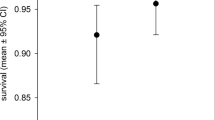
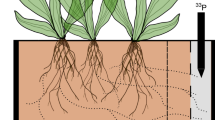
Abstract
Rhizobial nitrogen fixation in legumes provides spillover benefits to neighbouring plants such as pasture grasses. Generally, it is understood to be unidirectional between plant functional groups, providing a benefit from legumes to grasses. We question whether bidirectional complementarity also exists in terms of exploiting the wider soil nutrient pool. We test this hypothesis using soil cores with their component vegetation assemblages sampled from a hill country pasture in South Island, New Zealand. The soil was deficient in key essential elements: P, S, B, Mo and Ni. Facilitation from grasses to clovers was evident; legume–grass mixtures procured more nutrients from the soil than when either species was growing alone. When grasses and clover grow together in unfertilized grassland, more nitrogen is procured by the plant community, and other limiting plant nutrients in the soil are better exploited. Coexistence with grasses is favourable to clovers in terms of soil biogeochemistry.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The raw data supporting the findings of this study are available through https://data.lincoln.ac.nz/. Source data are provided with this paper.
References
Agricultural and Horticultural Land Use (StatsNZ, 2021); https://www.stats.govt.nz/indicators/agricultural-and-horticultural-land-use
Thom, E. R. Hill Country Symposium Grassland Research and Practice Series No. 16 (New Zealand Grassland Association, 2016).
Wardle, P. Vegetation of New Zealand (Cambridge Univ. Press, 1991).
Maxwell, T. M. R., Moir, J. L. & Edwards, G. R. Grazing and soil fertility effect on naturalized annual clover species in New Zealand high country. Rangel. Ecol. Manage. 69, 444–448 (2016).
Maxwell, T., Moir, J. & Edwards, G. Influence of environmental factors on the abundance of naturalised annual clovers in the South Island hill and high country. J. N. Z. Grassl. 72, 165–170 (2010).
Nölke, I., Tonn, B., Komainda, M., Heshmati, S. & Isselstein, J. The choice of the white clover population alters overyielding of mixtures with perennial ryegrass and chicory and underlying processes. Sci. Rep. 12, 1155 (2022).
Fay, P. A. et al. Grassland productivity limited by multiple nutrients. Nat. Plants 1, 15080 (2015).
Zhang, W., Maxwell, T. M. R., Robinson, B. & Dickinson, N. Legume nutrition is improved by neighbouring grasses. Plant Soil (in the press).
Smith, S. E. & Read, D. J. Mycorrhizal Symbiosis (Academic Press, 2008)
Phoenix, G. K., Johnson, D. A., Muddimer, S. P., Leake, J. R. & Cameron, D. D. Niche differentiation and plasticity in soil phosphorus acquisition among co-occurring plants. Nat. Plants 6, 349–354 (2020).
Lambers, H., Clements, J. C. & Nelson, M. N. How a phosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am. J. Bot. 100, 263–288 (2013).
Li, X. et al. Long-term increased grain yield and soil fertility from intercropping. Nat. Sustain. 4, 943–950 (2021).
Homulle, Z., George, T. & Karley, A. Root traits with team benefits: understanding belowground interactions in intercropping systems. Plant Soil 471, 1–26 (2021).
Burrows, C. J. Processes of Vegetation Change (Unwin Hyman, 1990).
Fornara, D. & Tilman, D. Plant functional composition influences rates of soil carbon and nitrogen accumulation. J. Ecol. 96, 314–322 (2008).
Lynch, J. P., Strock, C. F., Schneider, H. M., Sidhu, J. S. & Ajmera, I. Root anatomy and soil resource capture. Plant Soil 466, 21–63 (2021).
Lambers, H., Wright, I. J., Caio, G. & Pereira, P. J. Leaf manganese concentrations as a tool to assess belowground plant functioning in phosphorus-impoverished environments. Plant Soil 461, 43–61 (2021).
Liu, G. & Martinoia, E. How to survive on low potassium. Nat. Plants 6, 332–333 (2020).
Anke, M. & Seifert, M. The biological and toxicological importance of molybdenum in the environment and in the nutrition of plants, animals and man. Part 1: molybdenum in plants. Acta Biol. Hung. 58, 311–324 (2007).
Gylfadóttir, T., Helgadóttir, Á. & Høgh-Jensen, H. Consequences of including adapted white clover in northern European grassland: transfer and deposition of nitrogen. Plant Soil 297, 93–104 (2007).
Maxwell, T. M. L. R. Ecology and Management of Adventive Annual Clover Species in the South Island Hill and High Country of New Zealand. PhD thesis, Lincoln Univ. (2013).
Li, C. et al. Syndromes of production in intercropping impact yield gains. Nat. Plants 6, 653–660 (2020).
McLaren, R. G. & Cameron, K. C. Soil Science: Sustainable Production and Environmental Science (Oxford Univ. Press, 1996).
Chang, X., Duan, C. & Wang, H. Root excretion and plant resistance to metal toxicity. J. Appl. Ecol. 11, 315–320 (2000).
Puschenreiter, M., Gruber, B. & Wenzel, W. W. Phytosiderophore-induced mobilization and uptake of Cd, Cu, Fe, Ni, Pb and Zn by wheat plants grown on metal-enriched soils. Environ. Exp. Bot. 138, 67–76 (2017).
Lu, J. Y. et al. Rhizosphere priming effects of Lolium perenne and Trifolium repens depend on phosphorus fertilization and biological nitrogen fixation. Soil Biol. Biochem. 150, 108805 (2020).
Wang, L., Chen, F. & Wan, K. Y. Research progress and prospects of plant growth efficiency and its evaluation. Soils 42, 164–170 (2010).
Tian, X. Y. et al. Physiological and molecular advances in magnesium nutrition of plants. Plant Soil 468, 1–17 (2021).
Wainwright, M. Sulfur oxidation in soils. Adv. Agron. 37, 349–376 (1984).
Kaiser, B. N., Gridley, K. L., Ngaire Brady, J., Phillips, T. & Tyerman, S. D. The role of molybdenum in agricultural plant production. Ann. Bot. 96, 745–754 (2005).
Khobra, R. & Singh, B. Phytosiderophore release in relation to multiple micronutrient metal deficiency in wheat. J. Plant Nutr. 41, 679–688 (2018).
Erenoglu, B., Eker, S., Cakmak, I., Derici, R. & Römheld, V. Effect of iron and zinc deficiency on release of phytosiderophores in barley cultivars differing in zinc efficiency. J. Plant Nutr. 23, 1645–1656 (2000).
Chen, C., Chaudhary, A. & Mathys, A. Nutritional and environmental losses embedded in global food waste. Resour. Conserv. Recycl. 160, 104912 (2020).
Nyfeler, D., Huguenin-Elie, O., Suter, M., Frossard, E. & Lüscher, A. Grass–legume mixtures can yield more nitrogen than legume pure stands due to mutual stimulation of nitrogen uptake from symbiotic and non-symbiotic sources. Agric. Ecosyst. Environ. 140, 155–163 (2011).
Zhang, C., Postma, J. A., York, L. M. & Lynch, J. P. Root foraging elicits niche complementarity-dependent yield advantage in the ancient ‘three sisters’ (maize/bean/squash) polyculture. Ann. Bot. 114, 1719–1733 (2014).
Ghestem, M., Sidle, R. C. & Stokes, A. The influence of plant root systems on subsurface flow: implications for slope stability. BioScience 61, 869–879 (2011).
Huo, C., Luo, Y. & Cheng, W. Rhizosphere priming effect: a meta-analysis. Soil Biol. Biochem. 111, 78–84 (2017).
Coskun, D., Britto, D. T., Shi, W. & Kronzucker, H. J. How plant root exudates shape the nitrogen cycle. Trends Plant Sci. 22, 661–673 (2017).
Grelet, G. et.al. Regenerative Agriculture in Aotearoa New Zealand: Research Pathways to Build Science-Based Evidence and National Narratives (Landcare Research New Zealand, 2021).
Sergei Schaub, R. F. et al. Plant diversity effects on forage quality, yield and revenues of semi-natural grasslands. Nat. Commun. 11, 768 (2020).
Brooker, R. W. et al. Improving intercropping: a synthesis of research in agronomy, plant physiology and ecology. N. Phytol. 206, 107–117 (2015).
Bardgett, R. D. et al. Combatting global grassland degradation. Nat. Rev. Earth Environ. 2, 720–735 (2021).
Acknowledgements
The research team are grateful to the Miss E. L. Hellaby Indigenous Grasslands Research Trust for grant funding to support Z.W. for his PhD stipend and operational funds. Special thanks also to S. Stephen, B. Richards, E. Huang, L. Hassall and R. Cresswell for practical support.
Author information
Authors and Affiliations
Contributions
All authors contributed to the planning and execution of the project and to manuscript preparation. Z.W. carried out the practical work and data analysis as part of his PhD programme supervised by N.D., T.M. and B.R. N.D. and Z.W. are responsible for the final manuscript draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Plants thanks Rafael Clemente, Chris Anderson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Table 1 and Figs. 1 and 2.
Supplementary Data 1
Data for Supplementary Fig. 1.
Supplementary Data 2
Data for Supplementary Fig. 2.
Source data
Source Data Fig. 1
Processed data for Fig. 1.
Source Data Fig. 2
Processed data for Fig. 2.
Source Data Fig. 3
Processed data for Fig. 3.
Source Data Fig. 4
Processed data for Fig. 4.
Source Data Fig. 5
Processed data for Fig. 5.
Source Data Fig. 6
Processed data for Fig. 6.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, Z., Maxwell, T., Robinson, B. et al. Grasses procure key soil nutrients for clovers. Nat. Plants 8, 923–929 (2022). https://doi.org/10.1038/s41477-022-01210-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-022-01210-1
This article is cited by
-
Invasive weed disrupts facilitation of nutrient uptake in grass-clover assemblage
Soil Ecology Letters (2024)
-
Companion species mitigate nutrient constraints in high country grasslands in New Zealand
Plant and Soil (2023)