Abstract
During double fertilization in angiosperms, the pollen tube delivers two sperm cells into an embryo sac; one sperm cell fuses with an egg cell, and the other sperm cell fuses with the central cell. It has long been proposed that the preference for fusion with one or another female gamete cell depends on the sperm cells and occurs during gamete recognition. However, up to now, sperm-dependent preferential fertilization has not been demonstrated, and results on preferred fusion with either female gamete have remained conflicting. To investigate this topic, we generated Arabidopsis thaliana mutants that produce single sperm-like cells or whose egg cells are eliminated; we found that although the three different types of sperm-like cell are functionally equivalent in their ability to fertilize the egg and the central cell, each type of sperm-like cell fuses predominantly with the egg cell. This indicates that it is the egg cell that controls its preferential fertilization. We also found that sperm-activating small secreted EGG CELL 1 proteins are involved in the regulation of egg-cell-dependent preferential fertilization, revealing another important role for this protein family during double fertilization.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data are available in the main text, the extended data or the supplementary information. Source data are provided with this paper.
References
Berger, F. & Twell, D. Germline specification and function in plants. Annu. Rev. Plant Biol. 62, 461–484 (2011).
Hafidh, S. & Honys, D. Reproduction multitasking: the male gametophyte. Annu. Rev. Plant Biol. 72, 581–614 (2021).
Dresselhaus, T., Sprunck, S. & Wessel, G. M. Fertilization mechanisms in flowering plants. Curr. Biol. 26, 125–139 (2016).
Sprunck, S. Twice the fun, double the trouble: gamete interactions in flowering plants. Curr. Opin. Plant Biol. 53, 106–116 (2020).
Roman, H. Directed fertilization in maize. Proc. Natl Acad. Sci. USA 34, 36–42 (1948).
Russell, S. D. Preferential fertilization in Plumbago: ultrastructural evidence for gamete-level recognition in an angiosperm. Proc. Natl Acad. Sci. USA 82, 6129–6132 (1985).
Chen, G., Hu, Z., Guo, F. & Guan, X. Male gametophyte development in Plumbago zeylanica: cytoplasm localization and cell determination in the early generative cell. Protoplasma 186, 79–86 (1995).
Saito, C. et al. Angiosperm species that produce sperm cell pairs or generative cells with polarized distribution of DNA-containing organelles. Sex. Plant Reprod. 15, 167–178 (2002).
Pagnussat, G. C., Yu, H. J. & Sundaresan, V. Cell-fate switch of synergid to egg cell in Arabidopsis eostre mutant embryo sacs arises from misexpression of the BEL1-like homeodomain gene BLH1. Plant Cell 19, 3578–3592 (2007).
Ingouff, M. et al. The two male gametes share equal ability to fertilize the egg cell in Arabidopsis thaliana. Curr. Biol. 19, 19–20 (2009).
Kong, J., Lau, S. & Jurgens, G. Twin plants from supernumerary egg cells in Arabidopsis. Curr. Biol. 25, 225–230 (2015).
Hamamura, Y. et al. Live-cell imaging reveals the dynamics of two sperm cells during double fertilization in Arabidopsis thaliana. Curr. Biol. 21, 497–502 (2011).
Hamamura, Y., Nagahara, S. & Higashiyama, T. Double fertilization on the move. Curr. Opin. Plant Biol. 15, 70–77 (2012).
Yang, W. C., Shi, D. Q. & Chen, Y. H. Female gametophyte development in flowering plants. Annu. Rev. Plant Biol. 61, 89–108 (2010).
Skinner, D. J. & Sundaresan, V. Recent advances in understanding female gametophyte development. F1000Res. 7, 804 (2018).
Hater, F., Nakel, T. & Gross-Hardt, R. Reproductive multitasking: the female gametophyte. Annu. Rev. Plant Biol. 71, 517–546 (2020).
Sharma, V., Clark, A. J. & Kawashima, T. Insights into the molecular evolution of fertilization mechanism in land plants. Plant Reprod. 34, 353–364 (2021).
Friedman, W. E. Developmental and evolutionary hypotheses for the origin of double fertilization and endosperm. C. R. Acad. Sci. III 324, 559–567 (2001).
Scott, R. J., Armstrong, S. J., Doughty, J. & Spielman, M. Double fertilization in Arabidopsis thaliana involves a polyspermy block on the egg but not the central cell. Mol. Plant 1, 611–619 (2008).
Grossniklaus, U. Polyspermy produces tri-parental seeds in maize. Curr. Biol. 27, 1300–1302 (2017).
Nagahara, S., Takeuchi, H. & Higashiyama, T. Polyspermy block in the central cell during double fertilization of Arabidopsis thaliana. Front. Plant Sci. 11, 588700 (2021).
Ngo, Q. A., Moore, J. M., Baskar, R., Grossniklaus, U. & Sundaresan, V. Arabidopsis GLAUCE promotes fertilization-independent endosperm development and expression of paternally inherited alleles. Development 134, 4107–4117 (2007).
Leshem, Y. et al. Molecular characterization of the glauce mutant: a central cell-specific function is required for double fertilization in Arabidopsis. Plant Cell 24, 3264–3277 (2012).
Zhao, P. et al. Two-step maternal-to-zygotic transition with two-phase parental genome contributions. Dev. Cell 49, 882–893 (2019).
Song, Q., Ando, A., Jiang, N., Ikeda, Y. & Chen, Z. J. Single-cell RNA-seq analysis reveals ploidy-dependent and cell-specific transcriptome changes in Arabidopsis female gametophytes. Genome Biol. 21, 178 (2020).
Susaki, D. et al. Dynamics of the cell fate specifications during female gametophyte development in Arabidopsis. PLoS Biol. 19, e3001123 (2021).
Sprunck, S. et al. Egg cell-secreted EC1 triggers sperm cell activation during double fertilization. Science 338, 1093–1097 (2012).
Cyprys, P., Lindemeier, M. & Sprunck, S. Gamete fusion is facilitated by two sperm cell-expressed DUF679 membrane proteins. Nat. Plants 5, 253–257 (2019).
Wang, W. et al. DMP8 and 9 regulate HAP2/GCS1 trafficking for the timely acquisition of sperm fusion competence. Proc. Natl Acad. Sci. USA 119, e2207608119 (2022).
Yu, X. et al. Fertilized egg cells secrete endopeptidases to avoid polytubey. Nature 592, 433–437 (2021).
Hamamura, Y. et al. Live imaging of calcium spikes during double fertilization in Arabidopsis. Nat. Commun. 5, 4722 (2014).
Denninger, P. et al. Male–female communication triggers calcium signatures during fertilization in Arabidopsis. Nat. Commun. 5, 4645 (2014).
Frank, A. C. & Johnson, M. A. Expressing the diphtheria toxin A subunit from the HAP2(GCS1) promoter blocks sperm maturation and produces single sperm-like cells capable of fertilization. Plant Physiol. 151, 1390–1400 (2009).
Chen, Z., Tan, J. L., Ingouff, M., Sundaresan, V. & Berger, F. Chromatin assembly factor 1 regulates the cell cycle but not cell fate during male gametogenesis in Arabidopsis thaliana. Development 135, 65–73 (2008).
Iwakawa, H., Shinmyo, A. & Sekine, M. Arabidopsis CDKA;1, a cdc2 homologue, controls proliferation of generative cells in male gametogenesis. Plant J. 45, 819–831 (2006).
Nowack, M. K. et al. A positive signal from the fertilization of the egg cell sets off endosperm proliferation in angiosperm embryogenesis. Nat. Genet. 38, 63–67 (2006).
Kim, H. J. et al. Control of plant germline proliferation by SCF(FBL17) degradation of cell cycle inhibitors. Nature 455, 1134–1137 (2008).
Aw, S. J., Hamamura, Y., Chen, Z., Schnittger, A. & Berger, F. Sperm entry is sufficient to trigger division of the central cell but the paternal genome is required for endosperm development in Arabidopsis. Development 137, 2683–2690 (2010).
Li, L. et al. The egg cell is preferentially fertilized in Arabidopsis double fertilization. J. Integr. Plant Biol. 64, 2039–2046 (2022).
Kim, S. Y. et al. Exploring membrane-associated NAC transcription factors in Arabidopsis: implications for membrane biology in genome regulation. Nucleic Acids Res. 35, 203–213 (2007).
Morishita, T. et al. Arabidopsis NAC transcription factor, ANAC078, regulates flavonoid biosynthesis under high-light. Plant Cell Physiol. 50, 2210–2222 (2009).
Gladman, N. P., Marshall, R. S., Lee, K.-H. & Vierstra, R. D. The proteasome stress regulon is controlled by a pair of NAC transcription factors in Arabidopsis. Plant Cell 28, 1279–1296 (2016).
Kim, S. G. et al. Probing protein structural requirements for activation of membrane-bound NAC transcription factors in Arabidopsis and rice. Plant Sci. 178, 239–244 (2010).
Takahashi, T. et al. The male gamete membrane protein DMP9/DAU2 is required for double fertilization in flowering plants. Development 145, dev170076 (2018).
Ingouff, M., Hamamura, Y., GourgueS, M., Higashiyama, T. & Berger, F. Distinct dynamics of HISTONE3 variants between the two fertilization products in plants. Curr. Biol. 17, 1032–1037 (2007).
Rotman, N. et al. A novel class of MYB factors controls sperm-cell formation in plants. Curr. Biol. 15, 244–248 (2005).
Brownfield, L. et al. A plant germline-specific integrator of sperm specification and cell cycle progression. PLoS Genet. 5, e1000430 (2009).
Borg, M. et al. The R2R3 MYB transcription factor DUO1 activates a male germline-specific regulon essential for sperm cell differentiation in Arabidopsis. Plant Cell 23, 534–549 (2011).
Borg, M. et al. An EAR-dependent regulatory module promotes male germ cell division and sperm fertility in Arabidopsis. Plant Cell 26, 2098–2113 (2014).
Mori, T., Igawa, T., Tamiya, G., Miyagishima, S. Y. & Berger, F. Gamete attachment requires GEX2 for successful fertilization in Arabidopsis. Curr. Biol. 24, 170–175 (2014).
Mori, T., Kuroiwa, H., Higashiyama, T. & Kuroiwa, T. GENERATIVE CELL SPECIFIC 1 is essential for angiosperm fertilization. Nat. Cell Biol. 8, 64–71 (2006).
von Besser, K., Frank, A. C., Johnson, M. A. & Preuss, D. Arabidopsis HAP2 (GCS1) is a sperm-specific gene required for pollen tube guidance and fertilization. Development 133, 4761–4769 (2006).
Steffen, J. G., Kang, I. H., Macfarlane, J. & Drews, G. N. Identification of genes expressed in the Arabidopsis female gametophyte. Plant J. 51, 281–292 (2007).
Xiong, H. X., Wang, W. & Sun, M. X. Endosperm development is an autonomously programmed process independent of embryogenesis. Plant Cell 33, 1151–1160 (2021).
Beale, K. M., Leydon, A. R. & Johnson, M. A. Gamete fusion is required to block multiple pollen tubes from entering an Arabidopsis ovule. Curr. Biol. 22, 1090–1094 (2012).
Kasahara, R. D. et al. Fertilization recovery after defective sperm cell release in Arabidopsis. Curr. Biol. 22, 1084–1089 (2012).
Maruyama, D. et al. Independent control by each female gamete prevents the attraction of multiple pollen tubes. Dev. Cell 25, 317–323 (2013).
Pan, T. et al. A novel WEE1 pathway for replication stress responses. Nat. Plants 7, 209–218 (2021).
Zhang, X. et al. A female in vivo haploid-induction system via mutagenesis of egg cell-specific peptidases. Mol. Plant 16, 471–480 (2023).
Mao, Y. B. et al. ECS1 and ECS2 suppress polyspermy and the formation of haploid plants by promoting double fertilization. eLife 12, e85832 (2023).
Spielman, M. & Scott, R. J. Polyspermy barriers in plants: from preventing to promoting fertilization. Sex. Plant Reprod. 21, 53–65 (2008).
Tekleyohans, D. G., Mao, Y. B., Kagi, C., Stierhof, Y. D. & Gross-Hardt, R. Polyspermy barriers: a plant perspective. Curr. Opin. Plant Biol. 35, 131–137 (2017).
Tian, H. Q., Zhang, Z. J. & Russell, S. D. Sperm dimorphism in Nicotiana tabacum L. Sex. Plant Reprod. 14, 123–125 (2001).
Yang, Y. H. et al. Isolation of two populations of sperm cells and microelectrophoresis of pairs of sperm cells from pollen tubes of tobacco (Nicotiana tabacum). Sex. Plant Reprod. 18, 47–53 (2005).
Shiba, Y. et al. Behavior of male gamete fusogen GCS1/HAP2 and the regulation in Arabidopsis double fertilization. Biomolecules 13, 208 (2023).
Huang, J. L., Ju, Y., Wang, X. F., Zhang, Q. & Sodmergen A one-step rectification of sperm cell targeting ensures the success of double fertilization. J. Integr. Plant Biol. 57, 496–503 (2015).
Baroux, C., Spillane, C. & Grossniklaus, U. Evolutionary origins of the endosperm in flowering plants. Genome Biol. 3, reviews1026.1 (2002).
Baroux, C. & Grossniklaus, U. Seeds—an evolutionary innovation underlying reproductive success in flowering plants. Curr. Top. Dev. Biol. 131, 605–642 (2019).
Jiang, J. H. et al. Egg cell-secreted aspartic proteases ECS1/2 promote gamete attachment to prioritize the fertilization of egg cells over central cells in Arabidopsis. J. Integr. Plant Biol. 64, 2047–2059 (2022).
Zhang, X. C., Yu, X. B., Shi, C., Dresselhaus, T. & Sun, M. X. Do egg cell-secreted aspartic proteases promote gamete attachment? J. Integr. Plant Biol. 65, 3–6 (2023).
Rademacher, S. & Sprunck, S. Downregulation of egg cell-secreted EC1 is accompanied with delayed gamete fusion and polytubey. Plant Signal. Behav. 8, e27377 (2013).
Sprunck, S., Hackenberg, T., Englhart, M. & Vogler, F. Same but different: sperm-activating EC1 and ECA1 gametogenesis-related family proteins. Biochem. Soc. Trans. 42, 401–407 (2014).
Flores-Tornero, M. et al. Transcriptomics of manually isolated Amborella trichopoda egg apparatus cells. Plant Reprod. 32, 15–27 (2019).
Chen, J. et al. Zygotic genome activation occurs shortly after fertilization in maize. Plant Cell 29, 2106–2125 (2017).
Yan, H. et al. Ribosomal protein L18aB is required for both male gametophyte function and embryo development in Arabidopsis. Sci. Rep. 6, 31195 (2016).
Alonso, J. M. et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657 (2003).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Wu, J. J. et al. Mitochondrial GCD1 dysfunction reveals reciprocal cell-to-cell signaling during the maturation of Arabidopsis female gametes. Dev. Cell 23, 1043–1058 (2012).
Karimi, M., Inze, D. & Depicker, A. GATEWAYTM vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci. 7, 193–195 (2002).
Bensmihen, S. et al. Analysis of an activated ABI5 allele using a new selection method for transgenic Arabidopsis seeds. FEBS Lett. 561, 127–131 (2004).
Wang, Z. P. et al. Egg cell-specific promoter-controlled CRISPR/Cas9 efficiently generates homozygous mutants for multiple target genes in Arabidopsis in a single generation. Genome Biol. 16, 144 (2015).
Oliveros, J. C. et al. Breaking-Cas—interactive design of guide RNAs for CRISPR–Cas experiments for ENSEMBL genomes. Nucleic Acids Res. 44, 267–271 (2016).
Liu, W. Z. et al. DSDecode: a web-based tool for decoding of sequencing chromatograms for genotyping of targeted mutations. Mol. Plant 8, 1431–1433 (2015).
Waterhouse, A. M., Procter, J. B., Martin, D. M. A., Clamp, M. & Barton, G. J. Jalview Version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 (2009).
Acknowledgements
We thank F. Berger (Gregor Mendel Institute of Molecular Plant Biology, Vienna, Austria) and Y. Zhang (University of Shandong Agricultural University) for providing the marker lines and S. P. Yan (Huazhong Agricultural University) for providing the fbl17-3/+ seeds. We thank I. Fuchs for her help in identifying the 4xec1 mutant. This work was supported by National Natural Science Foundation of China grants (no. 32130031 to M.-X.S., no. 32200702 to H.X. and no. 31800264 to W.W.) and by the German Research Foundation to S.S. (Collaborative Research Center SFB924, TP A04; ICIPS research unit FOR 5098, P5).
Author information
Authors and Affiliations
Contributions
W.W., H.X., S.S. and M.-X.S. designed the experiments. W.W., H.X., R.M., P.C., M.L. and S.T. performed the experiments. W.W., H.X., R.M., M.L., P.C., K.S., X.Z., S.T., S.S. and M.-X.S. analysed the data. W.W., H.X., S.S. and M.-X.S. wrote the manuscript. All the authors have approved this submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Hong-Ju Li and Tomoko Igawa for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Promoter activity analysis using the nuclear marker H2B-GFP expressed by the NTL11 promoter.
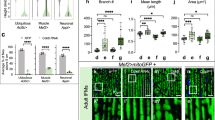
a-d, NTL11 promoter-driven H2B-GFP reporter fluorescence in nuclei of roots (a), root hairs (b), leaves (c), and trichomes (d), respectively. e, NTL11 promoter activity analysis during male gametophyte development. The H2B-GFP signals were visible in the microspore nucleus (MN), and in the generative cell nucleus (GN) and the vegetative cell nucleus (VN) of bicellular pollen (BC). In tricellular pollen (TC), the reporter signal became weak in the VN. In contrast, the signal remained persistently detectable in the sperm nuclei (SN) of tricellular pollen and germinated pollen tubes. f, Quantification of the GFP signal intensity shown in e from the microspore stage to the tricellular pollen stage (n = 22, 32, 32, 30 and 30, from left to right). Identical settings were used for microscopy, fluorophore excitation and detection. The central lines in the box plots represent the median, the box represents the interquartile range (IQR) and the whiskers extend to minima and maxima. Significant differences by two-sided Student’s t-test are indicated. g, NTL11 promoter-driven H2B–GFP reporter fluorescence in unfertilized and fertilized ovules. The H2B-GFP signals were visible in the egg cell nucleus (ECN), the central cell nucleus (CCN) and synergid cell nucleus (SCN) in mature ovules and could be detected in the zygote nucleus (ZYN) and endosperm nucleus (ESN) after fertilization. Scale bars, 25 µm in a, 10 µm in b and g, 20 µm in c, 60 µm in d and 4 µm in e.
Extended Data Fig. 2 Sperm cell development and fertility is not affected in the ntl4 ntl11 double mutant.
a, NTL4 and NTL11 gene structure showing the loci of T-DNA insertion in ntl4 (nac53-1) and ntl11 (nac78-1). b, DAPI staining of wild type (n = 466) and ntl4 ntl11 (n = 396) pollen. The mean percentage of pollen containing two sperm nuclei (SN) is shown (with SD in brackets). VN, vegetative cell nucleus. c, The ntl4 ntl11 mutant has a seed set comparable to that of the wild type (Col-0). Mean quantities of seeds formed (in %) with SD in brackets are indicated at the upper right. n = 9 siliques (597 ovules) for Col-0 and 10 siliques (664 ovules) for ntl4 ntl11. Scale bars, 10 µm in b and 1 mm in c.
Extended Data Fig. 3 Impact of 11ΔC on sperm cells, pollen tube guidance and seed development.
a, Representative images of sperm cells in growing pollen tubes. Pollen from 11ΔC plants expressing the sperm nuclear marker HTR10-mRFP were germinated in vitro, revealing either two sperm nuclei (2SN) or one sperm nucleus (1SN). b, Percentage of pollen tubes with a single SLC, analyzed at three time points. The number of pollen tubes evaluated is noted in the respective column. c, Pollen tube guidance in wild type ovules, pollinated with pLAT52::GFP and 11ΔC/pLAT52::GFP pollen. Arrows point at released pollen tube contents. d, Percentage of ovules attracting pollen tubes at 20 hours after pollination (5 siliques for Col-0, 6 siliques for 11ΔC-4 and 6 siliques 11ΔC-5). The number of evaluated ovules is noted in the respective column. e, Representative images of ovules co-expressing pDD45::GFP (egg cell/zygote marker) and pDD22::CFP (central cell/endosperm marker), 48 hours after pollination with HTR10-mRFP and 11ΔC/HTR10-mRFP pollen. Seeds fertilized by HTR10-mRFP pollen show successful double fertilization with a developing embryo (Emb) and dividing endosperm nuclei (indicated by asterisks). When 11ΔC/HTR10-mRFP pollen was used, three categories of seeds were observed: seeds derived from double fertilization; seeds with egg cell-single fertilization, indicated by a developing embryo and a central cell nucleus (CCN); seeds with a long, atypical zygote (Zy) and a central cell nucleus but no division, also no unfused sperm cells. The mean percentage of each category of seed (with SD in brackets) is indicated at the upper right of each image (2.6% unfertilized ovules for Col-0, 5.8% unfertilized ovules and 0.2% central cell-single fertilized seeds for 11ΔC are not shown; the number of siliques/ovules was 5/305 and 9/417 for each). In b and d, data represent the mean ± SD and significant differences by two-sided Student’s t-test are indicated. Scale bars are 5 µm in a, 15 µm in c and 20 µm in e.
Extended Data Fig. 4 Phenotypic analysis of fbl17-3/+ mutants and KRP1 overexpressing lines in male and seed development.
a, In contrast to two sperm cell nuclei in wild type (Col-0) pollen, some fbl17-3/+ and pDMP9::KRP1 pollen grains contain a single SLC nucleus. Nuclei of sperm cells and SLCs are labeled by HTR10-mRFP. b, Statistical analysis of pollen containing a single SLC for Col-0 (n = 275), fbl17-3/+ (n = 361) and different pDMP9::KRP1 lines (n = 446 for K1-2; n = 688 for K1-3; n = 723 for K1-4; n = 758 for K1-5). c, Representative images of sperm cells in growing pollen tubes. Pollen from wild type and fbl17-3/+ mutant plants were germinated in vitro. The sperm cells were marked by HTR10-mRFP. d, Percentage of growing pollen tubes with a single SLC, analyzed at three time points. The total number of pollen tubes is noted above or in the column. e, Relative expression levels of KRP1 in different overexpressing (pDMP9::KRP1) lines from three independent replicates. The expression level of KPR1 in the K1-3 line was set to 1. f, Seed morphology in self-crossed siliques in wild type, fbl17-3/+ mutant plants and KRP1 overexpressing lines (K1-4 and -5). Arrowheads point at aborted seeds and arrows indicate undeveloped seeds. g, Quantification of developed seeds, aborted seeds and undeveloped seeds shown in f. The number of siliques (n) was 10 for Col-0 (599 ovules), 17 for fbl17-3/+ (956 ovules), 10 for K1-3 (607 ovules), 8 for K1-2 (465 ovules), 9 for K1-4 (507 ovules) and 13 for K1-5 (701 ovules). In b, d, e and g, data are the means ± SD and significant differences by two-sided Student’s t-test are indicated. Scale bars, 10 µm in a, 5 µm in c, 0.5 mm in f.
Extended Data Fig. 5 fbl17-3 and K1 SLCs have sperm cell identity.
a, Relative expression levels of representative genes related to gamete fusion and male germline development (HAP2/GCS1, GEX2, DMP9, DUO1, DAZ1 and DAZ2) in opening flowers of wild type (Col-0) and pDMP9::KRP1 transgenic lines, investigated by real-time PCR from three biological replicates. The expression level in wild type was set to 1. b, Relative expression levels of FBL17 and representative genes related to male germline development in wild type (Col-0) and fbl17-3 opening flowers, from three independent replicates. The expression level in wild type was set to 1. c, Confocal images of HAP2/GCS1-RFP, GEX2-RFP and DMP9-GFP in wild type (Col-0) and fbl17-3 pollen. Identical settings were used for microscopy, fluorophore excitation and detection. Scale bars, 5 µm. d, Normalized signal intensities of HAP2/GCS1-RFP, GEX2-RFP and DMP9-GFP in wild type and fbl17-3 SLCs. From left to right, n = 90, 100, 167, 164, 130 and 125 pollen for each. In a and b, data are the means ± SD; in d, the central lines in the box plots represent the median, the box represents the interquartile range (IQR) and the whiskers extend to minima and maxima. Significant differences by two-sided Student’s t-test are indicated.
Extended Data Fig. 6 Over-expression of DAZ1 and DAZ2 in sperm cells does not affect gamete fusion.
a-b, qPCR analyses of DAZ1 (a) and DAZ2 (b) in opening flowers of plants expressing pDMP9::DAZ1 or pDAF1::DAZ2. The expression level of DAZ1 and DAZ2 in the wild type (Col-0) was set to 1. Data are the means ± SD from three independent experiments. c-d, Quantitative analyses of pollen containing two sperm cells in different pDMP9::DAZ1 and pDAF1::DAZ2 lines. The number of sperm cells marked by HTR10-RFP was counted in mature pollen grains (from left to right, n = 502, 509, 445, 456, 493, 535 and 439, respectively). e, Representative images of gamete fusion events in WT ovules 8-10 hours after pollination with WT, pDMP9::DAZ1 or pDAF1::DAZ2 pollen. The decondensed sperm chromatin (arrowheads) was taken as a sign of gamete fusion. Arrows indicate pollen tube contents (labeled by pLAT52::GFP). f, Quantitative analyses of double or single fertilization 8-10 HAP in WT ovules, when pollinated with WT, pDMP9::DAZ1 or pDAF1::DAZ2 pollen. From left to right, n = 166, 143, 146, 167, 190, 173 and 156 ovules. g, Seed morphology in self-crossed siliques in wild type, pDMP9::DAZ1 and pDAF1::DAZ2 lines. h, Quantification of developed seeds shown in g (n = 10 siliques for each). From left to right, the total number of seeds is 584, 568, 548, 590, 559, 677 and 640, respectively. In a, b, d, f and h, data are the means ± SD and significant differences by two-sided Student’s t-test are indicated. Scale bars, 10 µm in c, e and 0.5 mm in g. Abbreviations: CC, central cell; EC, egg cell.
Extended Data Fig. 7 fbl17 and K1 SLCs also preferentially fertilize the egg cell.
a, Ovules co-expressing pDD45::GFP and pDD22::CFP, 30 hours after pollination (HAP) with fbl17-3/+ or pDMP9::KRP1 pollen. (i), Enlarged ovule containing endosperm nuclei (asterisks) and a zygote (Zy). (ii) Ovule with single egg cell fertilization. (iii) Ovule with single central cell fertilization. (iv) Ovule with a zygote and delayed endosperm (EN) development. EC, egg cell; CCN, central cell nucleus. b, Quantification of ovule phenotypes in a, showing that single fertilization mainly relates to the egg cell. The number of siliques (n) was 6 for Col-0 (308 ovules), 11 for fbl17-3/+ (576 ovules), 10 for K1-4 (499 ovules) and 10 for K1-5 (532 ovules). EM, embryo. c, Representative images of wild type ovules, 24 HAP with fbl17-3/+ pollen expressing pRPL18aB::H2B-GFP. Three types of ovules were observed: double fertilized seeds with H2B-GFP signals in the nucleus of the zygote (ZYN) and endosperm nuclei (ESN); ovules with fertilized zygote but unfertilized central cell nucleus (CCN); ovules with H2B-GFP in divided ESN but no fluorescence in the unfertilized egg cell; ovules with fertilized zygote surrounded by ESN lacking H2B-GFP, indicating autonomous endosperm (AE) formation. Here the ovules without GFP signals were not counted. Please note that a pair of sperm cells was visible in central cell-single fertilized ovules, indicating secondary pollen tube entry. SN, sperm nucleus. d, Quantification of different types of fertilization in c. The number of siliques/ovules was 12/520. e, Quantitative assessment of gamete fusion events in wild type and ECcollapsed ovules, 8-10 HAP using fbl17-3/ + , K1-4 and K1-5 pollen, respectively. Note that in wild type ovules, single fertilization by SLCs mainly relates to the egg cell, whereas in ECcollapsed ovules it mainly relates to the central cell. From left to right, the number of siliques/ovules was 16/819, 12/645, 10/531, 10/563, 12/603 and 11/679. In b and d, data are the means ± SD; in e, the central lines in floating bars represent the mean, the bound of box extend to minima and maxima. Significant differences by two-sided Student’s t-test are indicated. Scale bars=20 µm.
Extended Data Fig. 8 Generation of ec1 quintuple mutants.
a, T-DNA insertion lines used to create the homozygous ec1 quadruple knockout (4xec1). T-DNA positions in the single exons of EC1.1, EC1.3, EC1.4 and EC1.5 and position of primer pairs used for RT-PCRs (blue) shown in Fig. 5b are indicated. b, Scheme of the EC1.2 gene, with regions coding for the N-terminal secretion signal and the two EC1 signature motifs S1 and S227. Three different single guide RNAs (sgRNAs) were used for CRISPR/Cas9 gene editing of EC1.2 in the 4xec1 background. Gene editing events in three independent lines homozygous for ec1.2 are shown below. The homozygous and Cas9-free 5xec1 line CR1-B-1 has a 14 bp deletion in EC1.2, in the region of the cleavage site of the secretion signal. This results in a frameshift mutation and a premature stop codon. Amino acids of the secretion signal (CR1-B-1) are shown in grey. Position of primer pair (blue) used for RT-PCR (Fig. 5b) is indicated. Homozygous and Cas9-free 5xec1 lines CR3-B-76 and CR5-G-61 have a deletion of 2 bp and an insertion of 1 bp, respectively. This results in a frameshift mutation in the region coding for motif S1 and S2, respectively, and in premature stop codons. c, Reduced seed set in T3 progenies of the homozygous and Cas9-free 5xec1 lines CR1-B-1, CR3-B-76, and CR5-G-61. Mean number of seeds per silique were counted for twelve individual plants per line. Data are the means ± SD. Means that do not share a letter differ significantly (P < 0.0001) in multiple comparisons using one-way ANOVA and Tukey’s range test. d, Average seed set (%) and total number of analyzed siliques shown in (c) for Col-0 (596 seeds), CR1-B-1 (981 seeds), CR3-B-76 (928 seeds), and CR5-G-61 (1035 seeds).
Extended Data Fig. 9 Seed set in 5xec1 expressing EC1.1-GFP and detection of EC1.1-GFP fusion protein when secreted by the central cell.
a, Seed morphology in self-crossed siliques in wild type (Col-0), 5xec1 and 5xec1 lines expressing pEC1.1::EC1.1-GFP, pDD22::EC1.1-GFP and pDD36::EC1.1-GFP, indicating that EC1.1-GFP cannot complement the 5xec1 mutant. Arrowheads indicate aborted seeds and arrows indicate undeveloped seeds. Scale bars, 0.5 mm. b, Quantification of seed set for Col-0, 5xec1 and different transgenic lines expressing pEC1.1::EC1.1-GFP, pDD22::EC1.1-GFP and pDD36::EC1.1-GFP in the 5xec1 mutant, proving that EC1.1-GFP is not functional. Seed set from T1 lines (heterozygous for the complementation constructs) are shown. From left to right, the number of siliques/seeds was 10/581, 13/654, 10/453, 10/440, 10/411, 10/441, 12/568, 11/529, 11/529, 10/477, 10/494, 10/484, and 10/462. Data are the means ± SD and significant differences compared with 5xec1 (two-sided Student’s t-test) are indicated. c, Ovules from pDD22::EC1.1-GFP and pDD36::EC1.1-GFP transgenic lines 24 hour after emasculation. Arrowheads point at fluorescent signals, indicating that EC1.1-GFP is secreted by the central cell before fertilization. Scale bars, 20 µm. Abbreviations: CC, central cell; EC, egg cell; SC, synergid cell.
Supplementary information
Supplementary Table 1
Primers used in this study.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Unprocessed gels for Fig. 5b and statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, W., Malka, R., Lindemeier, M. et al. EGG CELL 1 contributes to egg-cell-dependent preferential fertilization in Arabidopsis. Nat. Plants 10, 268–282 (2024). https://doi.org/10.1038/s41477-023-01616-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-023-01616-5