Abstract
Above-optimal growth temperatures, usually referred to as heat stress (HS), pose a challenge to organisms’ survival as they interfere with essential physiological functions and disrupt cellular organization. Previous studies have elucidated the complex transcriptional regulatory networks involved in plant HS responses, but the mechanisms of organellar remodelling and homeostasis during plant HS adaptations remain elusive. Here we report a non-canonical function of ATG8 in regulating the restoration of plant Golgi damaged by HS. Short-term acute HS causes vacuolation of the Golgi apparatus and translocation of ATG8 to the dilated Golgi membrane. The inactivation of the ATG conjugation system, but not of the upstream autophagic initiators, abolishes the targeting of ATG8 to the swollen Golgi, causing a delay in Golgi recovery after HS. Using TurboID-based proximity labelling, we identified CLATHRIN LIGHT CHAIN 2 (CLC2) as an interacting partner of ATG8 via the AIM–LDS interface. CLC2 is recruited to the cisternal membrane by ATG8 to facilitate Golgi reassembly. Collectively, our study reveals a hitherto unanticipated process of Golgi stack recovery from HS in plant cells and uncovers a previously unknown mechanism of organelle resilience involving ATG8.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the results in this study are available in the Article and its Supplementary Information files. The raw mass spectrometry data were searched against the TAIR10 database (https://www.arabidopsis.org/). The proteomics data have been deposited at the ProteomeXchange Consortium (http://proteomecentral.proteomexchange.org) via the iProX partner repository83 (identifier, PXD039608). Source data are provided with this paper.
References
Zandalinas, S. I., Fritschi, F. B. & Mittler, R. Global warming, climate change, and environmental pollution: recipe for a multifactorial stress combination disaster. Trends Plant Sci. 26, 588–599 (2021).
Hasanuzzaman, M., Nahar, K., Alam, M. M., Roychowdhury, R. & Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 14, 9643–9684 (2013).
Kotak, S. et al. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 10, 310–316 (2007).
Jung, H. et al. Arabidopsis cargo receptor NBR1 mediates selective autophagy of defective proteins. J. Exp. Bot. 71, 73–89 (2020).
Thirumalaikumar, V. P. et al. Selective autophagy regulates heat stress memory in Arabidopsis by NBR1-mediated targeting of HSP90.1 and ROF1. Autophagy 17, 2184–2199 (2021).
Zhou, J. et al. NBR1-mediated selective autophagy targets insoluble ubiquitinated protein aggregates in plant stress responses. PLoS Genet. 9, e1003196 (2013).
Sedaghatmehr, M. et al. A regulatory role of autophagy for resetting the memory of heat stress in plants. Plant Cell Environ. 42, 1054–1064 (2019).
Takeshige, K., Baba, M., Tsuboi, S., Noda, T. & Ohsumi, Y. Autophagy in yeast demonstrated with proteinase-deficient mutants and conditions for its induction. J. Cell Biol. 119, 301–311 (1992).
Nakatogawa, H., Suzuki, K., Kamada, Y. & Ohsumi, Y. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat. Rev. Mol. Cell Biol. 10, 458–467 (2009).
Li, H. et al. Shedding light on the role of phosphorylation in plant autophagy. FEBS Lett. 596, 2172–2185 (2022).
Fischer, T. D., Wang, C., Padman, B. S., Lazarou, M. & Youle, R. J. STING induces LC3B lipidation onto single-membrane vesicles via the V-ATPase and ATG16L1-WD40 domain. J. Cell Biol. 219, e202009128 (2020).
Fletcher, K. et al. The WD40 domain of ATG16L1 is required for its non-canonical role in lipidation of LC3 at single membranes. EMBO J. 37, e97840 (2018).
Gao, Y. et al. Golgi-associated LC3 lipidation requires V-ATPase in noncanonical autophagy. Cell Death Dis. 7, e2330 (2016).
Hooper, K. M. et al. V-ATPase is a universal regulator of LC3-associated phagocytosis and non-canonical autophagy. J. Cell Biol. 221, e202105112 (2022).
Xu, Y. et al. A bacterial effector reveals the V-ATPase-ATG16L1 axis that initiates xenophagy. Cell 178, 552–566 (2019).
Nieto-Torres, J. L., Leidal, A. M., Debnath, J. & Hansen, M. Beyond autophagy: the expanding roles of ATG8 proteins. Trends Biochem. Sci. 46, 673–686 (2021).
Martinez, J. et al. Microtubule-associated protein 1 light chain 3 alpha (LC3)-associated phagocytosis is required for the efficient clearance of dead cells. Proc. Natl Acad. Sci. USA 108, 17396–17401 (2011).
Heckmann, B. L. et al. LC3-associated endocytosis facilitates beta-amyloid clearance and mitigates neurodegeneration in murine Alzheimer’s disease. Cell 178, 536–551.e14 (2019).
Jia, M. et al. Noncanonical ATG8–ABS3 interaction controls senescence in plants. Nat. Plants 5, 212–224 (2019).
Cerrato, G. et al. Oleate-induced aggregation of LC3 at the trans-Golgi network is linked to a protein trafficking blockade. Cell Death Differ. 28, 1733–1752 (2021).
Gomes-da-Silva, L. C. et al. Recruitment of LC3 to damaged Golgi apparatus. Cell Death Differ. 26, 1467–1484 (2019).
Niso-Santano, M. et al. Unsaturated fatty acids induce non-canonical autophagy. EMBO J. 34, 1025–1041 (2015).
Huang, S. & Wang, Y. Golgi structure formation, function, and post-translational modifications in mammalian cells. F1000Res 6, 2050 (2017).
Colanzi, A. & Corda, D. Mitosis controls the Golgi and the Golgi controls mitosis. Curr. Opin. Cell Biol. 19, 386–393 (2007).
Tang, D. & Wang, Y. Cell cycle regulation of Golgi membrane dynamics. Trends Cell Biol. 23, 296–304 (2013).
Radulescu, A. E., Siddhanta, A. & Shields, D. A role for clathrin in reassembly of the Golgi apparatus. Mol. Biol. Cell 18, 94–105 (2007).
Petrosyan, A. & Cheng, P. W. Golgi fragmentation induced by heat shock or inhibition of heat shock proteins is mediated by non-muscle myosin IIA via its interaction with glycosyltransferases. Cell Stress Chaperones 19, 241–254 (2014).
Robinson, D. G., Albrecht, S. & Moriysu, Y. The V-ATPase inhibitors concanamycin A and bafilomycin A lead to Golgi swelling in tobacco BY-2 cells. Protoplasma 224, 255–260 (2004).
Luo, Q. et al. Nondegradable ubiquitinated ATG9A organizes Golgi integrity and dynamics upon stresses. Cell Rep. 40, 111195 (2022).
Ayala, I., Mascanzoni, F. & Colanzi, A. The Golgi ribbon: mechanisms of maintenance and disassembly during the cell cycle. Biochem. Soc. Trans. 48, 245–256 (2020).
Lee, I. et al. Membrane adhesion dictates Golgi stacking and cisternal morphology. Proc. Natl Acad. Sci. USA 111, 1849–1854 (2014).
Meyer, H. H. Golgi reassembly after mitosis: the AAA family meets the ubiquitin family. Biochim. Biophys. Acta 1744, 108–119 (2005).
Xiang, Y. & Wang, Y. GRASP55 and GRASP65 play complementary and essential roles in Golgi cisternal stacking. J. Cell Biol. 188, 237–251 (2010).
Tachikawa, M. & Mochizuki, A. Golgi apparatus self-organizes into the characteristic shape via postmitotic reassembly dynamics. Proc. Natl Acad. Sci. USA 114, 5177–5182 (2017).
Latijnhouwers, M., Hawes, C. & Carvalho, C. Holding it all together? Candidate proteins for the plant Golgi matrix. Curr. Opin. Plant Biol. 8, 632–639 (2005).
Kang, B. H. et al. A glossary of plant cell structures: current insights and future questions. Plant Cell 34, 10–52 (2022).
Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy 17, 1–382 (2021).
Wallace, E. W. et al. Reversible, specific, active aggregates of endogenous proteins assemble upon heat stress. Cell 162, 1286–1298 (2015).
Yano, H. et al. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J. Biol. Chem. 268, 25846–25856 (1993).
Sou, Y. S., Tanida, I., Komatsu, M., Ueno, T. & Kominami, E. Phosphatidylserine in addition to phosphatidylethanolamine is an in vitro target of the mammalian Atg8 modifiers, LC3, GABARAP, and GATE-16. J. Biol. Chem. 281, 3017–3024 (2006).
Durgan, J. et al. Non-canonical autophagy drives alternative ATG8 conjugation to phosphatidylserine. Mol. Cell 81, 2031–2040 (2021).
Schumacher, K. & Krebs, M. The V-ATPase: small cargo, large effects. Curr. Opin. Plant Biol. 13, 724–730 (2010).
Wang, P., Chen, X., Goldbeck, C., Chung, E. & Kang, B. H. A distinct class of vesicles derived from the trans-Golgi mediates secretion of xylogalacturonan in the root border cell. Plant J. 92, 596–610 (2017).
Takano, J., Miwa, K., Yuan, L., von Wirén, N. & Fujiwara, T. Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc. Natl Acad. Sci. USA 102, 12276–12281 (2005).
Fujiwara, T., Oda, K., Yokota, S., Takatsuki, A. & Ikehara, Y. Brefeldin A causes disassembly of the Golgi complex and accumulation of secretory proteins in the endoplasmic reticulum. J. Biol. Chem. 263, 18545–18552 (1988).
Branon, T. C. et al. Efficient proximity labeling in living cells and organisms with TurboID. Nat. Biotechnol. 36, 880–887 (2018).
Protter, D. S. W. & Parker, R. Principles and properties of stress granules. Trends Cell Biol. 26, 668–679 (2016).
Yu, J. & Zhou, J. Vacuolar accumulation and colocalization is not a proper criterion for cytoplasmic soluble proteins undergoing selective autophagy. Plant Signal. Behav. 16, 1932319 (2021).
Ji, C. et al. AtNBR1 is a selective autophagic receptor for AtExo70E2 in Arabidopsis. Plant Physiol. 184, 777–791 (2020).
Tian, Y., Chang, J. C., Fan, E. Y., Flajolet, M. & Greengard, P. Adaptor complex AP2/PICALM, through interaction with LC3, targets Alzheimer’s APP-CTF for terminal degradation via autophagy. Proc. Natl Acad. Sci. USA 110, 17071–17076 (2013).
Wang, J., Cai, Y., Miao, Y., Lam, S. K. & Jiang, L. Wortmannin induces homotypic fusion of plant prevacuolar compartments. J. Exp. Bot. 60, 3075–3083 (2009).
Zheng, J., Han, S. W., Rodriguez-Welsh, M. F. & Rojas-Pierce, M. Homotypic vacuole fusion requires VTI11 and is regulated by phosphoinositides. Mol. Plant 7, 1026–1040 (2014).
Staehelin, L. A. & Kang, B. H. Nanoscale architecture of endoplasmic reticulum export sites and of Golgi membranes as determined by electron tomography. Plant Physiol. 147, 1454–1468 (2008).
Kang, B. H., Nielsen, E., Preuss, M. L., Mastronarde, D. & Staehelin, L. A. Electron tomography of RabA4b- and PI-4Kbeta1-labeled trans Golgi network compartments in Arabidopsis. Traffic 12, 313–329 (2011).
Dettmer, J., Hong-Hermesdorf, A., Stierhof, Y. D. & Schumacher, K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18, 715–730 (2006).
Uemura, T., Suda, Y., Ueda, T. & Nakano, A. Dynamic behavior of the trans-Golgi network in root tissues of Arabidopsis revealed by super-resolution live imaging. Plant Cell Physiol. 55, 694–703 (2014).
Goh, T. et al. Autophagy promotes organelle clearance and organized cell separation of living root cap cells in Arabidopsis thaliana. Development 149, dev200593 (2022).
Engel, B. D. et al. In situ structural analysis of Golgi intracisternal protein arrays. Proc. Natl Acad. Sci. USA 112, 11264–11269 (2015).
Sagiv, Y., Legesse-Miller, A., Porat, A. & Elazar, Z. GATE-16, a membrane transport modulator, interacts with NSF and the Golgi v-SNARE GOS-28. EMBO J. 19, 1494–1504 (2000).
Tang, D., Mar, K., Warren, G. & Wang, Y. Molecular mechanism of mitotic Golgi disassembly and reassembly revealed by a defined reconstitution assay. J. Biol. Chem. 283, 6085–6094 (2008).
Kaksonen, M. & Roux, A. Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 19, 313–326 (2018).
Radulescu, A. E. & Shields, D. Clathrin is required for postmitotic Golgi reassembly. FASEB J. 26, 129–136 (2012).
Wang, C. et al. Clathrin light chains regulate clathrin-mediated trafficking, auxin signaling, and development in Arabidopsis. Plant Cell 25, 499–516 (2013).
Huang, X. et al. Genetic analyses of the Arabidopsis ATG1 kinase complex reveal both kinase-dependent and independent autophagic routes during fixed-carbon starvation. Plant Cell 31, 2973–2995 (2019).
Zhuang, X. et al. A BAR-domain protein SH3P2, which binds to phosphatidylinositol 3-phosphate and ATG8, regulates autophagosome formation in Arabidopsis. Plant Cell 25, 4596–4615 (2013).
Geldner, N. et al. Rapid, combinatorial analysis of membrane compartments in intact plants with a multicolor marker set. Plant J. 59, 169–178 (2009).
Nelson, B. K., Cai, X. & Nebenfuhr, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 51, 1126–1136 (2007).
Clough, S. J. & Bent, A. F. Floral dip: a simplified method for Agrobacterium‐mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998).
Kang, B. H. Electron microscopy and high-pressure freezing of Arabidopsis. Methods Cell Biol. 96, 259–283 (2010).
Ma, J. et al. Friendly mediates membrane depolarization-induced mitophagy in Arabidopsis. Curr. Biol. 31, 1931–1944 (2021).
Toyooka, K. & Kang, B. H. Reconstructing plant cells in 3D by serial section electron tomography. Methods Mol. Biol. 1080, 159–170 (2014).
Mastronarde, D. N. & Held, S. R. Automated tilt series alignment and tomographic reconstruction in IMOD. J. Struct. Biol. 197, 102–113 (2017).
Yoo, S. D., Cho, Y. H. & Sheen, J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2, 1565–1572 (2007).
Chung, T., Phillips, A. R. & Vierstra, R. D. ATG8 lipidation and ATG8-mediated autophagy in Arabidopsis require ATG12 expressed from the differentially controlled ATG12A and ATG12B loci. Plant J. 62, 483–493 (2010).
Mair, A., Xu, S. L., Branon, T. C., Ting, A. Y. & Bergmann, D. C. Proximity labeling of protein complexes and cell-type-specific organellar proteomes in Arabidopsis enabled by TurboID. eLife 8, e47864 (2019).
Tyanova, S., Temu, T. & Cox, J. The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat. Protoc. 11, 2301–2319 (2016).
Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote) omics data. Nat. Methods 13, 731–740 (2016).
Ashburner, M. et al. Gene Ontology: tool for the unification of biology. Nat. Genet. 25, 25–29 (2000).
Gene Ontology Consortium. The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Res. 49, D325–D334 (2021).
French, A. P., Mills, S., Swarup, R., Bennett, M. J. & Pridmore, T. P. Colocalization of fluorescent markers in confocal microscope images of plant cells. Nat. Protoc. 3, 619–628 (2008).
Bolte, S. & Cordelières, F. P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 (2006).
Gilles, J. F., Dos Santos, M., Boudier, T., Bolte, S. & Heck, N. DiAna, an ImageJ tool for object-based 3D co-localization and distance analysis. Methods 115, 55–64 (2017).
Ma, J. et al. iProX: an integrated proteome resource. Nucleic Acids Res. 47, D1211–D1217 (2019).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (grant nos. 32061160467, 32270291 and 31870171) and Fok Ying-Tong Education Foundation for Young Teachers in the Higher Education Institutions of China (grant no. 171014) to C.G., the National Science Foundation of China (grant no. 31600288) and the Basic Research Program of Guangzhou (grant no. 202201010508) to J.Z., and Hong Kong Research Grant Council (grant nos. GRF14113921, GRF14109222, N_CUHK462/22 and C4002-20W) to B.-H.K. We acknowledge the support of the 2022 Guangdong–Hong Kong–Macao Greater Bay Area Exchange Programs of SCNU.
Author information
Authors and Affiliations
Contributions
J.Z., J.M., B.-H.K. and C.G. designed the experiments. J.Z., J.M., C.Y., X. Zhu, J. Li, X. Zheng, X.L., S.C. and J. Liao performed the experiments. J.Z., J.M., J. Li, L.F., P.W., M.I.H., W.M., X. Zhuang, L.J., B.-H.K. and C.G. analysed the data. J.M., J. Li, P.W., M.I.H., W.M. and B.-H.K. contributed to the EM analysis. J.Z., J.M., C.Y., F.L., C.W., X. Zhuang, L.J., B.-H.K. and C.G. wrote and edited the paper. All authors reviewed the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Yohann Boutte, Tamar Avin-Wittenberg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Acute heat stress induces the formation of ATG8 puncta.
a, Analysis of the patterns of GFP-ATG8a puncta at different temperatures. Five-day-old GFP-ATG8a transgenic seedlings were exposed to various temperatures for 5 mins, then recovered for 3 hrs at 22 °C before confocal imaging. The intensity profiles were calculated along the dotted line indicated in 5x Enlargement. Scale bar, 20 µm. b, Confocal images of HS-induced GFP-ATG8a puncta in different tissues. Five-day-old GFP-ATG8a seedlings were exposed to 45 °C for 5 mins, then recovered for 3 hrs at 22 °C before confocal imaging. c, Analyzing the response of various ATG8 isoforms (ATG8a-i) to HS. Five-day-old GFP-ATG8a, YFP-ATG8b/c/d/e/g/h/i and mCherry-ATG8f transgenic seedlings were exposed to 45 °C for 5 mins, then recovered for 3 hrs at 22 °C before confocal imaging. Scale bars, 20 µm. Similar confocal imaging results in (a–c) were obtained in at least 10 individual roots/leaves with three times of replicates.
Extended Data Fig. 2 Characterize the dynamics of ATG8 puncta.
a, A schematic workflow of heat stress regimes applied to probe YFP-ATG8e response. b, The effect of heat duration on YFP-ATG8e puncta formation. Five-day-old YFP-ATG8e seedlings were exposed to 45 °C for the indicated time points, then recovered for 3 hrs at 22 °C before confocal imaging. Scale bar, 20 µm. c, Time series analysis of the formation of YFP-ATG8e puncta after HS treatment (45 °C, 5 mins). Scale bar, 25 µm. d, The specific response of the mCherry-ATG8f to HS treatment. Five-day-old GFP x mCherry-ATG8f double transgenic seedlings were incubated in liquid 1/2 MS medium without/with 45 °C HS treatment for 5 mins, then visualized under the confocal microscopy after 3 hrs recovery at 22 °C. Scale bar, 20 µm. e, Immunolabeling with ATG8 antibodies showing the punctate structures of native ATG8 in wild-type Col-0 root meristematic cells upon HS treatment. Scale bars, 20 µm. The experiments (b–e) were repeated independently three times with consistent results.
Extended Data Fig. 3 ATG8 puncta induced by HS differ from conventional autophagosomes.
a, Colocalization analysis of mCherry-ATG8f and NBR1-GFP under normal condition or upon HS treatment. Arrowheads indicated colocalized structures, while arrows indicated those that did not colocalize. Scale bars, 20 µm. b, Quantification of colocalization ratio showing in (a). The PSC coefficient was calculated by Image J with the PSC Colocalization plugin. Data represent means ± SD. n = 5 confocal microscopic images (112.5 µm x 112.5 µm) from individual roots. P values were determined by two-tailed unpaired Student’s t-test. c, GFP-ATG8a cleavage assay showing the autophagic activity during the HS recovery phase. The ratio of free GFP to GFP-ATG8a was quantified by Image J. Ponceau S staining indicated the protein loading. Asterisk indicated an unknown band. d, HS-induced GFP-ATG8a puncta were sensitive to ConcA. A schematic diagram illustrating the HS treatment and the addition of ConcA (1 μM) was given. Scale bar, 20 µm. e, Time-course analysis the disappearance of GFP-ATG8a puncta following ConcA application. A schematic diagram illustrating the HS treatment and the addition of ConcA was given on the left. White arrowheads indicated the representative disappearing GFP-ATG8a puncta. Scale bar, 50 µm. f, HS reduced autophagic body formation. The schematic diagram showed the time points of HS treatment and ConcA addition. Scale bar, 20 µm. g, Quantify the ATG8 positive bodies shown in (f). Data represent means ± SD. n = 10 confocal microscopic images (112.5 µm x 112.5 µm) from individual roots. P values were determined by two-tailed unpaired Student’s t-test. The confocal imaging (a, d–f) and western blot (c) were repeated independently three times with consistent results.
Extended Data Fig. 4 Possible role of ATG16 in HS-induced ATG8 puncta formation.
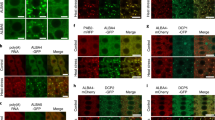
a, Interaction analysis of ATG16 and V-ATPase subunits by Y2H assay. The TGN-localized V0 subunits VHA-a1 and VHA-e1 as well as V1 subunits VHA-A and VHA-C were test for their interaction with full-length ATG16 and the WD40 domain of ATG16. SD-2, synthetic dropout medium lacking Leu and Trp. SD-4, synthetic dropout medium lacking Leu, Trp, His and Ade. b, Response of YFP-ATG16 to HS treatment in Col-0, atg5-1 and atg7-2 root cells. Five-day-old YFP-ATG16/Col-0, YFP-ATG16/atg5-1 and YFP-ATG16/atg7-2 seedlings were incubated in liquid 1/2 MS medium with 45 °C HS treatment for 5 mins, then visualized under the confocal microscopy after recovery at 22 °C for 3 hrs. Scale bar, 20 µm. c, Colocalization analysis of YFP-ATG16 and mCherry-ATG8f or VHA-a1-RFP under normal condition or upon HS treatment. Five-day-old YFP-ATG16 x mCherry-ATG8f and YFP-ATG16 x VHA-a1-RFP double transgenic plants were incubated in liquid 1/2 MS medium at 45 °C for 5 mins, then visualized under the confocal microscopy after recovery for 3 hrs. Scale bar, 20 µm. d, Colocalization analysis of RFP-SYP32 and ATG5-GFP or ATG12A-GFP under normal condition or upon HS treatment. Five-day-old ATG5-GFP x RFP-SYP32 and ATG12A-GFP x RFP-SYP32 double transgenic plants were incubated in liquid 1/2 MS medium at 45 °C for 5 mins, then visualized under the confocal microscopy after recovery for 3 hrs. Scale bar, 20 µm. The experiments were repeated at least three times with similar results.
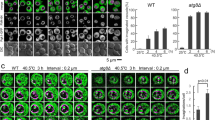
Extended Data Fig. 5 Restacking of Golgi cisternae is delayed in atg5-1 mutant.
a, Representative TEM images of Golgi apparatus in Col-0 and atg5-1 root cells at different recovery time points (3, 6 and 12 hrs). Arrowheads indicate restacking of Golgi cisternae. Similar TEM results were obtained in four individual EM blocks with three times of replicates. Scale bars, 500 nm. b, Quantification of the numbers of Golgi with stacks per cell showing in (a). Data represent means ± SD. n = 20 cells from four individual root blocks were used for quantification analysis. P values were determined by two-tailed unpaired Student’s t-test. c, Positional relationship between VHA-a1-YFP and RFP-SYP32 at different stages of recovery. The intensity profiles were calculated along the dotted arrow indicated in 5x Enlargement. Similar confocal imaging results were obtained in at least 10 individual roots with three times of replicates. Scale bar, 10 µm. d, Quantification of association between VHA-a1-YFP and RFP-SYP32 in (c). The center-to-center distance between VHA-a1-YFP and RFP-SYP32 was calculated with Image J plugin DiAna. n = 1634 closest objects from ten confocal microscopic images (112.5 µm × 112.5 µm). The median (solid line) and quartiles (dotted line) were given in violin plot. P values correspond to two-tailed unpaired Student’s t-test.
Extended Data Fig. 6 HS-induced Golgi swelling inhibits the post-Golgi trafficking.
a, A cell wall polysaccharide, xyloglucan, accumulated in the swollen Golgi lumen after HS treatment. Two xyloglucan-specific antibodies (anti-CCRCM1 and anti-XG) were used for immuno-EM analysis. CW, cell wall; G, Golgi apparatus; M, mitochondria; P, plastid; V, Vacuole. Red arrows indicate gold particles (10 nm). Scale bars, 500 nm. Similar immuno-EM results were obtained in at least four individual root blocks. b, HS-induced punctate signals of SEC-GFP accumulated inside the cells. Five-day-old SEC-GFP seedlings were incubated in liquid 1/2 MS medium with 45 °C HS treatment for 5 mins, then visualized under confocal microscopy after recovery at 22 °C for 3 hrs. Scale bar, 10 µm. c, HS inhibited the internalization of BOR1-GFP in response to high-boron treatment. The BOR1-GFP transgenic plants were grown on low-boron medium (1 µM boric acid) for 5 days and then transferred to high-boron (150 µM boric acid) liquid medium with/without 50 µM BFA for 1 hr before confocal imaging. Scale bar, 20 µm. d, Quantitative analysis of the ratio of BOR1-GFP signal intensity. The fluorescence intensity was calculated with Image J from thirteen root meristematic regions (n = 13). Data represent means ± SD. P values were determined by two-tailed unpaired Student’s t-test. e-f, FRAP analysis of BOR1-GFP dynamics in response to HS. The 5-day-old BOR1-GFP seedlings were treated with CHX (50 μM) for 30 mins prior to FRAP experiments (e), followed by relative fluorescent intensity analysis of BOR1-GFP dynamics (f). Scale bars, 10 μm. Data represent means ± SD from three individual roots (n = 3). The experiments were repeated three times with similar results.
Extended Data Fig. 7 HS abolishes GFP-SYP32 and VHA-a1-RFP response to BFA.
a-b, Effect of HS and BFA on the localization of mCherry-ATG8f and GFP-SYP32 (a), as well as GFP-ATG8a and VHA-a1-RFP (b). The schematic diagrams illustrating the HS treatment and the addition of BFA were given on the left. Similar confocal imaging results were obtained in at least 10 individual roots with three times of replicates. Scale bars, 20 µm.
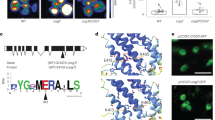
Extended Data Fig. 8 CLC2 interacts with all nine ATG8 isoforms (ATG8a-i).
a, Y2H examining the interaction between CLC2 with nine ATG8 isoforms (ATG8a-i). The Y2H assays were repeated three times with similar results. b, Multiple sequence alignment of the CLC proteins (CLC1 (ABF85787.1), CLC2 (AEC09770.1) and CLC3 (AEE78859.1)) using DNAMAN software. The AIM site in CLC2 was indicated with a red dashed box. Shaded regions show identical amino acid residues among CLC proteins. c, A working model for the in vivo recruitment assay. ATG8 was fused to ER-located calnexin (CNX) to generate a fusion protein CNX-RFP-ATG8. When co-expression of the potential interactors/cargoes of ATG8, they could be recognized by CNX-RFP-ATG8 and then recruited to ER, displaying a linear ER pattern. d, The ER-anchored ATG8e could recruit CLC2-YFP but not CLC2(ΔAIM)-YFP. The different plasmid combinations as indicated were transient expressed in mesophyll protoplasts. After incubated in the dark for 16 hrs, the protoplasts were heat treated at 45 °C for 5 mins, then recovered for 3 hrs at 22 °C before confocal imaging. Scale bar, 10 µm. The in vivo recruitment assays were repeated twice with similar results.
Extended Data Fig. 9 Characterize the dynamics of ATG8 puncta during long-term HS recovery.
a, Representative confocal images showing the patterns of GFP-ATG8a after recovery for 3 to 24 hrs. Five-day-old GFP-ATG8a transgenic seedlings were incubated in liquid 1/2 MS medium with 45 °C HS treatment for 5 mins, then visualized under the confocal microscopy after recovery at 22 °C for the indicated time points. Scale bar, 20 µm. b-c, Colocalization analysis of GFP-ATG8a and VHA-a1-RFP (b) as well as mCherry-ATG8f and NBR1-GFP (c) in root cells after recovery for 3, 6 and 12 hrs. Arrows indicate the separated dots. Scale bars, 20 µm. The colocalization ratio between VHA-a1-RFP and GFP-ATG8a was calculated with Image J plugin JACoP based on distance between centres of mass. Data represent means ± SD. n = 10 confocal microscopic images (112.5 µm × 112.5 µm) from individual roots. P values were analyzed with two-tailed unpaired Student’s t-test. Similar confocal imaging results (a–c) were obtained in at least 10 individual roots with three times of replicates.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2.
Supplementary Tables
Supplementary Tables 1–5.
Supplementary Video 1
Accumulation of GFP–ATG8a-labelled puncta in root cells after exposure to 45 °C for 5 minutes.
Supplementary Video 2
Accumulation of GFP–ATG8a-labelled puncta in mesophyll cells after exposure to 45 °C for 5 minutes.
Supplementary Video 3
Time-lapse imaging of YFP–ATG8e puncta formation after HS treatment.
Supplementary Video 4
ConcA dissociates ATG8 from the HS-induced punctate structures.
Supplementary Video 5
3D electron tomography analysis of the buds generated from the dilated Golgi cisternae after 4 h of recovery from HS.
Supplementary Video 6
Time-lapse imaging of a small dot released from mCherry–ATG8f puncta at 4 h after recovery from HS.
Source data
Source Data Unprocessed Blots
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, J., Ma, J., Yang, C. et al. A non-canonical role of ATG8 in Golgi recovery from heat stress in plants. Nat. Plants 9, 749–765 (2023). https://doi.org/10.1038/s41477-023-01398-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-023-01398-w
This article is cited by
-
Post-transcriptional dynamics and RNA homeostasis in autophagy and cancer
Cell Death & Differentiation (2023)
-
ATG8 keeps Golgi in shape after the heat
Nature Plants (2023)
-
Rescuing the Golgi from heat damages by ATG8: restoration rather than clean-up
Stress Biology (2023)
-
Studying plant autophagy: challenges and recommended methodologies
Advanced Biotechnology (2023)