number basis, one C. phytofermentans cell was produced from 16.5 to 19.1 cells of E. coli and on a mass basis,1 g of C. phytofermentans biomass was produced from 3.2 to 3.7 g of E. coli when added to mGS-2 medium. This figure assumed all E. coli cells were lysed and all necromass passed through the filter. This mechanism was likely responsible for the increased C. phytofermentans growth during both planktonic and biofilm growth.
Discussion
An artificial consortium was assembled using principles identified in naturally occurring consortia including division of labor between primary- and secondary-resource specialists, metabolite exchange with positive feedback, and enhanced resource extraction based on necromass catabolism27,41. The cellobiose-degrading consortium comprised of C. phytofermentans, the primary resource specialist, and E. coli, the secondary-resource specialist, demonstrated the emergent properties of enhanced substrate depletion, enhanced ethanol secretion, and enhanced biomass productivity relative to the sum of monoculture properties. For example, the synergistic interactions improved planktonic and biofilm biomass productivity approximately 121% and 153%, respectively, on a mass basis (Table 1 and Fig. 2e). A proposed model of the monoculture and consortium substrate preferences and interactions is illustrated in Fig. 9a–c. Consortial interactions also produced substantial, experimental changes in byproduct distributions after 72 h of cultivation (Table 1 and Fig. 9d–f). The consortium used wild-type microorganisms to achieve the enhanced properties. Use of traditional metabolic engineering approaches such as deleting inefficient metabolic routes could further optimize the system as well as be used to synthesis other valuable bioproducts39,40,61,62,63.
a C. phytofermentans monoculture, b E. coli monoculture, c C. phytofermentans, and E. coli binary consortia with necromass catabolism. d Experimental distribution of carbon products for C. phytofermentans monoculture after 72 h of cultivation. Areas represent percent of measured carbon moles. e Experimental distribution of carbon products for anoxic E. coli monoculture after 72 h of cultivation. Areas represent percent of measured carbon moles. d Experimental distribution of carbon products for anoxic consortium after 72 h of cultivation. Areas represent percent of measured carbon moles.
Enhanced biomass productivity was proposed to be the result of a few major mechanisms. First, C. phytofermentans released cellobiase enzyme which hydrolyzed cellobiose into glucose extracellularly (Fig. 1g). The presence of free glucose inhibited the production of additional cellulolytic enzymes (Fig. 6a, b); when E. coli was present, it catabolized the glucose relieving inhibition of cellulolytic enzyme synthesis and created a positive feed forward loop enhancing the degradation of cellulose-derived sugar (Fig. 1g). In the presence of O2, E. coli likely catabolized fermentation byproducts removing the inhibitory metabolites, creating a positive feedback loop enhancing substrate catabolism. C. phytofermentans readily formed spores, lysing the vegetative cells, and releasing necromass which was partially bioavailable for E. coli catabolism (Figs. 7 and 8b). Additionally, the anoxic to oxic switch (AOS) cultivation would have lysed C. phytofermentans cells in the oxic zone of the biofilm, releasing necromass (Figs. 2 and 7). The spore-forming and O2-lysed cells would also release cellobiase which remained active in the presence of O2 (Fig. 6a, b), producing additional glucose for E. coli catabolism. Moreover, E. coli grew readily on simple substrates including free amino acids, upgrading those resources into proteins and oligomers; this upgrading combined with E. coli cell lysis would make the otherwise inaccessible resources available for the fastidious C. phytofermentans, enhancing its growth and production of cellulolytic enzymes (Fig. 8a, c).
The turnover of biomass from the primary resource population and the release of necromass is a common mechanism in natural consortia and can drive flux of material and energy between trophic levels64,65,66,67,68. Biomass turnover, through mechanisms like senescence, inhibitor-based cell lysis, or viral predation, can result in increased energy acquisition rates in the systems. This is a predictor of competitive consortium function based on a theory known as the “Maximum Power” principle41,69,70.
A substantial increase in biomass productivity occurred when the consortium was transferred from anoxic to oxic conditions. One hundred and forty-seven percent more consortia mass was produced during AOS cultivation as compared to anoxic cultivation (Fig. 2e). The increase was substantially larger (153%) than the sum of the monoculture AOS productivities, quantifying the outcome of the synergistic interactions between the two species and the oxic environment. The use of agar plates for biofilm cultivation prevented direct measurement of cellobiose utilization and ethanol production, but they are proposed to scale with biomass productivity suggesting >2-fold increase in cellobiose catabolism and ethanol production compared to monocultures. The AOS cultivation is a relatively simple strategy with a large impact and can be integrated into cultivation systems via the introduction of O2 after the initial anoxic phase. This strategy could be applied readily to either solid phase or heterogenous (liquid + flocs) bioreactors. The timing of the anoxic to oxic transition would need to account for the system growth rates, biomass concentration, and the length scales for O2 diffusion71.
The simultaneous use of both anaerobic and aerobic chemistries within the biofilm provides opportunity for bioprocessing. The anoxic zone would favor the capture of sugar-derived electrons on reduced products like ethanol, while the oxic zone enables high energetic yields on byproducts like acetate and high metabolic rates which consume O2 maintaining the anoxic zone. E. coli is a convenient biotechnological host and provides opportunities for producing a wide range of biochemicals in the anoxic, oxic, or both zones of the biofilm. Obligate aerobic or facultative E. coli strains could be cultivated in biofilms to control vertical localization, generating laminated catalytic potential13,27.
This study constructed an artifical C. phytofermentans and E. coli consortium based on biomimicry of naturally occuring, microorganism interactions. The consortium demonstrated the emergent properties of enhanced substrate depletion, enhanced ethanol production, and enhanced biomass productivity. The assembled consortium had enhanced functioning during both planktonic and biofilm cultivation based on crossfeeding, positive feedback mechanisms, and the catabolism of necromass. These design features are powerful tools for improving bioprocesses and can likely be incorporated within existing bioprocesses.
Materials and methods
Bacterial strains and medium
C. phytofermentans ISDg (ATCC 700394) and E. coli K-12 MG1655 were used for all experiments. All reported planktonic and biofilm growth were performed in modified GS-2 media72 (mGS-2) with the following composition per liter: 1.5 g KH2PO4, 2.9 g K2HPO4, 2.1 g urea, 10 g MOPS, 3.0 g Na-Citrate, 1 g Resazurin, 1 g yeast extract, 1 g MgCl2·6H2O, 150 mg CaCl2·2H2O, 1.25 mg FeSO4·6H2O, 2.3 g glycerol, 5 g cellobiose and 10 mL of trace metal solution (per liter: 1.5 g FeCl2·4H2O, 70 mg ZnCl2, 0.1 g MnCl2·4H2O, 6 mg H3BO3, 0.19 g CoCl2·6H20, 2 mg CuCl2·2H2O, 24 mg NiCl2·H2O, 36 mg Na2MoO4·2H2O, 10 mL HCl (25%)). Salt solution (MgCl2·6H2O, CaCl2·2H2O, FeSO4·6H2O), cellobiose, yeast extract, and trace metal solution were sterilized separately by autoclave or filter sterilization and added after autoclaving. Initial pH of the basal components was adjusted to 6.9. When necessary, agar was added at 14 g L−1. Media was kept in an anaerobic chamber (Bactron II, Sheldon Manufacturing Inc.) with 5% H2, 5% CO2, and 90% N2 until it was used.
Planktonic culturing
Planktonic experiments were performed using 18 × 150 mm Balch anaerobic culture tubes in containing 10 mL of mGS-2 medium in a shaker operated at 150 revolutions per minute and 37 °C. Initial cultures of each strain were prepared from cryogenically (−80 °C) frozen stock. Inocula were prepared from fresh overnight cultures grown in mGS-2 medium. Initial OD600 of each strain was 0.01 OD600 for C. phytofermentans and 0.001 for E. coli after dilution. Samples were collected aseptically using a 1 mL syringe to analyzed for OD600, pH, CFU, and extracellular metabolite concentration. Total sampling volume collected was less than 20% of initial culture volume. CFUs of C. phytofermentans and E. coli monoculture were determined using drop plating on agarose (1.5%) with mGS-2 media plates under anaerobic conditions. For consortium CFU counts, selective plates were used. E. coli counts were performed on mGS-2 agar plates cultured under oxic conditions to prevent C. phytofermentans growth. C. phytofermentans counts were performed on mGS-2 agar plates containing 50 µg mL−1 kanamycin to prevent E. coli growth.
Data analysis used the following conversion factors to quantify biomass: E. coli: 1 OD600 = 0.45 g cell dry weight L−1, 1 OD600 = 9.15 × 108 CFU mL−1; C. phytofermentans: 1 OD600 = 1.55 g cell dry weight L−1, 1 OD600 = 7.55 × 108 CFU mL−1. Parameters were either experimentally determined or from literature73,74.
Colony biofilm culturing
Colony biofilm culturing systems consisted of 25 mm polycarbonate membrane disks with 0.22 µm pores (GVS Life Science, REF# 1215609) placed on mGS-2 agar plates75,76,77,78. Membranes were aseptically placed on mGS-2 agar plates and inoculated with 100 µL of planktonic cultures (0.01 OD600 for C. phytofermentans and 0.001 OD600 for E. coli). Biofilms were incubated at 37 °C in an anoxic chamber and/or oxic incubator depending on experiment. Biofilm cultures were aseptically transferred to a new medium plate every 2 days. Biofilm analysis was performed every 2 days using destructive sampling. Sampled colony biofilms were aseptically transferred to 5 mL of sterile phosphate-buffered saline (PBS) and vortexed vigorously for 30 s to separate cells from the membrane. The membrane was discarded, and the biofilm suspension was disaggregated using a high-performance dispersing instrument (T25 Ultra-Turrax, IKA) at 7000 revolutions per minute for 30 s. Further analysis (biomass, CFU and qPCR) was performed using this biofilm suspension.
Extracellular metabolite analysis
Extracellular metabolite concentrations (glucose, acetate, lactate, ethanol, succinate, and formate) from planktonic cultures were measured using an Agilent 1200 HPLC. Samples were filtered with 0.2 µm centrifuge filter to remove cell debris. Twenty microliters of filtered samples were injected on an HPX-87H column (Bio-Rad) at 40 °C with a 0.005 M H2SO4 mobile phase (0.6 mL min−1). Data were collected with a refractive index detector and analyzed with Agilent ChemStation software.
Spatial O2 concentrations within biofilms
Spatially resolved, in situ O2 concentrations were measured within biofilms using a MicroProfiling System from Unisense (Aarhus, Denmark). It consisted of a 25 µm O2 microsensor (OX-25), held by a motorized and computer-controlled micromanipulator (MM33-2) and microscope. The microsensor was calibrated with a strong reductant solution with both ascorbic acid and sodium hydroxide at a final concentration of 0.1 M and fully air saturated water with vigorous bubbling for 5 min. The O2 microsensor was positioned with the micromanipulator on the biofilm sample using a microscope. O2 gradients were measured every 25 µm from the top of the biofilm. Data were collected by SensorTrace Logger software from Unisense.
Cryoprocessing of biofilms
Colony biofilms were cryoembedded using Tissue-Tek. Optimal cutting temperature (OCT) compound (Sakra Finetechnical Co.), dry ice, and a stainless steel slide for enhanced heat transfer. Vertical transections of biofilms were obtained by sectioning biofilms embedded in solidified OCT with a cryomicrotome. Thin section (10 µm) of vertical transects of the biofilms were placed onto polyethylene naphthalate (PEN) membrane-coated stainless microscope slide (Leica microsystems Inc.). The microscope slides were kept at −20 °C until analysis.
Laser microdissection (LMD) of biofilm
Leica LMD6 (Leica microsystems Inc.) was used to dissect and capture sections from different regions within the biofilm. PEN membrane microscope slides containing biofilm were examined using lenses with objectives of ×10 to ×40 magnification. Samples were obtained using the laser cut and capture sequence which allow dissected samples to be captured into 20 µl of enzymatic lysis buffer (20 mM Tris-Cl, 2 mM sodium EDTA, 1.2% Triton X-100, 20 mg mL−1 lysozyme at pH 8.0). Samples were collected from three vertical positions (top, middle, bottom) at four to six different radial positions from a single biofilm.
qPCR analysis of species abundance and distribution
qPCR was performed to analyze species abundance in both planktonic and biofilm cultures. DNA was extracted and processed with DNeasy Kit or DNeasy Micro kit (Qiagen) using the manufacturer protocols. DNA samples were stored at −20 °C until analysis by qPCR. Primers for 16s rRNA genes (Table 3) were evaluated in silico using IDT Oligoanalyzer tool and NCBI’s primer Blast tool. Additionally, primer independency between C. phytofermentans and E. coli was confirmed both by 16s RNA sequence alignment using Mega7 software and by experimental testing using Rotor-Gene 3000 (Corbett Research) with QuantiFast SYBR Green PCR Kit (Qiagen). Genomic DNA from C. phytofermentans and E. coli monocultures were extracted and quantified with Qubit Fluorometer (Thermo Fisher) and used to create a standard DNA curve for each species. Cycling parameters were as follows: PCR initial heat activation at 95 °C for 5 min, 40 cycles of 95 °C for 10 s and 60 °C for 30 s. Data were acquired during 60 °C analyzing step and calculated threshold cycle (CT) values with Roto-Gene6 software. Equation (1) was used to calculate the DNA copy number for each species79,80:
DNA copy number was divided by the 16s rRNA copy number per chromosome (E. coli: 7 copies, C. phytofermentans: 8 copies) to calculate the total cell equivalents. Calibration curves can be found in Supplementary Fig. 6. The cell number could be converted to other quantities such as OD600, CFU L−1, and g cell dry weight L−1 using through conversion factors listed in the “Planktonic culturing” section.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Yang, B. & Wyman, C. E. Pretreatment: the key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Bioref. 2, 26–40 (2008).
Ko, J. K. & Lee, S.-M. Advances in cellulosic conversion to fuels: engineering yeasts for cellulosic bioethanol and biodiesel production. Curr. Opin. Biotechnol. 50, 72–80 (2018).
Lynd, L. R., Weimer, P. J., van Zyl, W. H. & Pretorius, I. S. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66, 506–577 (2002).
Lynd, L. R., van Zyl, W. H., McBride, J. E. & Laser, M. Consolidated bioprocessing of cellulosic biomass: an update. Curr. Opin. Biotechnol. 16, 577–583 (2005).
Mohite, B. V. & Patil, S. V. in New and Future Developments in Microbial Biotechnology and Bioengineering (ed. Gupta, V. K.) Ch. 4, 31–40 (Elsevier, 2016).
Brethauer, S. & Studer, M. Consolidated bioprocessing of lignocellulose by a microbial consortium. Energy Environ. Sci. 7, 1446–1453 (2014).
Du, R. et al. Cellulosic ethanol production by natural bacterial consortia is enhanced by Pseudoxanthomonas taiwanensis. Biotechnol. Biofuels 8, 10 (2015).
Zuroff, T. R. & Curtis, W. R. Developing symbiotic consortia for lignocellulosic biofuel production. Appl. Microbiol. Biotechnol. 93, 1423–1435 (2012).
Lindemann, S. R. et al. Engineering microbial consortia for controllable outputs. ISME J. 10, 2077–2084 (2016).
Olson, D. G., McBride, J. E., Joe Shaw, A. & Lynd, L. R. Recent progress in consolidated bioprocessing. Curr. Opin. Biotechnol. 23, 396–405 (2012).
Carlson, R. P. & Taffs, R. L. Molecular-level tradeoffs and metabolic adaptation to simultaneous stressors. Curr. Opin. Biotechnol. 21, 670–676 (2010).
Kneitel, J. M. & Chase, J. M. Trade-offs in community ecology: linking spatial scales and species coexistence. Ecol. Lett. 7, 69–80 (2004).
Patel, A., Carlson, R. P. & Henson, M. A. In silico metabolic design of two-strain biofilm systems predicts enhanced biomass production and biochemical synthesis. Biotechnol. J. 14, 1800511 (2019).
Bernstein, H. C. & Carlson, R. P. Microbial consortia engineering for cellular factories: in vitro to in silico systems. Comput. Struct. Biotechnol. J. 3, e201210017 (2012).
Fredrickson, J. K. Ecological communities by design. Science 348, 1425–1427 (2015).
Brenner, K., You, L. & Arnold, F. H. Engineering microbial consortia: a new frontier in synthetic biology. Trends Biotechnol. 26, 483–489 (2008).
Kato, S., Haruta, S., Cui, Z. J., Ishii, M. & Igarashi, Y. Network relationships of bacteria in a stable mixed culture. Microb. Ecol. 56, 403–411 (2008).
Girvan, M. S., Campbell, C. D., Killham, K., Prosser, J. I. & Glover, L. A. Bacterial diversity promotes community stability and functional resilience after perturbation. Environ. Microbiol. 7, 301–313 (2005).
Kanokratana, P. et al. Characterization of cellulolytic microbial consortium enriched on Napier grass using metagenomic approaches. J. Biosci. Bioeng. 125, 439–447 (2018).
Poszytek, K., Ciezkowska, M., Sklodowska, A. & Drewniak, L. Microbial Consortium with High Cellulolytic Activity (MCHCA) for enhanced biogas production. Front. Microbiol. 7, 324 (2016).
Zuroff, T. R., Xiques, S. B. & Curtis, W. R. Consortia-mediated bioprocessing of cellulose to ethanol with a symbiotic Clostridium phytofermentans/yeast co-culture. Biotechnol. Biofuels 6, 59 (2013).
Wang, W. et al. Characterization of a microbial consortium capable of degrading lignocellulose. Bioresour. Technol. 102, 9321–9324 (2011).
Jin, M., Balan, V., Gunawan, C. & Dale, B. E. Consolidated bioprocessing (CBP) performance of Clostridium phytofermentans on AFEX‐treated corn stover for ethanol production. Biotechnol. Bioeng. 108, 1290–1297 (2011).
Wongwilaiwalin, S. et al. Analysis of a thermophilic lignocellulose degrading microbial consortium and multi-species lignocellulolytic enzyme system. Enzym. Microb. Technol. 47, 283–290 (2010).
Minty, J. J. et al. Design and characterization of synthetic fungal-bacterial consortia for direct production of isobutanol from cellulosic biomass. Proc. Natl Acad. Sci. USA 110, 14592–14597 (2013).
Costerton, J. W., Lewandowski, Z., Caldwell, D. E., Korber, D. R. & Lappin-Scott, H. M. Microbial biofilms. Annu. Rev. Microbiol. 49, 711–745 (1995).
Bernstein, H. C., Paulson, S. D. & Carlson, R. P. Synthetic Escherichia coli consortia engineered for syntrophy demonstrate enhanced biomass productivity. J. Biotechnol. 157, 159–166 (2012).
Stewart, P. S. & Franklin, M. J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 6, 199–210 (2008).
Henson, M. A. Genome-scale modeling of microbial metabolism with temporal and spatial resolution. Biochem Soc. Trans. 43, 1164–1171 (2015).
Moons, P., Michiels, C. W. & Aertsen, A. Bacterial interactions in biofilms. Crit. Rev. Microbiol. 35, 157–168 (2009).
Rosche, B., Li, X. Z., Hauer, B., Schmid, A. & Buehler, K. Microbial biofilms: a concept for industrial catalysis? Trends Biotechnol. 27, 636–643 (2009).
Bernstein, H. C., Beam, J. P., Kozubal, M. A., Carlson, R. P. & Inskeep, W. P. In situ analysis of oxygen consumption and diffusive transport in high-temperature acidic iron-oxide microbial mats. Environ. Microbiol. 15, 2360–2370 (2013).
Warnick, T. A., Methé, B. A. & Leschine, S. B. Clostridium phytofermentans sp. nov., a cellulolytic mesophile from forest soil. Int. J. Syst. Evolut. Microbiol. 52, 1155–1160 (2002).
Petit, E. et al. Genome and transcriptome of Clostridium phytofermentans, catalyst for the direct conversion of plant feedstocks to fuels. PLoS ONE 10, e0118285 (2015).
Boutard, M. et al. Functional diversity of carbohydrate-active enzymes enabling a bacterium to ferment plant biomass. PLoS Genet. 10, e1004773 (2014).
Cantarel, B. L. et al. The Carbohydrate-Active EnZymes database (CAZy): an expert resource for glycogenomics. Nucleic Acids Res. 37, D233–D238 (2009).
Tolonen, A. C. et al. Physiology, genomics, and pathway engineering of an ethanol-tolerant strain of Clostridium phytofermentans. Appl. Environ. Microbiol. AEM.00619-15, https://doi.org/10.1128/AEM.00619-15 (2015).
Minty, J. J. et al. Design and characterization of synthetic fungal-bacterial consortia for direct production of isobutanol from cellulosic biomass. Proc.Natl Acad. Sci. USA 110, 14592–14597 (2013).
Pontrelli, S. et al. Escherichia coli as a host for metabolic engineering. Metab. Eng. 50, 16–46 (2018).
Wang, R., Zhao, S., Wang, Z. & Koffas, M. A. Recent advances in modular co-culture engineering for synthesis of natural products. Curr. Opin. Biotechnol. 62, 65–71 (2020).
Hunt, K. A., Jennings, R. M., Inskeep, W. P. & Carlson, R. P. Multiscale analysis of autotroph-heterotroph interactions in a high-temperature microbial community. PLOS Comput. Biol. 14, e1006431 (2018).
McInerney, M. J., Sieber, J. R. & Gunsalus, R. P. Syntrophy in anaerobic global carbon cycles. Curr. Opin. Biotechnol. 20, 623–632 (2009).
Morris, B. E. L., Henneberger, R., Huber, H. & Moissl-Eichinger, C. Microbial syntrophy: interaction for the common good. FEMS Microbiol Rev. 37, 384–406 (2013).
Schink, B. Synergistic interactions in the microbial world. Antonie Van. Leeuwenhoek 81, 257–261 (2002).
Stams, A. J. M. & Plugge, C. M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat. Rev. Microbiol. 7, 568–577 (2009).
Taffs, R. et al. In silico approaches to study mass and energy flows in microbial consortia: a syntrophic case study. BMC Syst. Biol. 3, 114 (2009).
Edwards, M. C. et al. Addition of genes for cellobiase and pectinolytic activity in Escherichia coli for fuel ethanol production from pectin-rich lignocellulosic biomass. Appl Environ. Microbiol. 77, 5184–5191 (2011).
Gross, R., Hauer, B., Otto, K. & Schmid, A. Microbial biofilms: new catalysts for maximizing productivity of long-term biotransformations. Biotechnol. Bioeng. 98, 1123–1134 (2007).
Stewart, P. S. A review of experimental measurements of effective diffusive permeabilities and effective diffusion coefficients in biofilms. Biotechnol. Bioeng. 59, 261–272 (1998).
Cao, L. et al. Engineering a novel glucose-tolerant β-glucosidase as supplementation to enhance the hydrolysis of sugarcane bagasse at high glucose concentration. Biotechnol. Biofuels 8, 202 (2015).
Prawitwong, P. et al. Direct glucose production from lignocellulose using Clostridium thermocellum cultures supplemented with a thermostable β-glucosidase. Biotechnol. Biofuels 6, 184 (2013).
Kosugi, A., Arai, T. & Doi, R. H. Degradation of cellulosome-produced cello-oligosaccharides by an extracellular non-cellulosomal β-glucan glucohydrolase, BglA, from Clostridium cellulovorans. Biochem. Biophys. Res. Commun. 349, 20–23 (2006).
Zuroff, T. R. Engineering a microbial consortium for lignocellulosic biofuel production. https://etda.libraries.psu.edu/catalog/22540 (2014).
Folsom, J. P., Parker, A. E. & Carlson, R. P. Physiological and proteomic analysis of escherichia coli iron-limited chemostat growth. J. Bacteriol. 196, 2748–2761 (2014).
Eichmiller, J. J., Best, S. E. & Sorensen, P. W. Effects of temperature and trophic state on degradation of environmental DNA in lake water. Environ. Sci. Technol. 50, 1859–1867 (2016).
Cowart, D. A., Murphy, K. R. & Cheng, C.-H. C. Metagenomic sequencing of environmental DNA reveals marine faunal assemblages from the West Antarctic Peninsula. Mar. Genomics 37, 148–160 (2018).
Collins, R. A. et al. Persistence of environmental DNA in marine systems. Commun. Biol. 1, 1–11 (2018).
Harrison, J. B., Sunday, J. M. & Rogers, S. M. Predicting the fate of eDNA in the environment and implications for studying biodiversity. Proc. R. Soc. B Biol. Sci. 286, 20191409 (2019).
Bennett, B. D. et al. Absolute metabolite concentrations and implied enzyme active site occupancy in Escherichia coli. Nat. Chem. Biol. 5, 593–599 (2009).
Neidhardt, F. C., Ingraham, J. L. & Schaechter, M. Physiology of the Bacterial Cell: A Molecular Approach (Sinauer Associates Inc., 1990).
Calhoun, M. W., Oden, K. L., Gennis, R. B., de Mattos, M. J. & Neijssel, O. M. Energetic efficiency of Escherichia coli: effects of mutations in components of the aerobic respiratory chain. J. Bacteriol. 175, 3020–3025 (1993).
Hua, Q., Yang, C., Baba, T., Mori, H. & Shimizu, K. Responses of the central metabolism in Escherichia coli to phosphoglucose isomerase and glucose-6-phosphate dehydrogenase knockouts. J. Bacteriol. 185, 7053–7067 (2003).
Trinh, C. T., Carlson, R., Wlaschin, A. & Srienc, F. Design, construction and performance of the most efficient biomass producing E. coli bacterium. Metab. Eng. 8, 628–638 (2006).
Dong, X. et al. Fermentative spirochaetes mediate necromass recycling in anoxic hydrocarbon-contaminated habitats. ISME J. 12, 2039–2050 (2018).
Liew, F. J. Prospecting fungi for methane biofiltration reveals high-efficiency capture by dried mycelia (necromass) https://conservancy.umn.edu/handle/11299/194651 (2017).
Müller, A. L. et al. Bacterial interactions during sequential degradation of cyanobacterial necromass in a sulfidic arctic marine sediment. Environ. Microbiol. 20, 2927–2940 (2018).
Schreiner, K. M., Blair, N. E., Buiser, A. & Egerton-Warburton, L. The contribution of fungal necromass to soil organic matter storage. AGU Fall Meet. Abstr. 2013, B33B–B30483 (2013).
Fazzino, L., Anisman, J., Chacón, J. M., Heineman, R. H. & Harcombe, W. R. Lytic bacteriophage have diverse indirect effects in a synthetic cross-feeding community. ISME J. 14, 123–134 (2020).
Beck, A. E., Bernstein, H. C. & Carlson, R. P. Stoichiometric network analysis of cyanobacterial acclimation to photosynthesis-associated stresses identifies heterotrophic niches. Processes 5, 32 (2017).
DeLong, J. P. The maximum power principle predicts the outcomes of two-species competition experiments. Oikos 117, 1329–1336 (2008).
Raghava Rao, K. S. M. S., Gowthaman, M. K., Ghildyal, N. P. & Karanth, N. G. A mathematical model for solid state fermentation in tray bioreactors. Bioprocess Eng. 8, 255–262 (1993).
Johnson, E. A., Madia, A. & Demain, A. L. Chemically defined minimal medium for growth of the anaerobic cellulolytic thermophile Clostridium thermocellum. Appl. Environ. Microbiol 41, 1060–1062 (1981).
Folsom, J. P. & Carlson, R. P. Physiological, biomass elemental composition and proteomic analyses of Escherichia coli ammonium-limited chemostat growth, and comparison with iron- and glucose-limited chemostat growth. Microbiology 161, 1659–1670 (2015).
Myers, J. A., Curtis, B. S. & Curtis, W. R. Improving accuracy of cell and chromophore concentration measurements using optical density. BMC Biophys. 6, 4 (2013).
Anderl, J. N., Franklin, M. J. & Stewart, P. S. Role of antibiotic penetration limitation in Klebsiella pneumoniae biofilm resistance to ampicillin and ciprofloxacin. Antimicrobial Agents Chemother. 44, 1818–1824 (2000).
Hamilton, M. The Biofilm Laboratory: Step-by-Step Protocols for Experimental Design, Analysis, and Data Interpretation (Cytergy, 2004).
Walters, M. C., Roe, F., Bugnicourt, A., Franklin, M. J. & Stewart, P. S. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 47, 317–323 (2003).
Zuroff, T. R. et al. Robustness analysis of culturing perturbations on Escherichia coli colony biofilm beta-lactam and aminoglycoside antibiotic tolerance. BMC Microbiol. 10, 185 (2010).
Whelan, J. A., Russell, N. B. & Whelan, M. A. A method for the absolute quantification of cDNA using real-time PCR. J. Immunological Methods 278, 261–269 (2003).
Lee, C., Kim, J., Shin, S. G. & Hwang, S. Absolute and relative QPCR quantification of plasmid copy number in Escherichia coli. J. Biotechnol. 123, 273–280 (2006).
Acknowledgements
This work was supported by the Army Research Office award W911NF-16-1-0463 and National Science Foundation award DMS 1361240. The authors would like to thank Tomas Gedeon for reading the manuscript and helpful discussions.
Author information
Authors and Affiliations
Contributions
Conception: M.A.H. and R.P.C.; design of work: H.P., K.A.H., and R.P.C.; acquisition and analysis: H.P., K.A.H., and R.P.C.; interpretation of data: H.P., A.P., K.A.H., M.A.H., and R.P.C.; drafting and revising of document: H.P., A.P., K.A.H., M.A.H., and R.P.C.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, H., Patel, A., Hunt, K.A. et al. Artificial consortium demonstrates emergent properties of enhanced cellulosic-sugar degradation and biofuel synthesis. npj Biofilms Microbiomes 6, 59 (2020). https://doi.org/10.1038/s41522-020-00170-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41522-020-00170-8
This article is cited by
-
Microbial engineering strategies to utilize waste feedstock for sustainable bioproduction
Nature Reviews Bioengineering (2023)
-
Microbes and microbial strategies in carcinogenic polycyclic aromatic hydrocarbons remediation: a systematic review
Environmental Science and Pollution Research (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.